Abstract
BACKGROUND
Objective cost estimates and source of cost differences are needed across the spectrum of cognition, including cognitively normal (CN), mild-cognitive-impairment (MCI), newly-discovered dementia, and prevalent dementia.
METHODS
Subjects were a subset of the Mayo Clinic Study of Aging stratified-random sampling of Olmsted County, MN, residents aged 70-89 years. A neurologist reviewed provider-linked medical records to identify prevalent-dementia (review date=index). Remaining subjects were invited to participate in prospective clinical/neuropsychological assessments; participants were categorized as CN, MCI, or newly-discovered-dementia (assessment date=index). Costs for medical services/procedures 1-year pre-index (excluding indirect and long-term care costs) were estimated using line-item provider-linked administrative data. We estimated contributions of care-delivery site and comorbid conditions (including and excluding neuropsychiatric diagnoses) to between-category cost differences.
RESULTS
Annual mean medical costs for CN, MCI, newly-discovered-dementia, and prevalent-dementia were $6,042, $6,784, $9,431, $11,678 respectively. Hospital inpatient costs contributed 70% of total costs for prevalent dementia and accounted for differences between CN and both prevalent and newly-discovered dementia. Ambulatory costs accounted for differences between CN and MCI. Age-, sex-, education-adjusted differences reached significance for CN versus newly-discovered and prevalent-dementia and for MCI versus prevalent-dementia. After considering all comorbid diagnoses, between-category differences were reduced (e.g., prevalent-dementia minus MCI (from $4,842 to $3,575); newly-discovered-dementia minus CN (from $3,578 to$711). Following exclusion of neuropsychiatric diagnoses from comorbidity adjustment, between-category differences tended to revert to greater differences.
CONCLUSIONS
Cost estimates did not differ significantly between CN and MCI. Substantial differences between MCI and prevalent dementia reflected high inpatient costs for dementia and appear partly related to co-occurring Mental Disorders. Such comparisons can help inform models aimed at identifying where, when, and for which individuals proposed interventions might be cost-effective.
Keywords: Dementia, Cognitive status, Mild cognitive impairment, Economics, Utilization, Cost
1. INTRODUCTION
The burden of Alzheimer's disease (AD) and related dementias on affected individuals, families, healthcare providers, and society is substantial and growing, both in the U.S. and elsewhere.[1,2] As life expectancy increases and the “Baby Boom” generation ages, the estimated five million Americans with AD in 2012 is projected to nearly triple to 14 million by 2050.[3] Total payments for health care, long-term care, and hospice for AD and other dementias in the U.S. are projected to increase 6-fold from 214 billion dollars in 2014 to 1.2 trillion dollars in 2050.[3] These projections are especially alarming because existing pharmacological efforts to prevent dementia onset, slow its progression, or mitigate its impact have been largely disappointing.
In response to the impending crisis, a National Alzheimer's Project Act was signed into law in 2011 and the National Plan to Address Alzheimer's Disease was released in May, 2012.[4] The first goal of the National Plan is to find effective ways to prevent and treat AD and other dementias. Reliable estimates of costs associated with cognitive decline will be needed to determine the net cost and/or cost-effectiveness of alternative therapies.
Of existing models constructed to evaluate the economics of dementia prevention, postponement, or treatment,[5-15] few appear to have had simultaneous access to two key elements: detailed objective data on costs and accurate assignment of cognitive status. Objective and complete estimates of direct medical costs can be obtained from billing data. However, reliance on diagnosis codes from billing data to identify dementia has serious limitations, and important biases have been demonstrated.[16-18]
Of those studies in which dementia was carefully assessed, the vast majority have estimated medical costs based solely on self- or proxy-report of utilization (e.g., number of hospitalizations, hospital days, emergency department [ED] visits, office visits) followed by application of average costs per unit obtained for the general population. Such cost estimates may be limited by recall bias and fail to consider higher unit costs for cognitively impaired individuals compared with unimpaired individuals with the same medical conditions.[3,19-21] The few exceptions with access to objective cost estimates using administrative data[22,23] have typically been limited to fee-for-service Medicare data, thus missing non-Medicare costs and those for the nearly 30% of Medicare managed care enrollees.[24]
Regardless of across-study differences and limitations, the devastating economic consequences of Alzheimer's disease and other dementias are observed for both direct (including medical and nursing home care) and indirect (informal) care. There is general agreement that mean direct medical cost differences between persons with and without dementia are greatest for hospital inpatient use and that comorbidity plays an important role. However, a majority of studies of comorbidity costs have been limited to a few self-reported conditions or medications. More objective data on a broader range of conditions are needed to inform where excess costs for individuals with dementia might be reduced.
There is less appreciation for the extent and source of excess medical costs associated with cognitive impairment that does not meet criteria for dementia. Depending on the question being addressed and where interventions may have the greatest impact, there is a need for estimates of costs across the spectrum of cognition, including the ability to distinguish cognitively normal individuals from those meeting criteria for mild cognitive impairment (MCI) and from those meeting criteria for previously undiagnosed dementia.[25-27] The difficulties noted above for assigning both cognitive status and objective cost estimates for dementia are magnified for these earlier stages. Of three reports estimating MCI-associated costs separately,[28-30] MCI cognitive status was determined using currently accepted criteria[31,32] in two.[28,29] One of the two was drawn from clinical trials, with MCI cases referred for informant-identified memory complaints.[28] Both were limited to comparisons between individuals with and without MCI and thus excluded comparisons that may be relevant for conversion from MCI to dementia. None of the three previous studies had access to objective cost estimates.
This study seeks to add to our understanding of direct medical costs (excluding long-term care costs) across the spectrum of cognitive decline by employing three unique population-based resources: a) a medical records-linkage infrastructure system that includes detailed clinical data for essentially all residents of Olmsted County, MN;[33,34] b) a prospective cohort study consisting of randomly sampled Olmsted County residents age 70-89 years who were assessed for cognitive status using neurologic evaluation and neuropsychological testing;[35] and c) provider-linked billing data consisting of line-item detail that affords direct cost estimates for essentially all medical services and procedures received by County residents (excluding long-term care).[36] These resources provide a rare opportunity to compare direct medical costs for individuals categorized as cognitively normal (hereafter referred to as CN), MCI, newly-discovered dementia, and prevalent dementia. The present study also investigates factors associated with between-category cost differences. Findings will help address the recognized need to inform future projections regarding which interventions might be most cost-effective for which individuals, in which settings, and at which stage of cognitive decline.[25,37]
2.METHODS
2.1. Design/setting/resources
Rochester Epidemiology Project (REP)
This population-based cross-sectional study was conducted in Olmsted County, MN. The capability for epidemiologic studies in this setting results from a unique set of circumstances. Rochester, the county seat (2010 census 144,248), is approximately 80 miles from the nearest major metropolitan area and home to Mayo Clinic, one of the world's largest medical centers. Mayo Clinic and Olmsted Medical Center (OMC), a second group practice, and their affiliated hospitals, provide essentially all medical care received by local residents. Since 1907, every Mayo patient is assigned a unique identifier. Detailed information from every contact (office, nursing home, emergency department, hospital inpatient and outpatient) is contained within a unit record for each patient. Information includes medical history, clinical assessments, consultations, surgical procedures, dismissal summaries, laboratory and radiology results, correspondence, death certificates, and autopsy reports. Diagnoses assigned at each visit are coded and entered into continuously updated files. Under auspices of the REP, the unique identifiers, diagnostic index, and records-linkage were expanded to include other medical providers, including OMC and the few private practitioners in the area.[34] Recent enhancements afford an essential enumeration of all Olmsted County residents on any given date from 1965 through present.[33]
Mayo Clinic Study of Aging (MCSA)
As described in detail elsewhere,[35] REP resources were used to construct an age- and sex-stratified sampling frame of Olmsted County residents aged 70–89 years. The population was initially sampled in 2004. To maintain cohort size, additional samplings have been conducted in subsequent years, employing procedures used in 2004. All inpatient and ambulatory medical records of sampled individuals are reviewed by a neurologist for prevalent dementia, defined using Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria.[38,39] After additionally excluding terminally ill individuals and those who cannot be contacted, remaining individuals are invited to participate in prospective in-person evaluations. Individuals who decline the in-person evaluation are invited to participate in a telephone interview.[35,40]
In-person evaluations consists of a nurse interview, a neurologic evaluation by a physician, and extensive cognitive testing by a psychometrist. The interview includes questions regarding age, education, marital status, etc. Questions about memory are administered to the participant. The Clinical Dementia Rating (CDR) scale[41] and Functional Activities Questionnaire (FAQ)[42] are administered to an informant. The neurologic evaluation includes the Short Test of Mental Status,[43] a medical history review, and a complete neurologic examination. Neuropsychological testing is performed to assess impairment in four cognitive domains: memory (three tests), executive function (two tests), language (two tests), and visuospatial skills (two tests). Domain scores are computed as previously described.[35,44]
MCSA diagnostic criteria
Performance in a cognitive domain is assessed by comparing the participant's domain score with scores for an independent sample of cognitively normal subjects from the Olmsted County population.[44] Cognitive impairment is considered if the score is ≥1.0 standard deviation below the mean. However, the final decision is based on consensus agreement among the examining physician, nurse, and neuropsychologist, taking into account education, prior occupation, visual or hearing deficits, and other information.[45]
Among in-person participants, a newly-discovered dementia is based on DSM-IV criteria.[39] MCI is determined as follows: cognitive concern by subject (from interview), informant (from the CDR scale), nurse, or physician; impairment in one or more of the four cognitive domains (from cognitive battery); essentially normal functional activities (from the CDR scale and FAQ); and absence of dementia. Subjects are categorized as CN if they perform within the normative range and do not meet criteria for MCI or dementia.[32,35,45] Further staging was precluded absent collection of bio-marker data during calendar years for this study.[46]
Subjects who elect to participate by telephone only are interviewed using the 50-item Modified Telephone Interview for Cognitive Status (TICS-m).[35,40,47,48] Based on a validation study in this cohort,[40] a TICS-m cutoff score ≤31 is used to define MCI and ≤27 is used to define newly-discovered dementia.
Olmsted County Healthcare Expenditure and Utilization Database (OCHEUD)
Due to the geographic isolation and limited number of providers, >95% of all medical care encounters by Olmsted County residents occur at either Mayo Clinic, OMC, or affiliated hospitals.[49] Through an electronic data-sharing agreement between Mayo Clinic and OMC, patient-level administrative data on healthcare utilization and associated billed charges incurred at these institutions are shared and archived for use in approved research studies. These electronically-linked data afford complete information on all hospital and ambulatory care delivered by these providers to area residents from 1/1/1987 through the present. The files include information on all patients (i.e., all ages and payer types, including the uninsured) and contain line-item detail on date, type, frequency, and billed charge for every good or service provided each individual. Long-term care costs are not included. OCHEUD's costing algorithm employs widely-accepted valuation techniques to generate standardized inflation-adjusted cost estimates for each service or procedure in constant dollars. A nationally-representative calendar-year-specific dollar cost is assigned each line item.[50] Present study estimates were adjusted to represent 2010 dollars. A detailed description of the costing methodology is provided elsewhere.[36]
2.2 Study sample
This study was approved by Mayo Clinic and OMC Institutional Review Boards. The sample consists of MCSA subjects identified for 2004 and 2008 sampling frames (n=6,682). Five hundred twelve individuals were excluded who were found upon medical record review to have resided outside Olmsted County or who refused authorization for use of medical records in research.[51] As described above, inpatient and ambulatory medical records were reviewed for prevalent dementia; remaining individuals were presumed to be dementia-free and were invited to participate in prospective evaluations. Four hundred eighty four individuals had met criteria for prevalent dementia, and 538 individuals were excluded due to terminal illness or inability to be contacted. Of the 5,148 who remained eligible for prospective assessment, 1,777 (34%) refused the invitation. There were 3,371 individuals who were prospectively assessed at baseline, either in person (n=2,447) or by telephone (n=924). Individuals with indeterminate cognitive status (12 in-person and 68 telephone subjects) were excluded from analysis, as were 184 enrolled in the telephone interview who did not return the Health Insurance Portability and Accountability Act form,[52] an institutional requirement for using survey results in research. A flow chart is provided in Appendix.
2.3. Data collection
For the present study, participants enrolled in the prospective portion of MCSA were assigned the cognitive status determined at the baseline (i.e., the first) assessment. The baseline assessment date was defined as the index date. For persons with prevalent dementia determined from record review, the date of record review was defined as the index date. Index dates ranged from 11/2/2004 through 8/2/2010. OCHEUD billing data were used to obtain line-item detail on all medical services and procedures, site of care delivery, and all International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes[53] assigned each individual the full year before index.
Site of care
Care-delivery site was determined using “location of service” codes from OCHEUD line-item detail. Site was categorized as hospital inpatient, hospital outpatient, emergency department (ED), or ambulatory (including office visits, outpatient laboratory and radiology tests, etc.). OCHEUD outpatient medication use/costs are not available electronically for the study period. The present study is limited to direct medical care; nursing home use and reimbursed costs will be provided in a subsequent manuscript.
Comorbid conditions
From the list of all diagnosis codes assigned 1-year before index, those for dementia were excluded. To explore differences among cognitive categories with respect to all other diagnoses, each non-dementia code assigned each individual was categorized into ICD-9-CM chapters and subchapters. To assess the contribution of comorbid conditions to direct medical costs, we used Johns Hopkins Adjusted Clinical Groups (ACG) System® software[54] to assign a Resource Utilization Band (RUB) value to each individual. ACG software first categorizes an individual's ICD-9-CM-coded diagnoses based on persistence, severity, and etiology of the condition, as well as diagnostic certainty, and need for specialty care.[54] RUB values are then assigned based on aggregations of ACGs that have similar expected resource use, with values ranging from 0 (no encounters), to 5 (diagnosis codes associated with very high use).[55]
2.4. Statistical analysis
Subject characteristics
Comparisons among cognitive categories for age, sex, education, proportions of individuals with any activity (overall and by site of care), and proportions of individuals with at least one diagnosis code in an ICD-9-CM chapter and subchapter were conducted using analysis of variance, Chi-square, and Fisher's exact tests. Comparisons among cognitive categories for RUB distributions were conducted using Mantel-Haenszel Chi-Square test. Statistical testing used the two-tailed alpha level of 0.05.
Costs
We estimated direct medical costs in the year before index across the spectrum of cognitive decline from CN through MCI, newly-discovered dementia, and prevalent dementia. Similar to REP studies of population-based cost-of-illness estimates for multiple medical conditions,[36,56-59] we first examined cost distributions within each cognitive category. We then utilized multivariable generalized linear models with a log link and a gamma distribution for the error term to account for skewed cost distributions. This approach enabled coefficients to be directly back transformed into the original dollar scale.[60,61]
We used the method of recycled predictions to analyze differences in costs between cognitive categories. For each between-category comparison, this study employed three separate models: the first with adjustment for age at index, sex, and education; the second with adjustment for age at index, sex, education, and the RUB measure of co-morbidity (after excluding dementia diagnoses); and the third with adjustment for age at index, sex, education, and RUB (excluding both dementia diagnoses and all diagnoses within the ICD-9-CM chapter Mental Disorders). Predicted mean differences and bootstrapped 95% confidence intervals (CI) were calculated.[62,63] All analyses were conducted in SAS version 9.2 (SAS Institute, Cary, NC).
3. RESULTS
3.1. Subject characteristics
Table 1 provides sample sizes and subject characteristics for the 3,591 individuals either identified from record review as prevalent dementia or assessed in-person or by telephone as CN, MCI, or newly-discovered dementia. There was no significant difference in gender distribution. Age increased significantly across cognitive categories. Individuals with newly-discovered or prevalent dementia were approximately 2 years older than those with MCI and 4 years older than CN individuals. Statistically significant declines in education were observed across cognitive categories; however, differences were relatively small, with a median of 12 years for MCI, newly-discovered and prevalent dementia.
Table 1.
Subject characteristics
| Characteristics | Cognitively normal (n=2,451) | Mild cognitive impairment (n=537) | Dementia Newly-discovered (n=119) | Prevalent (n=484) | P value |
|---|---|---|---|---|---|
| Male sex, n (%) | 1,144 (47%) | 277 (52%) | 52 (44%) | 220 (45%) | 0.13 |
| Age at index in years, mean (SD) | 79 (5.2) | 81 (5.0) | 83 (4.9) | 83 (4.5) | <0.001 |
| Years of education, mean (SD) | 14.8 (3.0) | 12.9 (3.1) | 12.0 (3.3) | 12.4 (3.2) | <0.001 |
| Percent of individuals with activity by site* | |||||
| Overall | 98% | 97% | 97% | 96% | 0.11 |
| Ambulatory setting† | 97% | 97% | 97% | 95% | 0.04 |
| Hospital outpatient | 45% | 48% | 40% | 29% | <0.001 |
| Emergency dept. | 26% | 35% | 40% | 52% | <0.001 |
| Hospital inpatient | 18% | 20% | 28% | 40% | <0.001 |
| # of inpatient stays, mean; median‡ | 1.4; 1.0 | 1.4; 1.0 | 1.4; 1.0 | 1.7; 1.0 | <0.001 |
| # of inpatient days, mean; median§ | 4.8; 3.0 | 4.9; 4.0 | 7.2; 4.0 | 11.2; 5.0 | <0.001 |
| Percent surgical stays§ | 8.3% | 7.4% | 6.4% | 3.0% | 0.02 |
Data are unadjusted for age, sex, and years of education
Ambulatory encounters include office visits, outpatient laboratory and radiology activity, etc.
Limited to persons with inpatient stays
Limited to inpatient stays
Site of care
For both overall and ambulatory encounters, at least 95% of individuals had some utilization 1-year before index (Table 1). The proportion with any utilization overall was similar between cognitive categories. For ED and hospital inpatient encounters, the proportions of individuals with any activity increased with increasing cognitive impairment. Number of inpatient stays per person was similar for CN, MCI, and newly-discovered dementia and higher for prevalent dementia. Among individuals with any inpatient stay, those with newly-discovered dementia and prevalent dementia experienced longer stays compared to CN and MCI individuals. The proportion of inpatient encounters that included surgery declined with increasing cognitive impairment. Compared with other categories, prevalent dementia had lower proportions of both hospital outpatient and ambulatory encounters.
Comorbid conditions
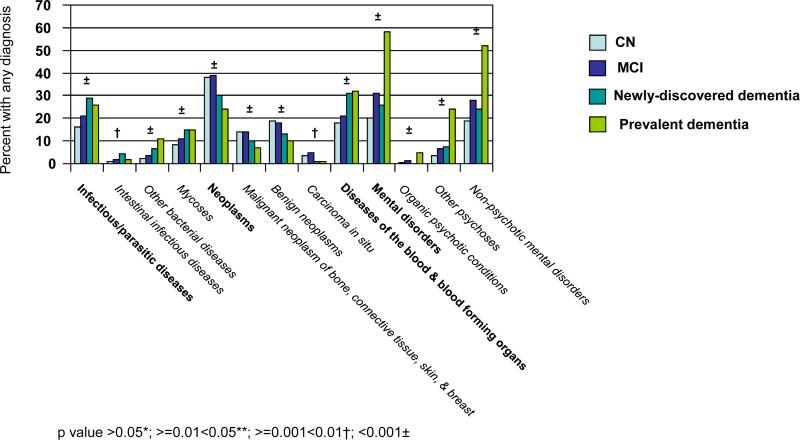
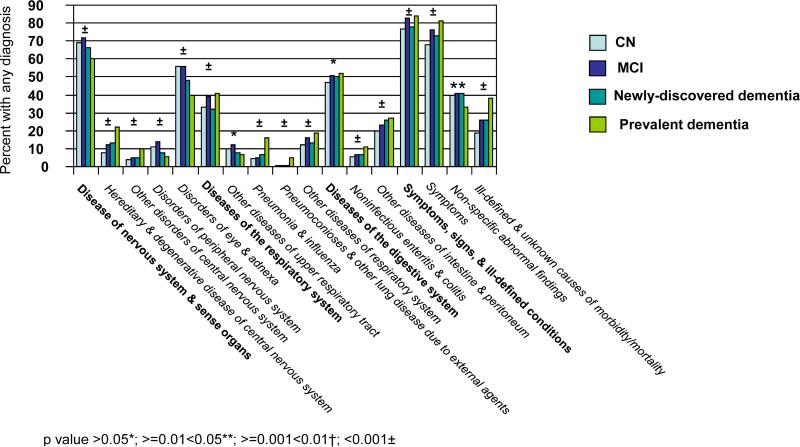
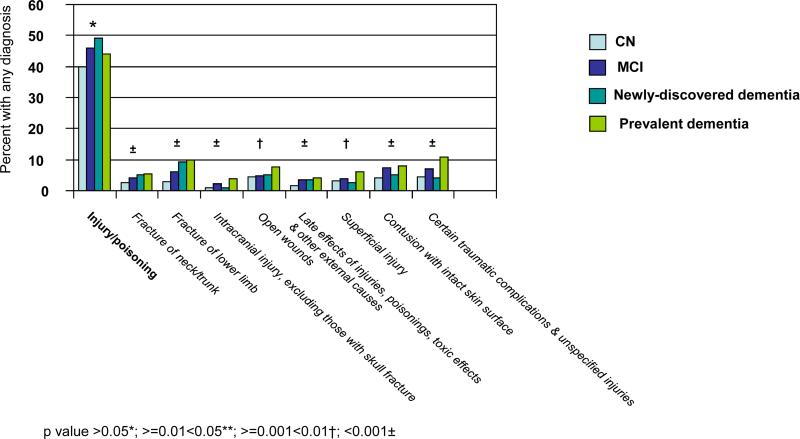
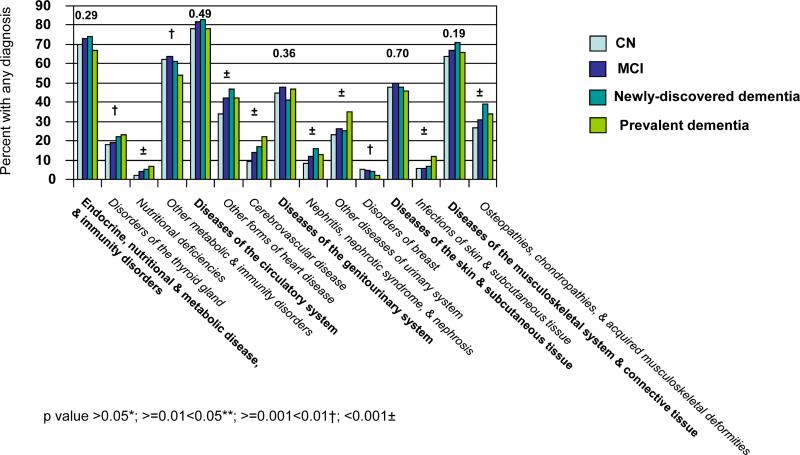
The ICD-9-CM categorization includes 17 chapters and 113 subchapters. Figure 1 provides unadjusted comparisons across cognitive categories of the proportions of individuals with ICD-9-CM clinical diagnoses in the year before index. Figure 1a is limited to the nine chapters with significant differences (p<0.05) across cognitive categories and associated significant subchapters. There was generally a positive correlation between increasing cognitive impairment and the proportion of individuals assigned a diagnosis. Exceptions included the chapter Neoplasms and associated subchapters ‘malignant neoplasms of bone, connective tissue, skin, and breast’; ‘benign neoplasms’; and ‘carcinoma in situ’; the chapter Diseases of Nervous System & Sense Organs and associated subchapters ‘disorders of peripheral nervous system’ and ‘disorders of eye & adnexa’; the subchapter ‘other diseases of upper respiratory tract’ and the subchapter ‘non-specific abnormal findings’.
Figure 1.
The proportion of individuals in each cognitive category with any significant across-category differences* in ICD-9-CM† chapters and/or subchapters assigned in the full year before index
Figure 1a is limited to the nine ICD-9-CM† chapters and associated subchapters for which there was a significant difference across cognitive categories.
Figure 1b is limited to the five ICD-9-CM† chapters for which there was no significant difference across cognitive categories but for which there was a significant difference across categories in one or more subchapters.
*Analyses were unadjusted and conducted using Chi-square and Fisher's exact test
†International Classification of Diseases, 9th Revision, Clinical Modification [53]
With the single exception of the subchapter ‘benign neoplasms’, the proportion of individuals with any diagnosis within each chapter and subchapter in Figure 1a appeared the same or higher for MCI compared to CN. The direction of the association appeared less consistent for comparisons between newly-discovered dementia and MCI. For the category prevalent dementia, the proportion of individuals with a diagnosis was higher for five of the nine significant chapters compared to other categories; the difference was especially marked for the chapter Mental Disorders and for each significant subchapter within that chapter.
Figure 1b is limited to the five chapters with no significant difference across cognitive categories, but for which there were significant differences within associated subchapters. Of these 10 significant subchapters, all but two revealed a general increase in the proportion of individuals with any diagnosis with increasing cognitive impairment. The exceptions were ‘other metabolic and immunity disorders’ and ‘disorders of breast’. ‘Disorders of breast’ was the only subchapter in Figure 1b for which the proportion of individuals with any diagnosis was less for MCI than for CN. Importantly, although the chapter Diseases of the Circulatory System was not itself significant, two subchapters revealed a significant increase in the proportion assigned a diagnosis with increasing cognitive impairment, including ’cerebrovascular disease’.
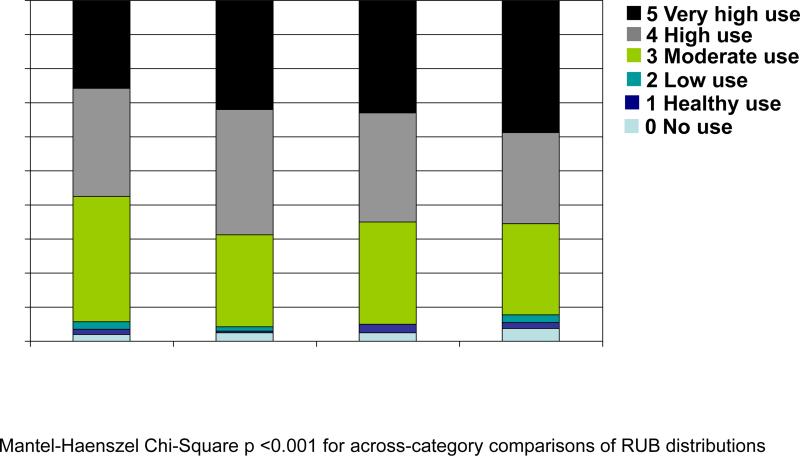
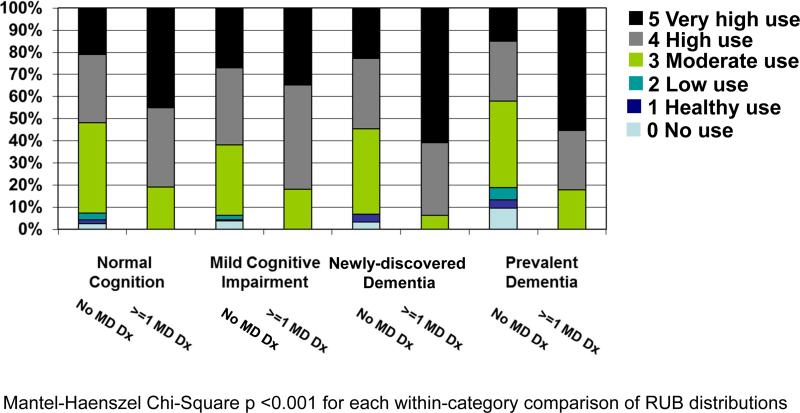
Figure 2 provides the distribution of ICD-9-CM diagnoses aggregated by RUB values. Figure 2a provides RUB distributions by cognitive category and includes all individuals and all diagnoses except dementia diagnoses. For each cognitive category (including CN) 90% of individuals had a RUB value ≥3 (i.e., diagnoses indicative of “moderate”, “high”, or “very high” resource use). The proportion with “very high” use increased with increasing cognitive impairment. Figure 2b provides RUB distributions (again excluding dementia diagnoses) within each cognitive category, comparing individuals who did and did not have any diagnoses in the ICD-9-CM chapter Mental Disorders. Within each cognitive category, the proportion of individuals with diagnoses indicative of very high use was higher for those with any Mental Disorder diagnosis compared with those with no Mental Disorder diagnosis (p<0.001).
Figure 2.
Distribution of Resource Utilization Band (RUB) [55] values*
Figure 2a compares RUB distributions across cognitive categories and includes all diagnosis codes (except dementia diagnoses) and all individuals.
Figure 2b provides RUB distributions within each cognitive category (again excluding dementia diagnoses) and compares individuals with and without any diagnose in the ICD-9-CM† chapter Mental Disorders.
*RUB 0 is limited to non-users. RUB 1 is limited to diagnosis codes in the “preventative/administrative”, eye and dental”, or “acute minor conditions” disease groups (e.g., noninfectious gastroenteritis) and no other diagnoses. There are multiple ways to fall into RUB 2-5. Some helpful examples are provided in the ACG Technical Reference Guide.[55] In our sample, examples of RUB assignments included: RUB 2: male age 72 with brief depressive reaction; female age 79 with central hearing loss; male age 80 with diabetes mellitus general medical exam. RUB 3: female age 74 with malignant neoplasm of breast; female age 79 with catatonic schizophrenia; male age 83 with panic disorder and urinary tract infection. RUB 4: male age 77 with hypertension, general medical examination, ischemic heart disease, congenital heart disease, cardiac valve disorders, gastrointestinal signs/symptoms, diverticular disease of colon, chest pain, and lower back pain. RUB 5: female age 78 with diabetes mellitus, general medical examination, cardiovascular symptoms, cardiac arrhythmia, sinusitis, abdominal pain, anorectal conditions, benign/unspecified neoplasm, otitis media, cholelithiasis, cholecystitis)
†International Classification of Diseases, 9th Revision, Clinical Modification [53]
3.2. Direct medical costs
Table 2 provides unadjusted cost distributions for each cognitive category, overall and by care-delivery site. Costs were highly skewed within each category. Overall costs ranged from $0-$173,937 (CN); $0-$69,882 (MCI); $0-$140,559 (newly-discovered dementia); and $0-$354,786 (prevalent dementia). Importantly, the distribution of costs generally increased with increasing cognitive impairment. Total unadjusted mean direct medical costs 1-year before index were 12% higher for MCI vs. CN, 39% higher for newly-discovered dementia vs. MCI, and 24% higher for prevalent dementia vs. newly-discovered dementia. Hospital inpatient costs accounted for >43% of all costs within each category, and fully 70% of all costs for prevalent dementia. ED costs accounted for <7% of all costs in each category. For both hospital inpatient and ED, the proportion of total costs within these sites generally increased with increasing cognitive impairment. By contrast, the proportion of all costs that occurred in hospital outpatient and ambulatory sites generally decreased with increasing cognitive impairment. In each site, a very few individuals experienced extremely high costs, and in some sites, >50% of individuals experienced no costs.
Table 2.
Distribution of unadjusted direct medical costs* 1 year before index for each cognitive category, overall and by site of care
| Cognitively normal (n=2,451) | Mild cognitive impairment (n=537) | Newly-discovered dementia (n=119) | Prevalent dementia (n=484) | |
|---|---|---|---|---|
| Total Costs | ||||
| Mean | $6,042 | $6,784 | $9,431 | $11,678 |
| Median | $2,218 | $2,767 | $2,028 | $3,168 |
| 25th, 75th percentile | $782, $5,993 | $1,084, 7,117 | $827, $9,248 | $764, $11,098 |
| Minimum, Maximum | $0, $173,937 | $0, $69,882 | $0, $140,559 | $0, $354,786 |
| Hospital Inpatient | ||||
| Mean | $2,751 | $2,956 | $5,471 | $8,203 |
| Median | $0 | $0 | $0 | $0 |
| 25th, 75th percentile | $0, $0 | $0, $0 | $0, $2,649 | $0, $7,108 |
| Minimum, Maximum | $0, $167,736 | $0, $55,708 | $0, $134,333 | $0, $299,227 |
| Percent of total costs | 46% | 44% | 58% | 70% |
| Hospital Outpatient | ||||
| Mean | $1,322 | $1,473 | $1,876 | $1,338 |
| Median | $0 | $0 | $0 | $0 |
| 25th, 75th percentile | $0, $1,563 | $0, $1,879 | $0, $1,146 | $0, $635 |
| Minimum, Maximum | $0, $48,246 | $0, $37,816 | $0, $47,713 | $0, $51,628 |
| Percent of total costs | 22% | 22% | 20% | 11% |
| Emergency Dept. | ||||
| Mean | $256 | $363 | $455 | $690 |
| Median | $0 | $0 | $0 | $157 |
| 25th, 75th percentile | $0, $69 | $0, $289 | $0, $362 | $0, $970 |
| Minimum, Maximum | $0, $8,768 | $0, $4,977 | $0, $5,287 | $0, $11,971 |
| Percent of total costs | 4% | 5% | 5% | 6% |
| Ambulatory Visits | ||||
| Mean | $1,713 | $1,992 | $1,629 | $1,446 |
| Median | $1,171 | $1,406 | $1,163 | $984 |
| 25th, 75th percentile | $602, $2,195 | $698, $2,525 | $603, $2,010 | $466, $1,803 |
| Minimum, Maximum | $0, $42,086 | $0, $45,015 | $0, $7,121 | $0, $14,064 |
| Percent of total costs | 28% | 29% | 17% | 12% |
Long-term care costs are excluded
For all sites combined, Table 3 provides mean predicted direct medical costs and mean predicted difference in costs for each between-category comparison for three separate models. All models were adjusted for age, sex, and education. For each model, between-category cost differences increased markedly with increasing impairment of the category being compared. In the model adjusted only for age, sex, and education, the confidence intervals excluded zero (i.e., reached statistical significance) for comparisons between CN and newly discovered dementia, CN and prevalent dementia, and MCI and prevalent dementia.
Table 3.
Predicted mean direct medical costs for each cognitive category and between-category cost differences one year before index,* adjusted for age, sex, education, and two formulations of comorbidity
| Referent Category | Comparison Category | Predicted Mean Costs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, Sex, and Education Adjusted | Age, Sex, Education, and RUB Adjusted | |||||||||
| RUB Excludes Dementia Diagnoses | RUB Excludes Dementia Diagnoses and all Mental Disorder Diagnoses | |||||||||
| Referent Category | Comparison Category | Comparison minus Referent (95%CI) | Referent Category | Comparison Category | Comparison minus Referent (95%CI) | Referent Category | Comparison Category | Comparison minus Referent (95%CI) | ||
| Cognitively Normal |
Mild Cognitive Impairment |
$6,054 | $6,837 | $783 (−$147, $1,763) |
$6,618 | $6,781 | $163 (−$849, $1,307) |
$6,602 | $6,987 | $385 (−$715, $1,506) |
| Newly Discovered Dementia |
$6,018 | $9,596 | $3,578 ($444, $7,431) |
$6,619 | $7,330 | $711 (−$1,059, $2,603) |
$6,663 | $7,354 | $691 (−$1,042, $2,527) |
|
| Prevalent Dementia |
$6,002 | $10,142 | $4,140 ($3,713, $8,579) |
$6,386 | $10,289 | $3,903 ($587, $6,971) |
$5,791 | $12,342 | $6,551 ($1,941, $10,240) |
|
| Mild Cognitive Impairment |
Newly Discovered Dementia |
$6,802 | $9,331 | $2,529 (−$479, $5,889) |
$7,777 | $8,380 | $603 (−$1,417, $2,920) |
$7,943 | $8,149 | $206 (−$1,794, $2,435) |
| Prevalent Dementia |
$6,783 | $11,625 | $4,842 ($2,625, $7,417) |
$7,208 | $10,783 | $3,575 (−$70, $5,778) |
$6,388 | $11,228 | $4,840 ($1,535, $7,118) |
|
| Newly Discovered Dementia |
Prevalent Dementia |
$9,048 | $11,959 | $2,911 (−858, 6,444) |
$8,493 | $11,046 | $2,553 (−$1,272, $5,215) |
$7,443 | $10,777 | $3,334 ($350, $5,899) |
Index was defined as record review date for prevalent dementia; for subjects assessed as cognitively normal, mild cognitive impairment, or newly-discovered dementia, cognitive status was that determined at their baseline (i.e., first) assessment, and index was defined as the assessment date. †Separate models were run for estimating each between-category difference. †Slight variability in predicted mean costs for the same category results from the methodology; recycled predictions sets all individuals to the referent category or the comparison category, while all other individual characteristics remain as observed [62, 63]. More than 95% of individuals in each category had some medical costs in the year before the index date, thus we did not employ two-part models as recommended if zero costs are of concern [84].
After adjustment for the RUB calculation of summary comorbidity that considered all diagnoses except dementia, the 95% confidence intervals excluded zero for only CN and prevalent dementia. Visual comparisons with models which adjusted only for age, sex, and education reveal markedly lower point- estimates for between-category differences in mean predicted costs, e.g., the difference between newly-discovered dementia and MCI decreased from $2,529 to $603. Importantly, however, as revealed by the right-most column in Table 3, much of the reductions in between-category cost differences following adjustment for all comorbid conditions were mitigated when diagnoses contained in the ICD-9-CM chapter Mental Disorders were excluded from the RUB adjusting variable.
4. DISCUSSION
This study provides objective estimates of direct medical care use and costs for persons across the spectrum of cognitive decline, from CN through MCI, newly-discovered dementia, and prevalent dementia. Mean direct annual cost estimates for individuals with prevalent dementia were $11,678, nearly twice those for CN individuals ($6,042). Greater than 95% of individuals within each category had some costs in the year before index. However, consistent with findings for health expenditures generally,[64] a large proportion of costs within each site were accrued by relatively few individuals. The single exception was ambulatory visits. Although 79% of CN and MCI individuals and >59% of individuals with prevalent dementia had no hospital inpatient encounter, hospital inpatient costs accounted for a substantial proportion of all costs within each cognitive category, and fully 70% of all costs for prevalent dementia.
For the vast majority of ICD-9-CM chapters/subchapters with significant across-category differences in the proportion of individuals with any diagnosis code, the proportions increased as cognitive impairment increased (Figure 1). The few exceptions prompt speculation that persons with prevalent dementia are less likely than CN or MCI individuals to be seen for minor conditions and preventive care. This suggestion is reinforced by observations that a) aggregation of individual's diagnosis codes into RUB groupings indicative of resource use revealed increasing proportions in the “very high use” group as cognitive impairment increased and b) rates of ambulatory and hospital outpatient encounters were relatively low for persons with dementia.
Although direct medical costs for individuals with MCI were higher than those for CN individuals within each care delivery setting under investigation, the overall annual age- sex- and education-adjusted predicted mean difference of $783 was not statistically significant. Age- sex- and education-adjusted predicted mean differences in costs reached significance for CN versus newly discovered dementia, CN versus prevalent dementia, and for MCI versus prevalent dementia. Addition of a summary measure of comorbidity to age- sex- and education-adjusted models revealed dramatic reductions in between-category cost differences. The only between-category difference that remained significant was that for CN versus prevalent dementia. Each of the other differences now had confidence intervals that included zero, leading us to conclude that much of the observed increases in overall costs with increasing cognitive impairment were attributable to comorbid conditions.
We specifically investigated the contribution of Mental Disorders (including psychosis, depression, anxiety, agitation, and several other neuropsychiatric conditions) to this reduction in cost differences by excluding diagnoses within that ICD-9-CM chapter from our calculation of RUB. In general, the reductions in between-category cost differences following adjustment for all comorbid conditions were less evident when Mental Disorders were not considered in the adjustment. Further investigation is needed to fully address the question of whether comorbid conditions associated with between-category cost differences are risk factors for, co-travelers with, and/or consequences of cognitive impairment. We concluded that significant cost increases for prevalent dementia relative to MCI and CN categories are concentrated within the hospital inpatient site and among relatively few individuals with high comorbidity.
Comparison with other cost studies is limited by marked between-study differences, including ascertainment of cognitive status, age range, study period, source of cost data, extent to which comorbid conditions were considered, and statistical analyses. Such differences have contributed to conflicting findings among previous studies, even for comparison between dementia and non-dementia.[65-68] Regarding comparisons between prevalent dementia and CN, our results reflect pooled conclusions by others that a) medical costs are higher for individuals with dementia compared to those without dementia; b) differences are especially great for hospital inpatient costs; c) among hospitalized patients, those with dementia are admitted for different reasons and longer stays; d) dementia subjects with selected comorbid conditions have higher costs than those for subjects with similar conditions but no dementia; and e) dementia-associated use is reduced following adjustment for comorbidity.[3,5,19,20,23,69-73]
We are aware of only two other studies [28,29] with estimates of direct medical costs associated with MCI in which MCI was identified using currently accepted diagnostic criteria.[31,32] Luppa et al.[29] identified German primary care patients age 75+ with (n=39) and without (n=413) MCI. No significant difference in direct medical costs was found, either overall or for any cost category except pharmaceuticals (p=0.047). The difference between subjects with and without MCI for total mean annual direct costs (after translating Euros to U.S. dollars) was similar to our estimate of $742.
By contrast, Zhu et al.[28] found substantial differences in baseline average annual direct medical cost per person between subjects with MCI ($6,499) and without MCI ($2,969). The number of selected self-reported medical conditions was associated with higher costs in both groups; however, with few exceptions (renal/genitourinary, neurological, and respiratory problems), presence of medical conditions were similar between participants with and without MCI.
The marked differences between our findings and those by Zhu et al. may reflect differences in study design. The age range of subjects in Zhu et al.'s study was 55-90 years versus 70-89 years in our study. Costs associated with cognitive impairment may be greater at younger ages. Subjects in Zhu et al.'s study were drawn from clinical trials; cases were referred for memory problems and selection of controls required absence of depression or other neurodegenerative conditions. As noted by the authors and others,[74] generalizability may be limited because community-dwelling older adults with MCI are typically older with more medical problems and rarely have their cognitive impairment identified.[74]
Studies by Luppa et al.[29] and Zhu et al. [28] consisted only of comparisons between normal and MCI individuals. Comparisons between MCI and dementia (newly discovered or prevalent) are needed to inform efforts to prevent or postpone cognitive decline across the full spectrum. Wimo et al.[30] used Mini Mental Status Examination scores to categorize individuals as normal (24-30), MCI (18-23), and dementia (<18). Consistent with our significant difference in annual costs between persons with MCI and those with prevalent dementia, Wimo et al. concluded that postponement between MCI and manifest dementia may result in short-term benefits (a few years) of about $5300.
4.1. Strengths
This study has several strengths. The sample is population-based. Cognitive status at baseline for CN, MCI, and newly-discovered dementia was assessed comprehensively using information from a neurologic evaluation by a physician, a nurse interview, and neuropsychological testing; the diagnosis was made by consensus. Hospital inpatient, outpatient, ED, and ambulatory care sites were included. Costs were based on provider-linked billing data containing detailed objective data for essentially all medical services and procedures provided each individual 1-year before index. Our analyses accounted for the extremely skewed nature of cost data and adjusted for differences between cognitive categories in age, sex, education, and clinically-diagnosed comorbid conditions. Analyses of comorbidity considered all hospital and ambulatory diagnoses assigned each individual over a full year; the RUB summary measure is a preferred measure for cost adjustment.[75]
4.2. Limitations
Study limitations include that estimates are for a single geographic population, which in 2010 was 86% white. Although limited to Olmsted County, MN, rates of chronic disease prevalence are very similar to those for Minnesota generally and all other upper midwest states.[76] Olmsted County age- sex- and racial-distributions are also similar to these geographic regions; however, Olmsted County residents exhibit higher income and education (Olmsted County vs. Minnesota respectively for 2000: median household income=$51,316 vs. $47,111; % with bachelor's degree or higher=35% vs. 27%).[76] Among Medicare eligible residents, the mean (SD) number of inpatient stays and inpatient days respectively are similar for Olmsted County [0.33 (0.83); 1.5 (5.9)] and non-Olmsted County Minnesota [0.30 (0.78); 1.4 (5.1)].[49] While no single geographic area is representative of all others, the under-representation of minorities and the fact that essentially all medical care is delivered by few providers compromises the generalizability of our study findings to different racial and socio-economic groups and different health care environments.
The present study was limited to eligible persons who did not refuse participation (see Appendix). For subjects with prevalent dementia based on record review, previous studies reveal that the proportion of all Olmsted County residents who refuse use of medical records for research is <5%.[51] For subjects who were eligible for participation in the prospective study, previous studies reveal that MCSA subjects who refused participation are older, more likely male, and more likely to have greater comorbidity. Likelihood of participation was not associated with history of stroke, hypertension, coronary heart disease, marital status, or prior clinical diagnosis of MCI or dementia.[35].
The study included both in-person and telephone participants. To assess the impact of including the latter, we reanalyzed data from Table 3, excluding telephone participants (data not shown, available upon request). The CI values overlapped between the two approaches. With respect to disparate conclusions regarding significant between-category comparisons; in the right hand column of Table 3, the only comparison that differed was that between newly-discovered and prevalent dementia. The point estimates were very similar, but the cost difference reached significance for analyses that included both in-person plus telephone participants [$3,334 (350 to 5,899)] but did not reach significance for the smaller subset that excluded telephone participants [$3,282 (-1,722 to 7,018)].
The study design was cross-sectional. Cognitive status was defined as of the index date; costs were accrued 1-year before. If some individuals categorized as MCI or dementia at index had progressed within the year before, between-category cost differences may be underestimated. Subsequent studies will follow MCSA subjects for costs accrued over sequential assessments, e.g. as they progress from MCI to newly-discovered dementia.
Prevalent dementia was identified based on neurologist's application of DSM-IV criteria following detailed medical record review;[38] information on duration and severity were not always reliably available; thus we were unable to estimate the contribution of these characteristics to increased costs.
This study did not include outpatient pharmaceutical costs. No indirect or long-term care costs were included. It is recognized that long-term care and indirect costs, including the burden for caregiver/spouses of affected individuals, contribute greatly to the excess costs associated with dementia.[3,23] Nursing home cost estimates will be afforded in future investigations with access to Centers for Medicare and Medicaid Services Minimum Data Set [MDS] for MCSA subjects.[77]. Our preliminary review of MDS data suggests that the proportion of MCSA subjects with ≥1 nursing home day in the year before index was 3.1%, 4.5%, 12%, and 35% for CN, MCI, newly-discovered dementia, and prevalent dementia respectively.[78] Consistent with findings by others,[28] it is unlikely that nursing home costs contribute greatly to MCI or CN costs.
4.3. Implications
Findings presented here for a single year reinforce the urgent need to address the impending crisis posed by rising numbers of persons within categories of CN and MCI who are currently at risk of dementia nationwide, and in the coming decades. Higher costs for both newly-discovered and prevalent dementia compared to CN and MCI categories appeared largely attributable to inpatient costs, with longer stays and a higher proportion of medical vs. surgical admissions. Based on reasons for admission recorded in billing data (i.e., principal discharge diagnosis codes), it is increasingly suggested that persons with dementia are over-hospitalized, and many hospitalizations are potentially preventable.[20,21,65,69,71-73,79]. Our findings do not appear to suggest excessive use of surgery in dementia patients—there is a possibility that surgery is underutilized. While our findings may lend support for potentially preventable medical stays, we caution that diagnosis codes may insufficiently capture all reasons for admission, including other medical conditions, behavioral and management issues, adjustment of complicated medication regimens, caregiver needs, and post-acute care reimbursement rules.
It has recently been noted that the current research focus on prevention of individual diseases largely ignores competing risk. It is suggested that greater reductions in morbidity, mortality, and federal spending would result from placing greater emphasis on the underlying biology of aging, with the goal of slowing the aging process generally.[80] The argument for reductions in federal spending, the focus of which is costs at the population level, is reinforced by our findings of extremely high direct medical costs observed for a very few individuals in every cognitive category (including CN). However, if the question under investigation has the individual as its focus (as is true for this study), it is important to note that direct medical cost differences between cognitive categories remained high after accounting for skewed distributions and adjusting for age, sex, education, and all comorbid conditions (Table 3).
To the extent that the argument for a paradigm shift from specific diseases to aging generally relates to medical costs, the argument is also reinforced by the marked reductions in between-category differences in medical costs following adjustment for comorbid conditions (Table 3). This finding (which importantly excludes indirect and long-term care costs) suggests that cognitive differences alone do not contribute greatly to medical cost differences and that focusing on co-occurring conditions could contribute to reductions in excess medical costs associated with increasing cognitive impairment. Moreover, as well recognized by others,[81-83] neuropsychiatric conditions (including depression, anxiety, agitation, psychosis, and other disorders) contribute substantially to the comorbidity associated with cognitive decline. This is consistent with findings in Figures 1a and 2b. When we excluded several such conditions from our calculation of the summary measure of comorbidity, between-category cost differences typically moved closer to estimates obtained absent adjustment for all comorbid conditions (Table 3), reinforcing suggestions that much of the excess burden associated with cognitive decline could be reduced by targeting relevant neuropsychiatric conditions.[82] However, it is important to note that annual costs for persons with prevalent dementia were nearly $4,000 higher than for CN individuals, even after adjustment for age, sex, education, and all diagnosed comorbid conditions.
We observed that, compared to CN individuals, persons with MCI exhibited a) a higher proportion with any diagnosis in 16 of 17 ICD-9-CM chapters, especially mental conditions, b) higher RUB values, and c) higher, but not significantly higher, medical costs. The small cost difference appeared concentrated in ambulatory costs, and diminished markedly following adjustment for RUB. Each of these findings is consistent with problems related to cognitive changes that have yet to reach the threshold for dementia.
The extent to which excess costs observed could be reduced with targeted cognitive testing and disease modifying interventions remains unclear. However, precise, reliable, and objective estimates of the sort provided here provide valuable data to help inform future projections of which interventions would be cost-effective for which individuals at which stage along the spectrum of cognitive decline. We believe that study findings can thus help inform decisions by individuals, providers, payers, researchers, and policy makers to ultimately realize the National Plan to Address Alzheimer's Disease's first goal of finding effective ways to prevent and treat AD and other dementias.
Supplementary Material
Research in Context.
Objective estimates of acute medical care costs are needed across the spectrum of cognition. Subjects were a subset of the Mayo Clinic Study of Aging stratified-random sampling of Olmsted County, MN, residents aged 70-89 years. Prevalent-dementia was identified following neurologist's provider-linked-medical-record review (review date=index). Remaining individuals were categorized as cognitively-normal (CN), mild-cognitive-impairment (MCI), or newly-discovered-dementia using clinical/neuropsychological assessments (assessment date=index). Using provider-linked administrative data, costs for all medical services/procedures 1-year pre-index were estimated. Source of differences (co-morbid conditions and site of care) were investigated. Unadjusted mean costs for CN, MCI, newly-discovered-dementia, and prevalent-dementia were $6,042, $6,784, $9,431, $11,678 respectively. Inpatient use accounted for 70% of prevalent-dementia costs. Age-sex-education-adjusted differences reached significance for CN versus newly-discovered and prevalent-dementia and MCI versus prevalent-dementia. Differences were markedly reduced following adjustment for co-morbid conditions, due largely to mental disorder diagnoses. Estimates reinforce the need for dementia prevention/postponement and inform economic models comparing alternative strategies.
ACKNOWLEDGMENTS
Funding/Support: The present study was funded by AbbVie, Department of Health Economics and Outcomes Research, with support from the Mayo Clinic Study on Aging (NIH U01 AG006786). Some study data were obtained from the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01 AG034676. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of the Sponsors: In support of the manuscript, the NIH had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. Separate from contributions provided by co-authors Hass and Duhig described above, AbbVie reviewed and approved the manuscript.
Financial Support: The present study was funded by AbbVie, Department of Health Economics and Outcomes Research (HEOR), with support from the Mayo Clinic Study on Aging (NIH U01 AG006786). Some study data were obtained from the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01 AG034676. All authors, except SLH, AMD, and KHL, were funded in part by AbbVie, Department of HEOR. SLH and AMD are employees of AbbVie, Department of HEOR. KHL has a subcontract with Mayo Clinic on the AbbVie, Department of HEOR-funded study.
Abbreviations
- ACG
Adjusted Clinical Groups
- AD
Alzheimer's disease
- CDR
Clinical Dementia Rating scale
- CI
Confidence Interval
- CN
Cognitively Normal
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, Fourth edition
- ED
Emergency Department
- FAQ
Functional Activities Questionnaire
- ICD-9-CM
International Classification of Disease, 9th Revision, Clinical Modification
- MCI
Mild Cognitive Impairment
- MCSA
Mayo Clinic Study of Aging
- MDS
Minimum Data Set
- OCHEUD
Olmsted County Healthcare Expenditure and Utilization Database
- OMC
Olmsted Medical Center
- REP
Rochester Epidemiology Project
- RUB
Resource Utilization Band
- SD
Standard Deviation
- TICS-m
Modified Telephone Interview for Cognitive Status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Parts of this manuscript were presented at the American Academy of Neurology Annual Meeting, April 22-27, New Orleans, LA, USA, the Alzheimer's Association International Conference, July 14-19, 2012, Vancouver, Canada, and the Gerontological Society of America 65th Annual Scientific Meeting, November 14-18, 2012, San Diego, CA Word count: Abstract=274; Text=5875
Author Contributions: Dr. Leibson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design and concept: Leibson, Long, Roberts, Hass, Duhig, Pankratz, Petersen
Acquisition of data: Ms. Ransom had full access to all data used in the statistical analyses and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Petersen and Roberts contributed to recruitment and the acquisition of clinical data for MCSA participants.
Analysis of data: Leibson, Long, Ransom, and Smith were each involved with analysis of the data. The analysis, evaluation of the study protocol, and pre-specified plan for data analysis was under the oversight of Dr. Pankratz, a PhD biostatistician at Mayo Clinic, who is a co-investigator on the funded proposal and, as such, received funding from the sponsor. The results of the independent statistical analysis are those reported in the manuscript.
Interpretation of data: Leibson, Long, Ransom, Roberts, Hass, Duhig, Smith, Emerson, Pankratz, Petersen
Additional Contributions: The authors are indebted to the participants in the MCSA study.
REFERENCES
- 1.Wimo A, Jonsson L, Bond J, Prince M, Winblad B. on behalf of Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9:1–11. doi: 10.1016/j.jalz.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer’s Disease International [August 18, 2014];The Global Voice on Dementia. Alzheimer’s Statistics. Available on-line at http://www.alz.co.uk/research/statistics.
- 3.Alzheimer's Association [September 21, 2014];Alzheimer's Disease Facts and Figures. 2014 doi: 10.1016/j.jalz.2014.02.001. Available on-line at http://www.alz.org/downloads/Facts_Figures_2014.pdf. [DOI] [PubMed]
- 4.U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation [September 21, 2014];National Plan to Address Alzheimer’s Disease. 2014 Update. Available on-line at http://aspe.hhs.gov/daltcp/napa/NatlPlan2014.pdf.
- 5.Lin PJ, Yang Z, Fillit HM, Cohen JT, Neumann PJ. Unintended benefits: the potential economic impact of addressing risk factors to prevent Alzheimer's disease. Health Aff (Millwood) 2014;33:547–54. doi: 10.1377/hlthaff.2013.1276. [DOI] [PubMed] [Google Scholar]
- 6.Valcarcel-Nazco C, Perestelo-Perez L, Molinuevo JL, Mar J, Castilla I, Serrano-Aguilar P. Cost-effectiveness of the use of biomarkers in cerebrospinal fluid for Alzheimer's disease. J Alzheimers Dis. 2014;42:777–88. doi: 10.3233/JAD-132216. [DOI] [PubMed] [Google Scholar]
- 7.Long KH, Moriarty JP, Mittelman MS, Foldes SS. Estimating the potential cost savings from the New York University Caregiver Intervention in Minnesota. Health Aff (Millwood) 2014;33:596–604. doi: 10.1377/hlthaff.2013.1257. [DOI] [PubMed] [Google Scholar]
- 8.Skoldunger A, Johnell K, Winblad B, Wimo A. Mortality and treatment costs have a great impact on the cost-effectiveness of disease modifying treatment in Alzheimer's disease--a simulation study. Curr Alzheimer Res. 2013;10:207–16. doi: 10.2174/1567205011310020011. [DOI] [PubMed] [Google Scholar]
- 9.Getsios D, Blume S, Ishak KJ, Maclaine G, Hernandez L. An economic evaluation of early assessment for Alzheimer's disease in the United Kingdom. Alzheimers Dement. 2012;8:22–30. doi: 10.1016/j.jalz.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Zhang K, Lin PJ, Clevenger C, Atherly A. A longitudinal analysis of the lifetime cost of dementia. Health Serv Res. 2012;47:1660–78. doi: 10.1111/j.1475-6773.2011.01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budd D, Burns LC, Guo Z, L'Italien G, Lapuerta P. Impact of early intervention and disease modification in patients with predementia Alzheimer's disease: a Markov model simulation. Clinicoecon Outcomes Res. 2011;3:189–95. doi: 10.2147/CEOR.S22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasuya M, Meguro K. Health economic effect of donepezil treatment for CDR 0.5 converters to Alzheimer's disease as shown by the Markov model. Arch Gerontol Geriatr. 2010;50:295–9. doi: 10.1016/j.archger.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Weimer DL, Sager MA. Early identification and treatment of Alzheimer's disease: social and fiscal outcomes. Alzheimers Dement. 2009;5:215–26. doi: 10.1016/j.jalz.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonsson L, Lindgren P, Wimo A, Jonsson B, Winblad B. Costs of Mini Mental State Examination-related cognitive impairment. Pharmacoeconomics. 1999;16:409–16. doi: 10.2165/00019053-199916040-00008. [DOI] [PubMed] [Google Scholar]
- 15.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–42. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin PJ, Kaufer DI, Maciejewski ML, Ganguly R, Paul JE, Biddle AK. An examination of Alzheimer's disease case definitions using Medicare claims and survey data. Alzheimers Dement. 2010;6:334–41. doi: 10.1016/j.jalz.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Taylor DH, Jr., Ostbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17:807–15. doi: 10.3233/JAD-2009-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newcomer R, Clay T, Luxenberg JS, Miller RH. Misclassification and selection bias when identifying Alzheimer's disease solely from Medicare claims records. J Am Geriatr Soc. 1999;47:215–9. doi: 10.1111/j.1532-5415.1999.tb04580.x. [DOI] [PubMed] [Google Scholar]
- 19.Hill JW, Futterman R, Duttagupta S, Mastey V, Lloyd JR, Fillit H. Alzheimer's disease and related dementias increase costs of comorbidities in managed Medicare. Neurology. 2002;58:62–70. doi: 10.1212/wnl.58.1.62. [DOI] [PubMed] [Google Scholar]
- 20.Lyketsos CG, Sheppard JM, Rabins PV. Dementia in elderly persons in a general hospital. Am J Psychiatry. 2000;157:704–7. doi: 10.1176/appi.ajp.157.5.704. [DOI] [PubMed] [Google Scholar]
- 21.Weiner M, Powe NR, Weller WE, Shaffer TJ, Anderson GF. Alzheimer's disease under managed care: implications from Medicare utilization and expenditure patterns. J Am Geriatr Soc. 1998;46:762–70. doi: 10.1111/j.1532-5415.1998.tb03814.x. [DOI] [PubMed] [Google Scholar]
- 22.Albert SM, Glied S, Andrews H, Stern Y, Mayeux R. Primary care expenditures before the onset of Alzheimer's disease. Neurology. 2002;59:573–8. doi: 10.1212/wnl.59.4.573. [DOI] [PubMed] [Google Scholar]
- 23.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–34. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bynum JP. The long reach of Alzheimer's disease: patients, practice, and policy. Health Aff (Millwood) 2014;33:534–40. doi: 10.1377/hlthaff.2013.1247. [DOI] [PubMed] [Google Scholar]
- 25.Lin PJ, Neumann PJ. The economics of mild cognitive impairment. Alzheimers Dement. 2013;9:58–62. doi: 10.1016/j.jalz.2012.05.2117. [DOI] [PubMed] [Google Scholar]
- 26.Sperling RA, Jack CR, Jr., Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3:111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Institute on Aging [September 21, 2014];Alzheimer’s disease progress report: seeking the earliest interventions. 2012–2013 Available on-line at http://www.nia.nih.gov/alzheimers/publication/2012-2013-alzheimers-disease-progress-report.
- 28.Zhu CW, Sano M, Ferris SH, Whitehouse PJ, Patterson MB, Aisen PS. Health-related resource use and costs in elderly adults with and without mild cognitive impairment. J Am Geriatr Soc. 2013;61:396–402. doi: 10.1111/jgs.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luppa M, Heinrich S, Matschinger H, Hensel A, Luck T, Riedel-Heller SG, et al. Direct costs associated with mild cognitive impairment in primary care. Int J Geriatr Psychiatry. 2008;23:963–71. doi: 10.1002/gps.2018. [DOI] [PubMed] [Google Scholar]
- 30.Wimo A, Winblad B. Pharmacoeconomics of mild cognitive impairment. Acta Neurol Scand Suppl. 2003;179:94–9. doi: 10.1034/j.1600-0404.107.s179.13.x. [DOI] [PubMed] [Google Scholar]
- 31.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–6. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 32.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 33.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173:1059–68. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melton LJ., 3rd. History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 35.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leibson CL, Katusic SK, Barbaresi WJ, Ransom J, O'Brien PC. Use and costs of medical care for children and adolescents with and without attention-deficit/hyperactivity disorder. JAMA. 2001;285:60–6. doi: 10.1001/jama.285.1.60. [DOI] [PubMed] [Google Scholar]
- 37.Alzheimer’s Association [September 21, 2014];Changing the trajectory of Alzheimer’s disease: A national imperative. http://www.alz.org/documents_custom/trajectory_appendix_a.pdf.
- 38.Knopman DS, Petersen RC, Rocca WA, Larson EB, Ganguli M. Passive case-finding for Alzheimer's disease and dementia in two U.S. communities. Alzheimers Dement. 2011;7:53–60. doi: 10.1016/j.jalz.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 40.Knopman DS, Roberts RO, Geda YE, Pankratz VS, Christianson TJ, Petersen RC, et al. Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology. 2010;34:34–42. doi: 10.1159/000255464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 42.Pfeffer RI, Kurosaki TT, Harrah CH, Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–9. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 43.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48:725–8. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 44.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, et al. Mayo's Older Americans Normative Studies: WAIS-R, WMS-R and AVLT norms for ages 56 through 97. Clin Neuropsychol. 1992;6:1–104. [Google Scholar]
- 45.Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75:889–97. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1993;6:103–10. [Google Scholar]
- 48.Cook SE, Marsiske M, McCoy KJ. The use of the Modified Telephone Interview for Cognitive Status (TICS-M) in the detection of amnestic mild cognitive impairment. J Geriatr Psychiatry Neurol. 2009;22:103–9. doi: 10.1177/0891988708328214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The Dartmouth Institute for Health Policy & Clinical Practice. Dartmouth Atlas of Healthcare; [September 21, 2014]. Available on-line at http://www.dartmouthatlas.org/. [Google Scholar]
- 50.Wagner JL, Alberts SR, Sloan JA, Cha S, Killian J, O'Connell MJ, et al. Incremental costs of enrolling cancer patients in clinical trials: a population-based study. J Natl Cancer Inst. 1999;91:847–53. doi: 10.1093/jnci/91.10.847. [DOI] [PubMed] [Google Scholar]
- 51.Melton LJ., 3rd. The threat to medical-records research. N Engl J Med. 1997;337:1466–70. doi: 10.1056/NEJM199711133372012. [DOI] [PubMed] [Google Scholar]
- 52.Government Printing Office [September 21, 2014]; Public Law 111-148--Mar.23, 2010. Available on line at http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf.
- 53.Centers for Disease Control and Prevention [September 21, 2014];International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) Available online at http://www.cdc.gov/nchs/icd/icd9cm.htm.
- 54.Johns Hopkins Bloomberg School of Public Health [September 21, 2014];The Johns Hopkins ACG® System. 2010 Available on-line at http://www.acg.jhsph.org/.
- 55.Johns Hopkins Bloomberg School of Public Health [September 21, 2014];The Johns Hopkins ACG® System: Excerpt from Technical Reference Guide, Version 9.0. 2009 Dec; Available on-line at http://www.healthpartners.com/ucm/groups/public/@hp/@public/documents/documents/dev_057914.pdf.
- 56.Long KH, Rubio-Tapia A, Wagie AE, Melton LJ, 3rd, Lahr BD, Van Dyke CT, et al. The economics of coeliac disease: a population-based study. Aliment Pharmacol Ther. 2010;32:261–9. doi: 10.1111/j.1365-2036.2010.04327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leibson CL, Long KH, Maraganore DM, Bower JH, Ransom JE, O'Brien PC, et al. Direct medical costs associated with Parkinson's disease: A population-based study. Mov Disord. 2006;21:1864–71. doi: 10.1002/mds.21075. [DOI] [PubMed] [Google Scholar]
- 58.Gabriel SE, Tosteson AN, Leibson CL, Crowson CS, Pond GR, Hammond CS, et al. Direct medical costs attributable to osteoporotic fractures. Osteoporos Int. 2002;13:323–30. doi: 10.1007/s001980200033. [DOI] [PubMed] [Google Scholar]
- 59.Begley CE, Famulari M, Annegers JF, Lairson DR, Reynolds TF, Coan S, et al. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia. 2000;41:342–51. doi: 10.1111/j.1528-1157.2000.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 60.Birnbaum HG, Ben-Hamadi R, Greenberg PE, Hsieh M, Tang J, Reygrobellet C. Determinants of direct cost differences among US employees with major depressive disorders using antidepressants. Pharmacoeconomics. 2009;27:507–17. doi: 10.2165/00019053-200927060-00006. [DOI] [PubMed] [Google Scholar]
- 61.Mullahy J. Much ado about two: reconsidering retransformation and the two-part model in health econometrics. J Health Econ. 1998;17:247–81. doi: 10.1016/s0167-6296(98)00030-7. [DOI] [PubMed] [Google Scholar]
- 62.Esposito D, Bagchi AD, Verdier JM, Bencio DS, Kim MS. Medicaid beneficiaries with congestive heart failure: association of medication adherence with healthcare use and costs. Am J Manag Care. 2009;15:437–45. [PubMed] [Google Scholar]
- 63.Basu A, Arondekar BV, Rathouz PJ. Scale of interest versus scale of estimation: comparing alternative estimators for the incremental costs of a comorbidity. Health Econ. 2006;15:1091–107. doi: 10.1002/hec.1099. [DOI] [PubMed] [Google Scholar]
- 64.Cohen SB, Uberoi N. Differentials in the concentration in the level of health expenditures across population subgroups in the U.S. [September 21, 2014];2010 Medical Expenditure Panel Survey. 2013 Aug; Statistical Brief #471 Available on-line at http://meps.ahrq.gov/mepsweb/data_files/publications/st421/stat421.shtml. [PubMed]
- 65.Caspi E, Silverstein NM, Porell F, Kwan N. Physician outpatient contacts and hospitalizations among cognitively impaired elderly. Alzheimers Dement. 2009;5:30–42. doi: 10.1016/j.jalz.2008.05.2493. [DOI] [PubMed] [Google Scholar]
- 66.Lamb VL, Sloan FA, Nathan AS. Dementia and Medicare at life's end. Health Serv Res. 2008;43:714–32. doi: 10.1111/j.1475-6773.2007.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ayyagari P, Salm M, Sloan FA. Effects of diagnosed dementia on Medicare and Medicaid program costs. Inquiry. 2007;44:481–94. doi: 10.5034/inquiryjrnl_44.4.481. [DOI] [PubMed] [Google Scholar]
- 68.Leibson C, Owens T, O'Brien P, Waring S, Tangalos E, Hanson V, et al. Use of physician and acute care services by persons with and without Alzheimer's disease: a population-based comparison. J Am Geriatr Soc. 1999;47:864–9. doi: 10.1111/j.1532-5415.1999.tb03846.x. [DOI] [PubMed] [Google Scholar]
- 69.Feng Z, Coots LA, Kaganova Y, Wiener JM. Hospital and ED use among medicare beneficiaries with dementia varies by setting and proximity to death. Health Aff (Millwood) 2014;33:683–90. doi: 10.1377/hlthaff.2013.1179. [DOI] [PubMed] [Google Scholar]
- 70.Callahan CM, Hendrie HC, Tierney WM. Documentation and evaluation of cognitive impairment in elderly primary care patients. Ann Intern Med. 1995;122:422–9. doi: 10.7326/0003-4819-122-6-199503150-00004. [DOI] [PubMed] [Google Scholar]
- 71.Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307:165–72. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin PJ, Biddle AK, Ganguly R, Kaufer DI, Maciejewski ML. The concentration and persistence of health care expenditures and prescription drug expenditures in Medicare beneficiaries with Alzheimer disease and related dementias. Med Care. 2009;47:1174–9. doi: 10.1097/MLR.0b013e3181b69fc1. [DOI] [PubMed] [Google Scholar]
- 73.Eaker ED, Mickel SF, Chyou PH, Mueller-Rizner NJ, Slusser JP. Alzheimer's disease or other dementia and medical care utilization. Ann Epidemiol. 2002;12:39–45. doi: 10.1016/s1047-2797(01)00244-7. [DOI] [PubMed] [Google Scholar]
- 74.Fowler NR. Accurate assessments of healthcare use along the course of cognitive decline. J Am Geriatr Soc. 2013;61:450–1. doi: 10.1111/jgs.12135. [DOI] [PubMed] [Google Scholar]
- 75.Clark DO, Von Korff M, Saunders K, Baluch WM, Simon GE. A chronic disease score with empirically derived weights. Med Care. 1995;33:783–95. doi: 10.1097/00005650-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 76.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–60. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Centers for Medicare & Medicaid Services [September 21, 2014];Minimum Data Set (MDS) – Version 2.0 for Nursing Home Resident Assessment and Care Screening. Available on line at http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/downloads/MDS20MDSAllForms.pdf.
- 78.Emerson J, Smith C, Roberts R, Hass S, Duhig A, Pankratz V, et al. Nursing home use and dependencey across the full trajectory of cognitive decline: normal, mild cognitive impairment, newly discovered, and prevalent dementia. Arch Clin Neuropsychol. 2013;28:592–3. [Google Scholar]
- 79.Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52:187–94. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- 80.Goldman DP, Cutler D, Rowe JW, Michaud PC, Sullivan J, Peneva D, et al. Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Aff (Millwood) 2013;32:1698–705. doi: 10.1377/hlthaff.2013.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Geda YE, Roberts RO, Knopman DS, Petersen RC, Christianson TJ, Pankratz VS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry. 2008;65:1193–8. doi: 10.1001/archpsyc.65.10.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geda YE, Schneider LS, Gitlin LN, Miller DS, Smith GS, Bell J, et al. Neuropsychiatric symptoms in Alzheimer's disease: past progress and anticipation of the future. Alzheimers Dement. 2013;9:602–8. doi: 10.1016/j.jalz.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, et al. Neuropsychiatric symptoms in Alzheimer's disease. Alzheimers Dement. 2011;7:532–9. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buntin MB, Zaslavsky AM. Too much ado about two-part models and transformation? Comparing methods of modeling Medicare expenditures. J Health Econ. 2004;23:525–42. doi: 10.1016/j.jhealeco.2003.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.