Abstract
Immunohistochemical and ex vivo anatomical studies have provided many glimpses of the variety, distribution, and signaling components of vertebrate retinal neurons. The beauty of numerous images published to date, and the qualitative and quantitative information they provide, indicate that these approaches are fundamentally useful. However, obtaining these images entailed tissue handling and exposure to chemical solutions that differ from normal extracellular fluid in composition, temperature, and osmolarity. Because the differences are large enough to alter intercellular and intracellular signaling in neurons, and because retinae are susceptible to crush, shear, and fray, it is natural to wonder if immunohistochemical and anatomical methods disturb or damage the cells they are designed to examine. Tissue fixation is typically incorporated to guard against this damage and is therefore critically important to the quality and significance of the harvested data. Here, we describe mechanisms of fixation; advantages and disadvantages of using formaldehyde and glutaraldehyde as fixatives during immunohistochemistry; and modifications of widely used protocols that have recently been found to improve cell shape preservation and immunostaining patterns, especially in proximal retinal neurons.
Keywords: formaldehyde, glutaraldehyde, retina, fixatives, antigen retrieval, quenching, sucrose, pH
1. Introduction
Structural studies that began more than a century ago have shown that retinal cell types differ in shape, dimensions, distribution, connections, and protein expression (Marc, 2008; Marc et al., 2013; Masland, 2001; Polyak, 1941; Ramón y Cajal, 1893; Siegert et al., 2009; Stell, 1972; Walls, 1963; Wässle, 2004). These studies, like those of other tissues, have relied on procedures termed "fixation" to preserve phenotypic properties in morphologically and chemically life-like states (Hopwood, 1985). Ideally, fixation stops physiological responses and metabolic processes within cells, and reduces tissue damage and distortion due to mechanical manipulation and osmotic stress during subsequent processing. Fixation is particularly helpful when examining fragile specimens, and it is necessary when the analytical methods are incompatible with live cells.
Successful fixation preserves tissue rapidly to capture its structure and state at the moment of exposure to the fixing medium, and for periods that permit post-hoc analysis. Although fixation should preserve both the natural shape of cells and the in vivo organization of tissue components (e.g., proteins), existing fixation protocols often strike compromises between morphological preservation and maintenance of the normal chemical environment (Eltoum et al., 2001). Moreover, although well-fixed preparations can be recognized on the basis of gross tissue dimensions and cell morphologies, it is less clear whether a given protocol accurately captures other properties of the same cells, whether the same protocol preserves the phenotypes of all cells, and whether a single protocol can clearly identify cells despite differences in species, age, and/or health.
Sections 2 and 3 describe mechanisms of fixation and immunohistochemistry. Sections 4 and 5 then focus on protocols that use chemical fixatives to define the morphology, organization, and light responses of retinal neurons at the light microscopic level, discussing both positive and negative impacts of chemical fixation on results obtained by immunohistochemistry. Lastly, Section 6 describes alternative fixation strategies that have recently been found to improve the pattern and intensity of immunostaining in proximal neurons of adult mammalian retinae. Three conclusions are supported. Firstly, formaldehyde has enabled investigators to detect and localize a larger variety of molecules that contribute to light sensitivity, synapses, and signal generation in the retina than any other fixative. Secondly, formaldehyde alone is not the best chemical fixative in multiple respects for certain studies. Thirdly, protocols that use formaldehyde-based fixatives can be modified in various ways to improve the preservation of cell phenotypes. Although we are unaware of previous reviews of these topics, we refer readers to two particularly helpful websites (Fisher, 2013; Marc, 2014) and to comparisons of eyes and retinae after fixation by various protocols (Chidlow et al., 2011; Eldred et al., 1983; Hageman and Johnson, 1991; Izumi et al., 2000; Latendresse et al., 2002; Margo and Lee, 1995; Peichl, 1989; Rasmussen, 1974; Webster et al., 1969).
2. Mechanisms of Fixation
Chemical and physical methods of fixation have been developed over more than a century of histological work. The most widely used fixatives are chemical, and these are classified as either crosslinking or coagulant based on their mode of action and effects on soluble proteins.
Crosslinking, non-coagulating chemical fixatives confer structural support without directly changing the overall solubility of individual proteins, linking adjacent tissue structures instead through intermediate molecules. Crosslinks occur at specific target regions, depending on the crosslinking agent used, and the crosslinked macromolecular complexes may have altered water solubility. Crosslinking fixatives include aldehydes and carbodiimides, with aldehydes preferentially crosslinking free amino groups on amino acid chains, and carbodiimides tending to crosslink adjacent carbonyl groups (Hopwood, 1985). Aldehydes are the most common crosslinking fixatives, with formaldehyde generally used for light microscopy and glutaraldehyde generally used for electron microscopy. They differ in their reaction speeds, rates of tissue penetration, avidity for non-protein molecules and, as discussed below, practical advantages and disadvantages. Carbodiimide has rarely been used in published retinal studies (Gastinger et al., 1999; Haverkamp and Wässle, 2000; Ivanova et al., 2013) and will not be considered further here.
Coagulant fixatives decrease protein solubility and initiate protein precipitation from solution, fixing precipitated proteins in place (Boon and Kok, 2008). Coagulant fixatives are fast-acting, so much so that when combined with formaldehyde, the coagulant components are thought to serve as the primary fixative while formaldehyde stabilizes the precipitated proteins into place (Wenk, 2006). Coagulant fixatives comprise two general types: dehydrating fixatives and acidic fixatives (Wenk, 2006).
Dehydrating coagulant fixatives remove the layer of water that normally separates adjacent amino acid chains in live tissue. This initiates intramolecular and intermolecular interactions that encourage spontaneous changes in protein structure and, in turn, water solubility (Boon and Kok, 2008). Ethanol, methanol, and acetone are commonly used dehydrating coagulants. Ethanol has been found to preserve structural features of human eyes at a gross level (Karma et al., 2007; Krauss, 1990). Acetone has been used as a retinal fixative and is compatible with a variety of antibodies (Terada et al., 2006; Terada et al., 2009). However, acetone and methanol are more commonly used as freeze-substitution media.
Acidic coagulating fixatives work differently, by breaking natural crosslinks between neighboring amino acid chains. This allows water to diffuse between adjacent regions, causing changes in protein structure and solubility (Wenk, 2006). Bouin’s fluid combines picric acid and acetic acid with formaldehyde. Its use as a retinal fixative predates modern immunohistochemical methods, although its use has waned due to fixation artifacts (French et al., 2008), the introduction of effective alternatives (Zamboni’s fixative: Stefanini et al., 1967; Zafra et al., 1995; modified Davidson’s fluid: Latendresse et al., 2002), and safety concerns (NIOSH, 2011, 2014).
Coagulant fixatives vary in their ability to precipitate amino acids and nucleotides, and generally have poor lipid fixing characteristics. Some coagulants fix nucleic acids well, but suffer from poor protein stabilization (e.g., acetic acid), while others are much better suited to protein fixation (e.g., Bouin's fluid; Bonin et al., 2005). Some investigators use mixtures of both types of fixative agents to take advantage of the strengths of each. Davidson’s fluid - a blend of ethanol, acetic acid, and formaldehyde - has yielded improved structural preservation relative to Bouin’s fluid in rabbit, rat, and monkey retinae (French et al., 2008; Latendresse et al., 2002) and is compatible with immunohistochemistry in retina (Chidlow et al., 2011; Stradleigh et al., 2011). Clarke's acetic ethanol - a blend of ethanol and acetic acid - has been found to reliably stabilize tissue and cellular structure (Kiernan, 2009), and act on a wide range of target molecules (e.g., glycoproteins and gangliosides; Bee, 1982). Zenker's fixative - a blend of mercuric chloride, potassium dichromate, and acetic acid - was used in early studies of human retinal degeneration (Verhoeff, 1931).
Physical methods of fixation change tissue without the primary action of an added chemical. The simplest such methods are heat and snap freezing. High heat causes protein chains to denature, resulting in altered structure and reduced solubility in water (Barnett et al., 1966; Pinheiro and Lockner, 1963). Microwave heating has been found useful for increasing the rate of heat fixation in ocular tissues (Izumi et al., 2000) and accelerating the action of chemical fixatives in retina (Contini and Raviola, 2003; Harahush et al., 2012; Wendt et al., 2004). We found that microwave heating significantly improved our ability to immunostain amacrine cells across all retinal eccentricities in adult rat (Partida et al., 2004). Conversely, tissue can be fixed by immersion in cold organic solvent (freeze-substitution; Feder and Sidman, 1958; Meissner and Schwarz, 1990; Mobius et al., 2010; Terada et al., 2006; Terada et al., 2009), by snap freezing in liquid nitrogen followed by the removal of water from the tissue via low-temperature vacuum (freeze-drying; Laties, 1966; Liang et al., 2004; Masland and Mills, 1979), or by metal contact rapid freezing (Usukura, 1993). Physical fixation methods have not been widely used in immunohistochemical studies of retina (for examples, see Terada et al., 2009; Yoshiki et al., 1993) and will therefore not be discussed further.
3. Immunohistochemistry
Fixation is widely used to prepare retinae for immunohistochemistry. Antibodies directed against a wide variety of specific antigen molecules (e.g., cytoskeletal components, enzymes, glycoproteins, second messengers, and neurotransmitters) are available for these studies. When coupled with a method to localize the antibody-antigen complex, antibodies can be used to determine the distribution and relative concentration of an antigen in situ. Provided adequate fixation, the antigen is immobilized within the tissue in a distribution assumed to be similar to that found in vivo. Before discussing problems associated with fixation, we briefly describe what antibodies bind to and how bound complexes are typically visualized.
3.1 Epitopes and visualizing antibody-antigen complexes
Antibodies bind to antigens to form an antibody-antigen complex through noncovalent interactions, and each individual antibody molecule recognizes a specific region on the antigen, known as an epitope. An antibody recognizes its particular epitope via the antigen’s spatial arrangement and the distribution of electronegative charges across its surfaces. Two types of epitopes are found in protein or polypeptide antigens. The simplest is linear, determined by the sequence of amino acid residues along the polypeptide chain. These linear epitopes are a minority population, discussed further in Section 4. Most epitopes - estimated to be 90% of known epitopes (Huang and Honda, 2006) - are conformational. These consist of discontinuous segments that form a protein's higher-order structure and, due to their chemical and structural complexity, pose several challenges in immunohistochemical methods.
Antibody-antigen complexes can be visualized in various ways, and all methods use high contrast probes to localize the antibody-antigen complexes against immunonegative backgrounds. These methods differ in usefulness and visualization techniques. The earliest light microscopic immunohistochemical methods deposited chromogenic substances within tissues (Bubenik et al., 1974; Nakane and Pierce, 1966; Riepe and Norenburg, 1977). In chromogenic immunohistochemistry, the antibodies directed against the antigen of interest ("primary antibodies") are visualized by binding with a “secondary antibody” covalently conjugated to an oxidizing enzyme (e.g., horseradish peroxidase), the antibody-enzyme complexes are incubated with the fixed tissue, and the tissue is exposed to a chromogenic reporter substrate (e.g., 3-3’-diaminobenzidine; DAB). The antibody-conjugated enzyme then oxidizes the reporter molecule, causing precipitation out of solution and deposition of reporter within the tissue near the antigen (Graham and Karnovsky, 1966). Deposited reporter appears brown or purple within the tissue, and is easily visualized on a light microscope. The amount of reporter substrate deposited increases with the amount of time the oxidation reaction proceeds, allowing for great amplification of low-concentration antigens. Chromogenic amplification is a powerful technique and is still used to study the distribution of various antigens in retina, e.g., HuC/D neuronal marker in fish (Rosillo et al., 2013), Bex1 and Brn3b in rat and mouse (Bernstein et al., 2006), and photooxidized DAB in human (da Silva Filho et al., 2013). Non-specific deposition of reporter in the vicinity of the targeted antigen and over-amplification have been found to spread reporter beyond the area immediately around the antibody-antigen complex in some studies (Novikoff et al., 1972), but not others (W.D. Eldred, personal communication).
Immunofluorescence is a popular alternative method for visualizing antigens within fixed tissue, offering an increase in the number of antibody-antigen complexes visualized in a field of view, increasing the spatial resolution in imaged sections, and allowing for quantitative signal analysis. The simplest immunofluorescence methods rely on direct conjugation of fluorescent dyes, or fluorophores, to antibodies directed against antigens of interest (Riggs et al., 1958). Following excitation by suitable illumination, the emitted fluorescence is imaged to map the antigen within the tissue. Signal can be amplified by indirect immunofluorescence methods in which fluorophores are conjugated to secondary antibodies, which in turn bind to the primary antibody-antigen complex. Many such secondary antibodies can bind a single primary antibody-antigen complex, resulting in increased sensitivity to low-concentration antigens while preserving spatial resolution.
Retinal vertical sections and flat mounts are often immunostained for two or three antigens, especially if primary antibodies raised in different host species are available. Although technically more difficult, some studies have successfully visualized the binding of antibodies directed against as many as seven antigens (see Cuenca et al., 2014; Deerinck, 2006; Fischer, 2008; Mills and Massey, 2014). Fluorescence intensities have been measured to assess levels (quantitatively or semi-quantitatively) of a wide variety of retinal antigens, including visual pigments, ion channels, and regulatory molecules (Galbinur et al., 2009; Goodyear et al., 2010; Rodger et al., 2005; Tanito et al., 2002; see also Sokolov et al., 2002). Quantitative measures are also possible in chromogenic methods (Tezel et al., 2003; Tezel and Wax, 2004), provided the activity of the antibodybound enzymes are strictly controlled. Fluorescence and chromogenic methods can be used interchangeably in both immunohistochemistry (Smith et al., 1983) and in situ hybridization (Saez et al., 2006) with no significant differences in sensitivity or specificity (Garcia-Caballero et al., 2010). Optimal visualization technique may depend upon factors such as embedding medium and post-fixation treatments (Gallegos Ruiz et al., 2007).
3.2. Purpose of fixation and associated problems
Although antibodies can bind to cell membranes and extracellular matrices of unfixed tissue, antibodies are relatively large (15–20 nm diameter) proteins and consequently diffuse slowly through tissue (e.g., to reach the inner plexiform layer when applied to the vitread or sclerad side of a flat-mounted retina, or to reach the middle of a transretinal section when applied to the section face and edges). In solutions typically used to dilute antibodies (phosphate-buffered saline supplemented with small amounts of detergent), unfixed tissue would degrade (Espina et al., 2009) during the relatively long incubation times that have been found to improve immunostaining (e.g., 5–10 days: Brecha et al., 1988; Massey and Mills, 1996). Immunostaining protocols therefore immerse retinae in chemical fixatives prior to incubation in antibodies. Ideal fixatives would not reduce accessibility or affinity of epitopes for antibodies, distort cell shape, or alter the linear dimensions of tissue. Although not commonly reported, aldehyde fixatives can cause immunostaining intensity to fall, retinae to shrink, and bead-like varicosities to form in cell dendrites and axons. These problems arise from fixation that is, in part, either insufficient or excessive. Both are discussed in Section 4, along with protocols modified to minimize or block these artifacts.
4. Formaldehyde
Formaldehyde has been used as a preservative agent to study retinae of all vertebrate classes and in many applications (e.g., immunohistochemistry, dye injections, expressed markers, histology, and pathology). Fixative solutions can be formed by diluting stock solutions of formalin (typically 37% weight-to-volume (w/v) formaldehyde with methanol added as a stabilizer) or from the hydrolysis of solid polymerized formaldehyde (paraformaldehyde). Whereas paraformaldehyde is insoluble by definition (Manoonkitiwongsa and Schultz, 2002), hydrolysis of the polymer results in a formaldehyde solution. The final product is commonly diluted to a concentration of 4% (w/v) in phosphate buffer or phosphate-buffered saline. Although some studies have used formaldehyde at concentrations as low as 1% (Brandon, 1987; Casini and Brecha, 1992; Kino et al., 2009; Klumpp et al., 1995; Macri et al., 2000; Margo and Lee, 1995; Tkatchenko, 2006; Wässle et al., 1993), we are unaware of any results demonstrating that 4% formaldehyde preserves the morphology or immunoreactivity of retinal neurons better than higher or lower concentrations. Use at 4% probably originated from the use of 10-fold dilutions of stock formalin (Fox et al., 1985).
4.1. Formaldehyde crosslinking
The formaldehyde molecule is composed of a single central carbon atom bound to an oxygen atom (via a double bond) and two hydrogen atoms. It is the simplest aldehyde, highly volatile and gaseous at room temperature. When dissolved in water, formaldehyde forms a methanediol that is reactive with a number of functional groups in solution. Its crosslinking activity results in the addition of a single carbon atom between two previously independent amino acid functional groups. Binding these amino acids occurs via an immonium cation intermediate or by the Mannich reaction, depending on the functional groups present, reaction temperature, and pH (Hermanson, 2013).
The immonium cation mechanism relies on formaldehyde reacting with a primary amine to form a quaternary ammonium salt. Primary amines possess a central nitrogen atom attached to two hydrogen atoms and a carbon chain, and are found at the N-terminus of amino acid chains, as well as in the side chains of glycine, glutamine, asparagine, arginine, and lysine. The ammonium intermediate spontaneously reacts to create an active immonium cation which is highly reactive towards primary amines in other molecules, and capable of reacting less vigorously with sulfhydryls, phenolic groups, and imidazole nitrogen atoms. The reaction yields a methylene bridge between the linked groups, binding macromolecules with a one-carbon linker (Fraenkel-Conrat and Olcott, 1948).
During the Mannich reaction, formaldehyde condenses with a primary or secondary amine-containing compound (and sometimes amide groups), and another compound containing an active hydrogen atom (as found on a phenol group). A common example of such a bond is found in crosslinks between arginine amino groups and tyrosine phenols (Sompuram et al., 2006; Sompuram et al., 2004). The Mannich reaction is inefficient at room temperature, typically requiring elevated temperatures to completely crosslink (e.g., incubation at 37–57°C for 2–24 hours; Hermanson, 2013).
Although the immonium ion mechanism and the Mannich reaction can occur simultaneously at room temperature, the majority of crosslinking in minimally fixed tissue probably occurs via the immonium ion pathway due to its faster reaction rate (Hermanson, 2013). Reducing the temperature of fixation below room temperature greatly favors the immonium ion reaction.
4.2. Rate of formaldehyde fixation
Saturation of small pieces of tissue and initial binding of formaldehyde to amino acids is complete in 24 hours (Helander, 1994), although complete methylene bridge formation in tissue requires 24–48 hours at room temperature (Werner et al., 2000). We know of no systematic tests of fixation time on structural preservation of retinal neurons. However, dye injection and immunohistochemical studies have routinely immersed retinae in formaldehyde-based fixatives for periods ranging from 5 min to overnight (Brandon, 1985b; Eldred et al., 1983; Morgans, 2001). Studies using brief fixations have reported that immunostaining intensity is reduced by longer fixations (Hack et al., 2001; Haverkamp and Wässle, 2000; Koulen et al., 1998). The rationale for immersing retinae in formaldehyde for several hours is that formaldehyde-stabilized antigens and structures are more resistant to unfixing and extraction during subsequent processing (Stradleigh et al., 2011).
The preferred duration of fixations can be limited by how tissues are exposed to fixatives. Immersion permits simple, fast, and thorough fixation provided the retina is directly exposed to fixative. Alternatively, tissue can be fixed by transcardial perfusion (Gage et al., 2012; Lamberts and Goldsmith, 1986). However, this requires large volumes of fixative and relatively long initial fixations (Kasukurthi et al., 2009).
Given access differences between antibodies and epitopes (e.g., those near the retinal surface as opposed to those buried within a given piece of retina; those facing extracellular space as opposed to those facing cytoplasm), it is difficult to state a priori a period of time that would be universally optimal for immersion fixation. Nevertheless, in the hope of avoiding excessive and insufficient fixation (e.g., De Marzo et al., 2002), problems attributable to each are discussed in Section 4.4.
4.3. Advantages of formaldehyde fixation
Fixatives containing 4% formaldehyde have been so widely used in retinal light microscopic immunohistochemistry that their usefulness is rarely questioned. Two primary attractions of using formaldehyde are that it is commonly available at reliably high purities and that formaldehyde-fixed retinae have been immunostained with antibodies directed against a wide range of functionally important proteins. The latter include light-sensitive pigments, cGMP-gated ion channels, and associated modulators (Hattar et al., 2002; Haverkamp et al., 2005; Molday et al., 1991; Philp et al., 1987; Seydewitz et al., 2004; Sokolov et al., 2002; Wikler and Rakic, 1990); neurotransmitters, neuromodulators, receptors, synthesizing enzymes, inactivating enzymes, and release-related proteins (Brandon, 1987; Brandstätter et al., 1999; Brecha et al., 1979; Famiglietti and Tumosa, 1987; Guo et al., 2010; Haverkamp et al., 2000; Hendrickson et al., 1985; Keyser et al., 1988; Wagner et al., 1993; Yamada et al., 1980); voltage-gated ion channels, auxiliary subunits, and connexins (Janssen-Bienhold et al., 1998; Klumpp et al., 1995; Morgans, 2001; Müller et al., 2003; Reyes et al., 2000; Stradleigh et al., 2011; Taylor and Morgans, 1998; Van Wart et al., 2007; Wollner and Catterall, 1986); transporters, cytoskeletal elements, anchoring proteins, and signaling cascade components (Grunert and Wässle, 1993; Haase et al., 1990; Haverkamp and Wässle, 2000; Hu and Wensel, 2004; Krizaj and Copenhagen, 1998; Morgans et al., 1998; Ogata et al., 2012; Partida et al., 2004; Röhrenbeck et al., 1989; Ryskamp et al., 2011; Stahl and Baskin, 1984; Volgyi et al., 2005; Zucker, 1998); and markers that have been transported, gene-gunned, or transiently expressed (Coombs et al., 2006; Dacey et al., 2003; Morgan et al., 2006; Rockhill et al., 2002; Siegert et al., 2009; Stradleigh et al., 2015; Stradleigh et al., 2011).
Downsides notwithstanding, formaldehyde has enabled investigators to detect and localize a larger variety of molecules that contribute to light sensitivity, synapses, and signal generation in the retina than any other fixative. This approach is powerful because it can (1) demonstrate the presence of proteins independently from other methods (e.g., electrophysiology); (2) detect protein isoforms that are difficult to distinguish by other methods (e.g., pharmacology); (3) do so in more cells than can be examined by single-cell methods; and (4) do so regardless of cell size, cell type, population density, or location (viz., retinal eccentricity, quadrant, and/or transretinal/vertical level). It has been possible to immunostain formaldehyde-fixed preparations with multiple primary antibodies (i.e., antibodies directed against more than one epitope) and thus test for colocalization of different antigens (Brandstätter et al., 2004; Li et al., 1986; Mills et al., 2001; Röhlich et al., 1994; Sassoe-Pognetto et al., 1995; Stradleigh et al., 2011), cell-specific expression (Hoshi et al., 2009; Lin and Masland, 2005; O'Brien et al., 2006; Rodriguez et al., 2014; Wässle et al., 2009), expression in identifiable subcellular compartments (Boiko et al., 2003; Greenberg et al., 2011; Jakobs et al., 2008; Rasband et al., 1999; Van Wart et al., 2007; Wu et al., 2013; Xu et al., 2008), and changes in the presence or localization of one antigen with opposite or no changes in another antigen (Elias et al., 2004; Ogata et al., 2012). Briefly stated, some of the most informative and aesthetically impressive images of immunostained retinae have been obtained with formaldehyde-fixed preparations that highlight specific populations of rods, cones (Bumsted and Hendrickson, 1999; Cuenca et al., 2014; Elias et al., 2004; Haverkamp et al., 2000; Haverkamp et al., 2005; Hornstein et al., 2004; Li and DeVries, 2004; O'Brien et al., 2012), bipolar cells (DeVries, 2000; Keeley and Reese, 2010; Kouyama and Marshak, 1992; Wässle et al., 2009; Young and Vaney, 1991), horizontal cells (Mills and Massey, 1994; O'Brien et al., 2006; Piccolino et al., 1984; Wässle et al., 2000), amacrine cells (Badea et al., 2009; Dacey, 1990; Lin and Masland, 2006; Lindstrom et al., 2009; Mills et al., 2001; Petrides and Trexler, 2008; Tauchi and Masland, 1984; Trexler et al., 2001; Voigt and Wässle, 1987), and ganglion cells (Coombs et al., 2006; Hattar et al., 2002; Jakobs et al., 2008; Kim et al., 2008; Nelson et al., 1978; O'Brien et al., 2002; Ogata et al., 2012; Rockhill et al., 2002; Van Wart et al., 2007; Vaney, 1991; Volgyi et al., 2005; Volgyi et al., 2009; Xu et al., 2008), as well as flat mounts and vertical sections stained to show multiple cell types (Cuenca, 2008; Deerinck, 2006; Fischer, 2008; Hoshi et al., 2009; Light et al., 2012; Majumdar et al., 2008; Mills and Massey, 2014; Morgan et al., 2006; Siegert et al., 2009; Zhang et al., 2005).
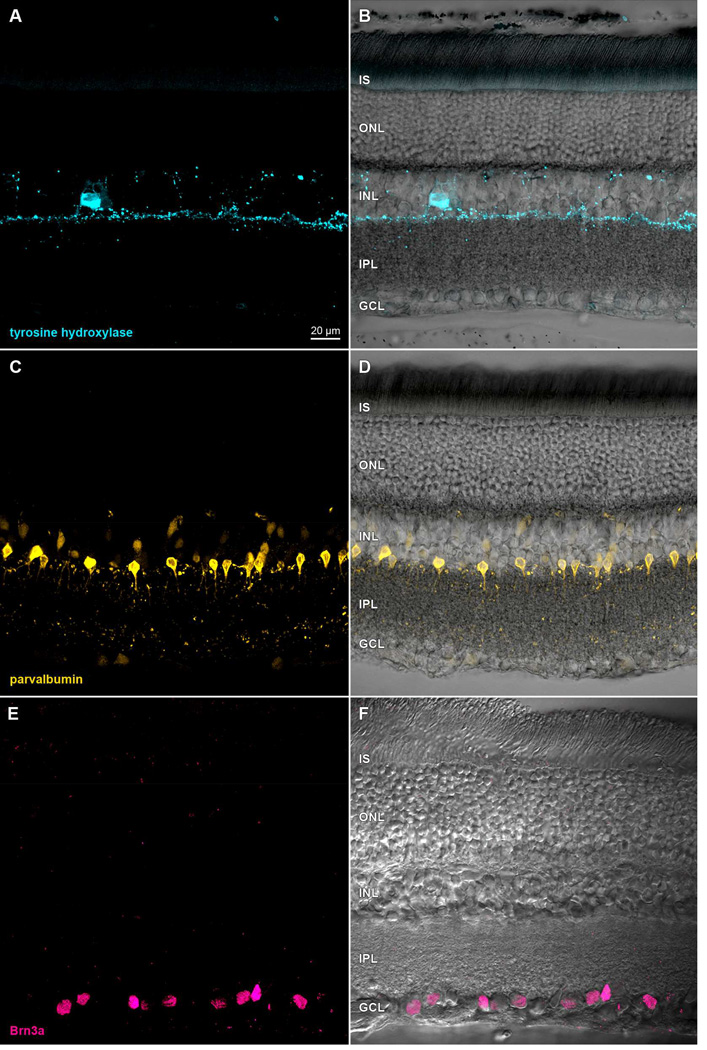
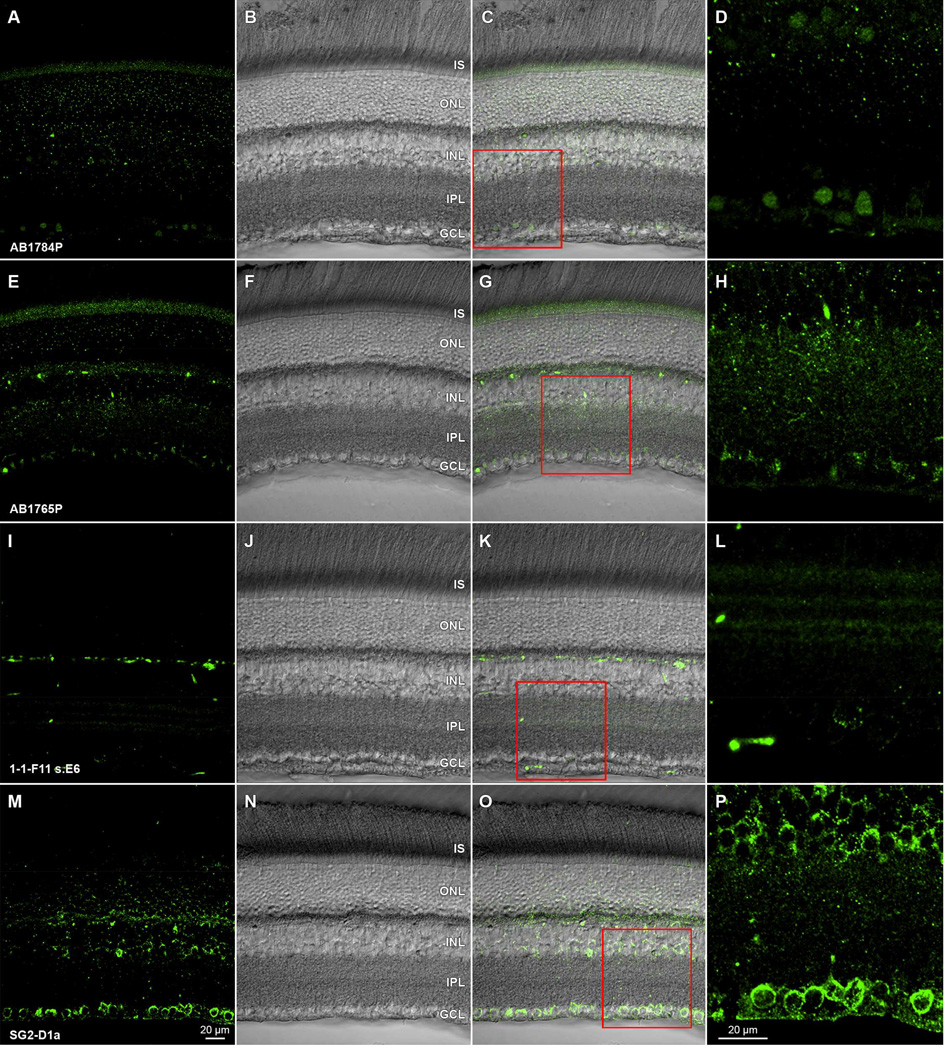
While these images highlight how vivid immunostaining can be in formaldehyde-fixed retinae, it is difficult to predict whether signal intensity will be high, or not, based on the information typically provided about immunogens. As illustrated in Fig. 1 by the images of adult rat retina incubated in mouse monoclonal antibodies against parvalbumin (clone PARV19; cat# P3088; Sigma-Aldrich) and tyrosine hydroxylase (clone LNC1; cat# MAB318; Millipore) and in polyclonal antibodies against Brn3a (clone sc-31984; Santa Cruz Biotechnology), we find that soluble and some hydrophilic antigens can immunostain well after fixation in 4% formaldehyde. Likewise, we have found that a mouse monoclonal antibody against D1 dopamine receptors (clone SG2-D1a; cat# NB110-60017; Novus Biologicals) also stains rat retinal ganglion cell somata (Fig. 2M–P; see also Hayashida et al., 2009) and a subsequent study (Van Hook et al., 2012) found similar binding of a polyclonal anti-D1a receptor antibody in rat retina. The monoclonal antibody bound to a protein band of the estimated molecular weight expected of D1a receptors (e.g., Huang et al., 1992) in western blots of whole retinal homogenate. By contrast, several polyclonal antibodies (cat#'s AB1765P and AB1784P; Millipore) and a rat monoclonal antibody (clone 1-1-F11 s.E6; cat# D187; Sigma-Aldrich) against the D1 dopamine receptor stained adult rat retina unreliably and were prone to negative immunostaining in tissue fixed for more than 2 hours at room temperature (Fig. 2A–L). The immunostaining pattern discrepancy between these antibodies is noteworthy because they each stain a protein band of 50–55 kDa in western blots (Hayashida et al., 2009; Free et al., 2007; Luedtke et al., 1999; Partida and Ishida, unpublished observations). It is not known why these antibodies yield such different staining patterns in tissue, but the similarity of their staining patterns on western blots indicates that formaldehdye fixation alters each epitope differentially.
Figure 1.
Immunoreactivity in formaldehyde-fixed rat retina. Transretinal vibratome sections incubated in (A) anti-tyrosine hydroxylase primary antibody and Alexa Fluor 488- conjugated anti-mouse secondary antibody, (C) anti-parvalbumin primary antibody and Alexa Fluor 488-conjugated anti-mouse secondary antibody, or (E) anti-Brn3a primary antibody and DyLight 549-conjugated anti-goat secondary antibody. Paired panels show single optical sections (A,C,E) of fields imaged under epifluorescence illumination on a laser scanning confocal microscope, and after merging these with the same fields under differential interference contrast optics (B,D,F). Acronyms positioned at the inner segment (IS), outer nuclear (ONL), inner nuclear (INL), inner plexiform (IPL), and ganglion cell (GCL) layers. Scale bar in (A) is 20 µm and applies to (A–F).
Figure 2.
Antibody-specific immunoreactivity effects of formaldehyde fixation on antidopamine D1 receptor antibodies in rat retina. Transretinal vibratome sections from a single retina incubated in (A) rabbit polyclonal AB1784P, (E) rabbit polyclonal AB1765P, (I) rat monoclonal 1-1-F11 s.E6, or (M) mouse monoclonal SG2-D1a. All preparations are visualized with Alexa Fluor 488-conjugated species-specific secondary antibodies. Paired panels show single optical sections (A,E,I,M) of fields imaged under epifluorescence illumination, the same fields under under differential interference optics (B, F, J, N), and merges of these in (C,G,K,O). High magnification details (D,H,L,P) of the ganglion cells layer, inner plexiform layer, and proximal inner nuclear layer are shown, and correspond to the red square regions of (C,G,K,O), respectively. Acronyms positioned at retinal layer levels as in Fig. 1. Scale bar in (M) is 20 µm and applies to (A–C, E–G, I–K, M–O). Scale bar in (P) is 20 µm and applies to (D,H,L,P). Lack of detectable GCL immunopositivity in A–L contrasts with GCL immunopositivity in M–P and with ganglion cell dopamine-sensitivity (Hayashida et al., 2009; Ogata et al., 2012).
The significance of unexpected immunostaining patterns like these can be checked in several ways. One is to run controls for non-specific binding of both primary and secondary antibodies (preferably processed side-by-side with tissue from which result images are collected). Negative controls for primary antibody binding may be obtained by pre-adsorption of primary antibodies with immunizing peptide before application to tissue, or by staining tissue from knock-out animals in which the antigen in nominally absent (Saper and Sawchenko, 2003). However, using knock-out organisms does not guarantee a reliable negative control, as the knock-out process is not always complete (Saper, 2009) and may change the distribution of other proteins that potentially cause false-positive staining (Lorincz and Nusser, 2008). Secondly, the ability of antibodies to selectively and specifically bind to protein of appropriate molecular weights can be checked by western blots prepared from the tissue being studied. A third approach would be to test for binding of antibodies directed against different epitopes of a given protein (Rhodes and Trimmer, 2006). A fourth approach is to functionally (e.g., electrophysiologically) test for the presence of proteins of interest (Hattar et al., 2002; Hayashida et al., 2009; Hornstein et al., 2004; Partida et al., 2012; Ryskamp et al., 2011; Stradleigh et al., 2011; Taylor and Morgans, 1998).
Lastly, antibodies can be optimized for compatibility with formaldehyde fixation. Antibodies are generally raised against amino acid sequences that are conjugated to carrier proteins through a crosslinking molecule. Glutaraldehyde was used classically as a crosslinker for antibody production (Avrameas and Ternynck, 1969), although antibodies can be directed against antigens crosslinked through carbodiimides or maleimides (Thermo, 2015). The crosslinking molecule becomes a part of an antibody’s epitope and, as might be expected, some antigens are modified by the activity of crosslinking fixatives (Pow, 1997). To circumvent formaldehyde-induced losses in immunoreactivity, primary antibodies have been raised against formaldehyde-bound antigens (Blom et al., 2012; de Vente et al., 1987; Harrach and Robenek, 1990), including neurotransmitters (Pow, 1997). Similarly, antibodies against acrolein-bound cyclic nucleotides have been developed for use in acrolein-fixed retinae (Wiemelt et al., 1997). Unfortunately, vendors of commercially available antibodies do not always identify the crosslinking molecules used in antibody production.
4.4. Drawbacks of formaldehyde fixation
At least three factors can limit formaldehyde's usefulness as a fixative. First, formaldehyde can interfere with specific antibody binding and increase non-specific signal, especially when attempting to fix antigens that crosslink with low efficiency. Secondly, fixation may be too slow to preserve dynamic and state-dependent processes, or it may be incomplete and not protect tissue from damage during subsequent processing. Thirdly, formaldehyde-based fixatives can induce artifactual physiological and anatomical changes. As described in Sections 4.4.1–4.4.6 (and as illustrated by results cited therein), these are largely due to either underfixation or overfixation.
4.4.1. Osmotic shock
The osmolarity of extracellular fluid in retina differs between species - e.g., rabbit (283 mOsm; Ames et al., 1965), rat (318 mOsm; Moran et al., 1991); and human (270 mOsm; Villegas, 1964). Although the osmolarity of buffers commonly used to dilute fixative agents and antibodies in retinal studies (300 mOsm) roughly matches extracellular fluid osmolarity, the osmolarity of 4% (w/v) formaldehyde solution (or 10% formalin) in water is 1300 mOsm (Fox et al., 1985). Consequently, the addition of formaldehyde to buffers significantly increases solution osmolarity. The slow rate of fixation by formaldehyde (especially at the neutral pH used in most studies) leaves the structure and functional state of cells vulnerable to disruption by hyperosmotic shock.
Not surprisingly, immersion of retina in hyper- or hypo-osmotic media has been found to cause cell and tissue shrinkage and swelling, respectively (Ames et al., 1965; Webster et al., 1969). Additional structural distortions include separation of the retina from the retinal pigment epithelium (Margo and Lee, 1995), shrinkage of retinal flatmounts (reportedly as high as 25%; Adams et al., 1974; Ammermüller and Weiler, 1988; Kino et al., 2009), shriveling of corneal endothelial cells (Doughty et al., 1997), swelling of inner nuclear layer somata (Izumi et al., 2000), and disruption of rod outer segments (Jones, 1974).
Retinae are less prone to detach from the RPE if the fixative osmolarity is reduced (Margo and Lee, 1995) or if the eye is immersed in a more rapidly acting chemical fixative (Latendresse et al., 2002). Lowering the formaldehyde concentration from 4% to 1% has been found to reduce linear shrinkage of whole eye volume (Margo and Lee, 1995). Whether other artifacts observed following exposure to 4% formaldehyde can be avoided by changes in the fixative osmolarity remains to be studied. In a recent test of this possibility, we found that immersion of freshly isolated retinae in 4% formaldehyde induces formation of bead-like varicosities in retinal ganglion cell dendrites and axons, and thinning of the neuritic segments connecting these varicosities; that this is not relieved by decreasing the formaldehyde concentration to as low as 0.8%; and that beading is not observed if 50% of the diluting buffer is replaced by isosmotic sucrose (Stradleigh et al., 2015). In some cells, the neurites connecting varicosities like these are almost undetectably thin (e.g., Huxlin and Goodchild, 1997). Moreover, exocitotoxicity-induced cell swelling following formaldehyde immersion can be avoided by infusing the eye with a glutamate receptor antagonist (DNQX; 6,7-dinitroquinoxaline-2,3-dione) or by microwaveassisted formaldehyde fixation (Izumi et al., 2000).
4.4.2. Uneven fixation
Immersion in formaldehyde leads to simultaneous crosslinking and tissue penetration. Although formaldehyde molecules are relatively small (FW = 30.03), crosslinking impedes their diffusion into and through tissue (Eldred et al., 1983). Consequently, immersion fixation of tissue will result in a gradient of fixation, with a relatively well-fixed outer shell and a relatively underfixed center (cf., Blankenship et al., 2007). This can, in turn, produce localized differences in immunoreactivity and structural preservation. This can be minimized by trimming tissue to blocks no greater than 2 mm in thickness before immersion fixation (Helander, 1994; Kiernan, 2000). The rate of formaldehyde diffusion can also be improved by altering the fixative pH (Berod et al., 1981; Eldred et al., 1983), as described in Section 6.2.
4.4.3. Slow fixation
The slow rate at which formaldehyde fixes tissue will not adversely affect the outcome of experiments examining static structural properties of cells, or the presence and location of epitopes that do not change over time. It has also been possible to monitor changes in the detectability, intracellular distribution, and phosphorylation of some proteins. Striking examples include the light-induced translocation of transducin and arrestin in rod and cone photoreceptors (Elias et al., 2004; Philp et al., 1987; Sokolov et al., 2002; Zhu et al., 2002), circadian changes in calcium-binding protein immunoreactivity in amacrine cells (Gabriel et al., 2004), light-induced changes in rod bipolar cell protein kinase C levels (Gabriel et al., 2001; see also Vaquero et al., 1996), light-induced changes in cAMP levels of goldfish photoreceptor and ganglion cell layer somata (Vaquero et al., 2001), carbon monoxide and nitric oxide-induced elevation of cGMP levels in turtle and mouse bipolar, amacrine, and ganglion cells (Blom et al., 2012; Blute et al., 1998), and activity-dependent phosphorylation of amacrine cell tyrosine hydroxylase (Witkovsky et al., 2004). The time between stimulus onset and the appearance of visualized signals (and the rate at which tissues return to pre-stimulus appearance after stimulus offset) has not been studied in detail by immunohistochemistry. The shortest times in figures published to date have been a few minutes (Blom et al., 2012; Elias et al., 2004; Witkovsky et al., 2004).
Other physiologically or experimentally driven changes in epitopes have been refractory to fixation by formaldehyde alone. For example, we did not find repeatable light-induced changes in the cAMP immunoreactivity of photoreceptor layer somata and ganglion cell somata in rat retinae fixed by formaldehyde (Stradleigh, Partida, and Ishida, unpublished observations). Because previous biochemical studies found light-induced decreases in mammalian photoreceptor cAMP levels (Farber et al., 1981) and marked losses of cAMP in unfixed tissue (Ortez et al., 1980), we tested the possibility that insufficient fixation by formaldehyde hampered immunohistochemical detection of changes in rat retinal cAMP. This is consistent with findings that formaldehyde crosslinking of nucleotides and nucleosides is reversible in aqueous media, with permanent crosslinking requiring several weeks in formaldehyde solution (Chaw et al., 1980; Hamazaki et al., 1993). As discussed in more detail in Section 6.1, we found repeatable differences in immunostaining of dark- and light-adapted adult rat retina with anti-cAMP antibodies following fixation in a mixture of 4% formaldehyde and 0.1% glutaraldehyde. Although we have not investigated why these light-induced cAMP changes were captured by formaldehyde in goldfish but not rat, these results are consistent with the possibility that signaling cascade component levels can change during fixation before settling at levels visualized subsequently. Previous studies have found that cAMP levels vary with fixation methods and attributed the differences to residual phosphodiesterase activity (Schmidt et al., 1971). Some studies have tested this notion with rapid freezing and with microwave irradiation, and found substantial signal loss after freezing and nearly total signal retention by microwave irradiation (e.g., Barsony and Marx, 1990). The latter has been used to study cAMP levels and DARPP-32 phosphorylation in brain (Mani et al., 2000) and DARPP-32 phosphorylation in rodent retina (Witkovsky et al., 2007).
4.4.4. Loss of immunoreactivity
Fixation can reduce antibody binding in various ways. For example, chemical crosslinking can physically alter antigen proteins and, in turn, disrupt formation of the antibody-antigen complex (Sternberger, 1979). These changes might occur within a single antigen molecule or between the antigen and adjacent molecules due to the formation of crosslinks between amino acids within the antibody’s epitope and other functional groups (Hua et al., 1985; Huebner, 2004; Kuby, 1994). Antigens enriched with lysine residues [e.g., the MHC class I molecule (H-2Kb; Hua et al., 1985) in visual cortex (Huh et al., 2000; Lee et al., 2014)] are particularly sensitive to formaldehyde crosslinking (Vani et al., 2006). Destruction of lysine-enriched epitopes renders some antibodies incompatible with formaldehyde fixation, necessitating the use of antibodies directed against different epitopes on the antigen of interest (Hua et al., 1985) and/or use of another fixative. Fixation can also alter the physical space separating the antigen from nearby tissue components. Such a change would sterically reduce access of the antibody to the antigen, resulting in a secondary reduction in immunoreactivity (e.g., loss of GFAP antigenicity; Vaughan et al., 1990).
Immunoreactivity losses can be tissue- and fixative-dependent. Staining with antiglial fibrillary acidic protein (GFAP) antibodies illustrate this for a protein considered to specifically mark retinal glial cells (Erickson et al., 1987; Schnitzer, 1985). For example, several different anti-GFAP antibodies bind to glial cells in grey matter after formaldehyde fixation but not white matter in the same preparations, and yet immunoreactivity is lost in grey matter fixed in acid-alcohol (Shehab et al., 1990). This differential effect suggests that loss of immunoreactivity at least partly depends on crosslinking to nearby molecules in a tissue-dependent manner. For other antigens, fixation-induced immunoreactivity losses are correlated with formaldehyde concentration or fixation time in a dose-dependent manner (Hoffman et al., 2010).
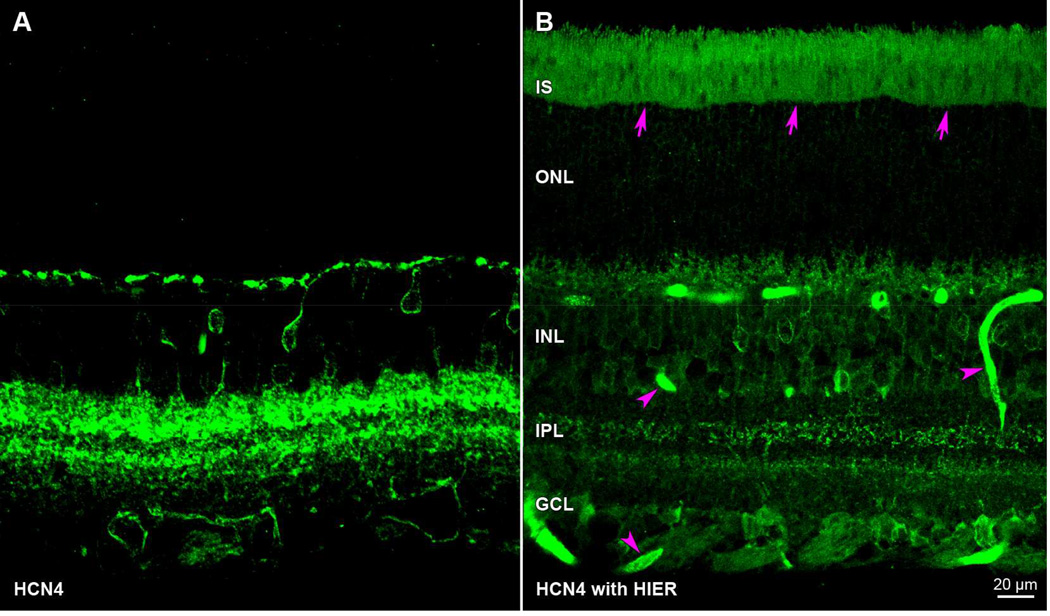
Three different approaches have been developed to improve signal-to-noise ratio (SNR). Because some antibodies might fail to recognize conjugated forms of lysine, one is to use a different antibody - viz., a different clone of a monoclonal antibody, or a polyclonal antibody raised against a different epitope on a macromolecular antigen. SNR should also increase with primary antibody titer, although the gain in signal is limited by steric hindrance, and by increases in non-specific antibody binding to the tissue. Post-fixation epitope retrieval methods have been developed to reverse immunoreactivity losses, e.g., by the application of proteolytic enzymes (Battifora and Kopinski, 1986; Huang et al., 1976) or sustained heating in a buffered solution (Shi et al., 1991). Heat-induced epitope retrieval (HIER) methods are most popular, and the methods may be optimized for a specific antigen by altering the reaction buffer pH (Koopal et al., 1998; Shi et al., 1994), adding metal cations (Evers and Uylings, 1994; Shi et al., 1991; Takahashi et al., 1993), adding calcium ion chelators (Balaton et al., 1995; Pileri et al., 1997), and/or altering the reaction temperature (Bankfalvi et al., 1996; Gown and Willingham, 2002; Man and Tavassoli, 1996). HIER may work through several mechanisms, such as disruption of a calcium ion shell surrounding epitopes (i.e., epitope unmasking; Jasani and Schmid, 1997; Shi et al., 1999) or by formaldehyde crosslink hydrolysis (Yamashita and Okada, 2005). The reaction temperatures employed in HIER must be controlled, as high temperatures can cause false-positive antibody staining and increase nonspecific background fluorescence (Ezaki, 2000). We do not use HIER in our studies of mammalian retinae because we find that it increases photoreceptor outer segment and blood vessel autofluorescence (Fig. 3B; cf., Stradleigh et al., 2011).
Figure 3.
Artifactual autofluorescence after heat induced epitope retrieval (HIER). Transretinal vibratome sections incubated in anti-HCN4 primary antibody and Alexa Fluor 488-conjugated anti-mouse secondary antibody. Paired panels show z-stacks of 5 optical sections under epifluorescence illumination. The fluorescence pattern in untreated retina (A) differs from HIER-treated tissue (B) in that HIER introduces extraneous staining in the photoreceptor inner and outer segments (arrows) as well as blood vessels (arrowheads). Sections in A and B were cut from opposite eyes of same animal (rat) and processed in parallel (aside from the HIER steps). Acronyms positioned at retinal layers as in Fig. 1. Scale bar in (B) is 20 µm and applies to (A,B).
4.4.5. Non-specific staining
Following exposure to formaldehyde-containing solutions, tissues are typically immersed in fixative-free solutions. Although this is intended to remove formaldehyde and stop crosslinking, the removal (i.e., efflux) is severely hampered by the crosslinking that has advanced since the fixation began. Prolonged washing might seem like a simple fix, except that this can reverse crosslinking (Helander, 1994). Partially crosslinked formaldehyde forms a reactive product that can bind to other amine groups in later steps. Notably, residual aldehyde activity can crosslink to reactive functional groups of antibody proteins, yielding false-positive immunoreactivity. Such non-specific staining is typically diffuse and increases the background signal. This reduces contrast between fluorescence due to the desired antibody-antigen complex and fluorescence due to non-specific antibody binding.
Residual aldehyde activity can be reduced by "quenching", i.e., by applying a substance enriched in free amino groups, binding the free ends of the partially-reacted aldehydes and forming a complete crosslink between tissue-bound molecules and the quenching agent. This neutralizes residual aldehyde reactivity and, in turn, can increase SNR achieved by subsequent antibody application. Common quenching agents include solutions containing the amino acids glycine or lysine, as well as the protein "blocking" solutions of normal animal sera or albumin. An alternative quenching protocol involves the use of sodium borohydride solution to neutralize free aldehydes; this reaction reduces free aldehydes to less reactive hydroxyl groups (Beisker et al., 1987). In Section 6.4, we show that alkaline Tris buffers are effective as quenching agents, too.
4.4.6. Autofluorescence
Catecholamines (e.g., epinephrine and dopamine), and some cellular proteins to a lesser extent, form autofluorescent products when exposed to formaldehyde (Eranko, 1955a, b; Falck and Torp, 1961). The Falck-Hillarp method for localizing catecholamines takes advantage of this phenomenon (Falck et al., 1962) and saw widespread use in retinal studies (Dowling and Ehinger, 1975; Häggendal and Malmfors, 1965; Negishi et al., 1979) before immunohistochemical methods became commonplace. This otherwise helpful autofluorescence is a liability in immunofluorescence studies, effectively increasing background fluorescence and decreasing SNR. This effect may be more pronounced in tissues that contain high levels of catecholamines (e.g., retinal dopamine). Moreover, the level of fixative-induced autofluorescence correlates with fixation time: excessively long fixation times will lead to unsuitably high autofluorescence intensity (Stewart et al., 2007).
Chemical and physical methods for reducing aldehyde-induced autofluorescence have been reported. Autofluorescence can be bleached by illuminating the fixed tissue before application of fluorophore-conjugated antibodies (Duong and Han, 2013; Neumann and Gabel, 2002). Alternatively, autofluorescence has been masked by use of dyes (e.g., trypan blue and Evan's Blue) or heavy metals (e.g., osmium), quenching the emission from the fluorescent aldehyde conjugate without affecting specific staining (Mosiman et al., 1997; Wan et al., 1993). However, trypan blue fluoresces at long visible wavelengths and may be incompatible with multi-label experiments, while heavy metals can pose health risks.
5. Glutaraldehyde
Some of the drawbacks of formaldehyde use can be circumvented by use of glutaraldehyde. Chief among the advantages are increased fixation rate and enhanced fixation strength, allowing for better tissue preservation and more reliable staining of some state-dependent antigens. At the same time, some drawbacks of formaldehyde use are seen with glutaraldehyde too, but at increased severity.
Glutaraldehyde was first synthesized by Harries and Tank (1908) and used in a histological study (Sabatini et al., 1963) to compare the fixation characteristics of several aldehydes in various tissues (including retina). The improved tissue morphology and preservation of microstructure (relative to formaldehyde) quickly led to widespread use of glutaraldehyde (Hopwood, 1972). Early methods of glutaraldehyde production resulted in multiple impurities (Anderson, 1967), leading to incorporation of steps (e.g., distillation) to purify glutaraldehyde solutions (Fahimi and Drochman, 1968). Some previously common contaminating compounds, such as acrolein, have crosslinking activities of their own and have been used as primary fixatives (Wiemelt et al., 1997). New methods of industrial synthesis and purification in the late 1970s resulted in nearly pure EM-grade reagents and eliminated a significant source of variability between production lots of glutaraldehyde.
5.1. Mechanisms of glutaraldehyde crosslinking
Glutaraldehyde is composed of 5 carbon atoms arranged in a trans-chain, with an aldehyde group on each terminus. Its dialdehyde structure allows for direct conjugation to biological molecules with fewer intermediate steps than a monoaldehyde molecule, while its 5-carbon backbone enables it to react with molecules across a wider distance than the single-carbon formaldehyde. These properties significantly increase the reaction rate of glutaraldehyde relative to formaldehyde. Glutaraldehyde also offers increased reactivity to lipids, carbohydrates, and nucleic acids.
Like formaldehyde, glutaraldehyde crosslinks well in basic and neutral solutions, and not in acidic solutions. Commercially available glutaraldehyde stock solutions are maintained at low pH to prevent homologous crosslinking and polymerization (Hopwood, 1972). Although storage at pH 8 encourages rapid polymerization and formation of a white precipitate (Hardy et al., 1969), low concentrations of soluble polymers form even at neutral and acidic pH. These polymers prove to have unique fixation properties, leading some histologists to "age" their glutaraldehyde solutions to encourage polymerization (Robertson and Schultz, 1970). Methods for accelerated glutaraldehyde polymerization have been devised for specialized fixation protocols (Nakagawa et al., 1989).
The exact form taken by glutaraldehyde in solution is unknown. Glutaraldehyde generally exists as a mixture of its linear monomer and its cyclic hemiacetal under acidic or neutral conditions, and becomes an unsaturated oligomer in basic conditions (Walt and Agayn, 1994). Binding of monomeric and linear polymeric forms of glutaraldehyde to proteins generally occurs via interaction with amino groups, and was initially hypothesized to proceed via Schiff base (Monsan et al., 1975) and Michael-type (Richards and Knowles, 1968) reactions. Others have suggested that the crosslinking reaction between two amine groups is due to glutaraldehyde dimerization and formation of a quaternary pyridinium compound (Hardy et al., 1976; Luftig and McMillan, 1981).
In free amino acid experiments, glutaraldehyde readily crosslinks lysine, especially near the amino acid's isoelectric point. This favors the use of a slightly basic solution for lysine crosslinking (Bowes and Cater, 1965; Quiocho and Richards, 1966), although reaction with lysine readily occurs at neutral pH. Glutaraldehdye also binds to tyrosine, tryptophan, phenylalanine (Bowes and Cater, 1965; Hopwood, 1970), histidine, proline, serine, glycine, and arginine (Chirita and Chisalita, 1971), although all at lower affinities than to lysine. Crosslinking experiments performed on whole proteins reveal that only lysine is crosslinked to a significant degree in neutral or slightly basic buffers, and that these bonds are generally irreversible (Ottesen and Svensson, 1971; Tomimatsu et al., 1971; Wang and Tu, 1969). Most proteins contain many lysine residues; these are generally on the exterior hydrophilic surface and are thus readily exposed to the fixative medium (Migneault et al., 2004).
5.2. Advantages of glutaraldehyde use
Given its increased reaction rate (Hopwood, 1969) and excellent retention of tissue-bound antigens, glutaraldehyde is well suited for experiments that require fast fixation of small, labile antigens. For example, glutaraldehyde is an excellent fixative for immobilization of neurotransmitters in retina (glutamate: Kageyama and Meyer, 1989; dopamine: Yanez and Anadon, 1994; glutamate, glycine, GABA: Marc and Jones, 2002). Its ability to crosslink epinephrine, norepinephrine, serotonin, and insulin in non-retinal tissues (Coupland and Hopwood, 1966; Grillo et al., 1971; Hopwood, 1967a) also raises the possibility that glutaraldehyde may be useful for localizing a broad range of neuroactive substances in retinae. Studies of other tissues have also shown that epitopes are retained better after fixation by glutaraldehyde than by formaldehyde (Hopwood, 1968; Vanha-Perttula and Grimley, 1970).
The molar masses of glutaraldehyde and formaldehyde are 100.12 and 30.03 g/mol, respectively. Correspondingly, a 4% (w/v) solution of glutaraldehyde in water is 455 mOsm (Rasmussen, 1974), while a 4% (w/v) solution of formaldehyde is 1300 mOsm (Fox et al., 1985). It is therefore easier to maintain iso-osmotic conditions when fixing in a lower concentration glutaraldehyde solution by using hypo-osmotic buffers (Doughty et al., 1997; Jones, 1974), and the addition of dilute glutaraldehyde will not significantly increase the osmolarity of solutions that contain other fixative agents (Margo and Lee, 1995).
5.3. Drawbacks of glutaraldehyde use
Glutaraldehyde shares many drawbacks with formaldehyde, and these are even more apparent due to glutaraldehyde's rapid reaction rate. For example, at the concentrations used to date, retinae shrink in glutaraldehyde (Famiglietti, 1985; Steinberg et al., 1973) as much as other studies reported for formaldehyde (Section 4.4.1). Also, glutaraldehyde is commonly thought to increase background immunofluorescence (Pow et al., 1995) and thereby reduce SNR and the resolution of specific immunofluorescent markers. Moreover, reactions between glutaraldehyde and catecholamines create autofluorescent compounds (Rost and Ewen, 1971), much like formaldehyde.
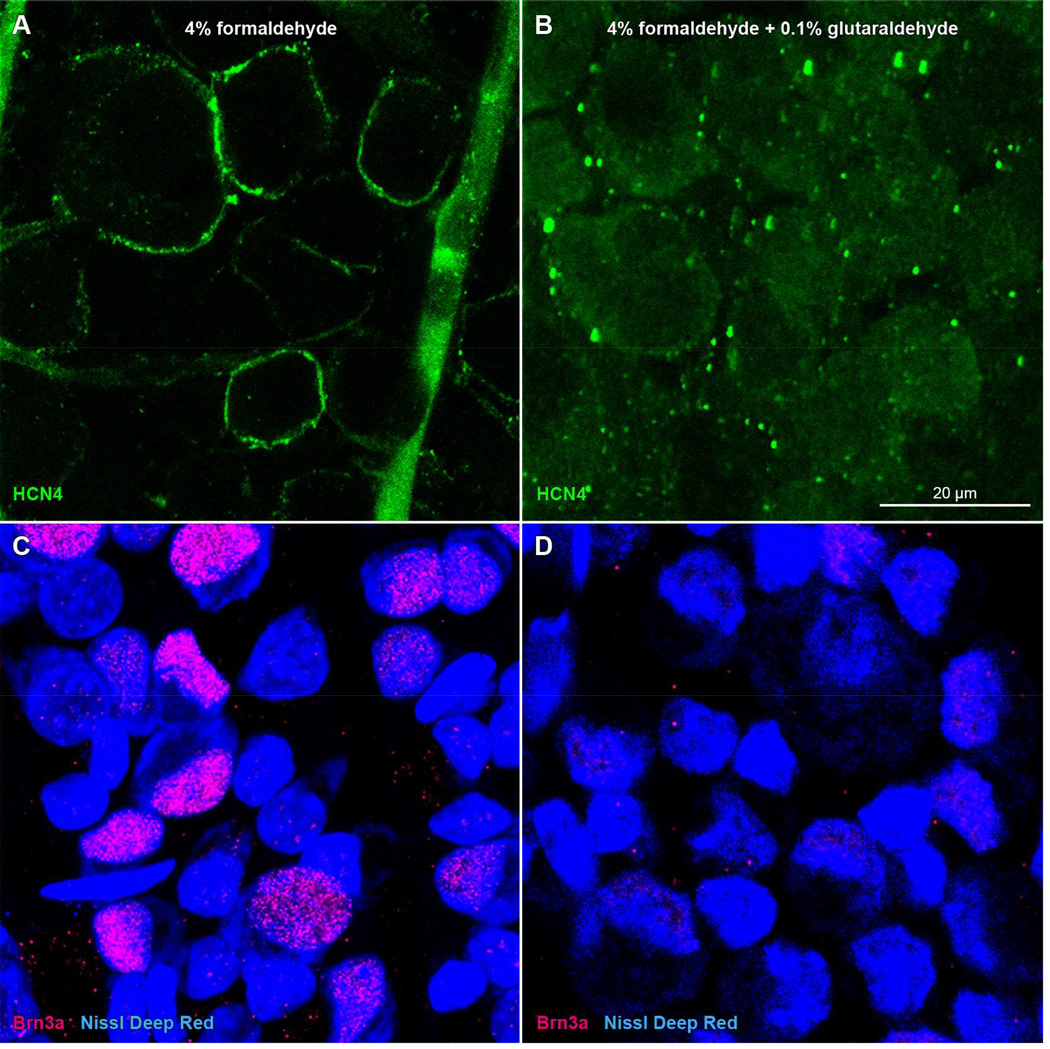
Some antigens are at greater risk of denaturation and reduced immunoreactivity when fixed in glutaraldehyde (instead of formaldehyde). One reason is that, for a given duration of fixation, glutaraldehyde is more likely to overfix tissue-bound antigens than formaldehyde. For example, mouse monoclonal antibodies directed against HCN channel proteins (HCN4, clone N114/10; cat# 73-150, NeuroMab) resisted formaldehyde fixation (Fig. 4A; see also Stradleigh et al., 2011) and yielded false-negative staining when tissue was treated with fixatives containing glutaraldehyde (Fig. 4B). We also noted decreased binding efficiency when using anti-Brn3a (cat# sc-31984; Santa Cruz) antibodies on glutaraldehyde-fixed tissue (Fig. 4D).
Figure 4.
Immunoreactivity loss in rat retinae in glutaraldehyde-containing fixative. Panels show single optical sections through the ganglion cell layer of the retinae of a single rat, one fixed in formaldehyde (A, C) and the other fixed in a mixture of formaldehyde and glutaraldehyde (B, D). Whole-mounted retinae were incubated in (A–B) mouse monoclonal anti-HCN4 antibody and DyLight 488-conjugated anti-mouse secondary antibody (green), or (C–D) goat anti-Brn3a antibody and DyLight 549-conjugated anti-goat secondary antibody (red), followed by NeuroTrace deep-red fluorescent Nissl stain (blue). Scale bar in (B) is 20 µm and applies to (A–D).
Secondly, glutaraldehyde has been found to decrease antibody penetration into fixed tissue, and thereby decrease antibody binding (Pow, 1997). This effect is exacerbated when fixing thick tissue pieces because glutaraldehyde diffuses into tissue more slowly than formaldehyde (Reale and Luciano, 1970; Thiessen et al., 1970). This results in a shell of over-fixed tissue on the exterior of the tissue block, and a core of poorly fixed tissue at the center (much like formaldehyde), although various methods for improving antibody penetration after fixation are available (e.g., whole mount electroimmunofluorescence (Liu and Kao, 2009); buffered-ethanol extraction; cryoprotection and subsequent freeze-thaw (Eldred et al., 1983; Voigt and Wässle, 1987).
The biggest drawbacks of glutaraldehyde - increased background fluorescence and loss of immunogenicity - can be avoided by use of lower concentrations. In combination with 4% formaldehyde, we find that 0.1% (v/v) glutaraldehdye suffices to accelerate fixation without noticeable deleterious effects. We have not experienced significant autofluorescence in our experiments at this concentration of glutaraldehyde. At the same time, free aldehyde groups added to tissue by glutaraldehyde (due to its dialdehyde structure) can increase the risk of non-specific binding of primary or secondary antibodies (i.e., non-specific immunofluorescence) and reduced SNR. We find that this can be minimized by quenching residual aldehyde activity with ample amounts of free amino groups prior to immersion in antibody-containing solutions (see Section 6.4).
Glutaraldehyde has also been found to distort the cytoskeleton, causing more tissue shrinkage than formaldehyde (Hopwood, 1967b). It is widely known that glutaraldehyde alters F-actin conformation (Lehrer, 1972; Prochniewicz-Nakayama and Yanagida, 1982) and destroys its phalloidin binding site (Borovikov, 1984). Although actin can not be stained by phalloidin in glutaraldehyde-fixed tissue, phalloidin has been used to study formaldehyde-fixed photoreceptors and retinal pigment epithelium (Daniele et al., 2007; Nagle et al., 1986), bipolar and horizontal cells (Job and Lagnado, 1998; Vaughan and Lasater, 1990), and retinal ganglion cells (Cristofanilli and Akopian, 2006).
6. Alternative Fixation/Processing Strategies
The vast majority of retinal immunohistochemistry studies have used formaldehyde, diluted to 4% in a phosphate-containing buffer at neutral pH, as the sole fixative. The major difference, if any, between these studies has been the duration of the exposure to the fixative. The results summarized above indicate that other fixatives can improve the quality (i.e., SNR) of immunostaining and of tissue preservation at the light microscopic level. We have found several modifications of formaldehyde-based fixatives, and at least one alternative fixative, to be particularly helpful.
6.1. Adding glutaraldehyde to formaldehyde
Many of glutaraldehyde's drawbacks can be avoided by diluting it in a mixture of other fixing agents. As mentioned above, the mixture of formaldehyde and glutaraldehyde combine the best qualities of each fixative: formaldehyde quickly penetrates tissues to stabilize molecules, while the slowly diffusing glutaraldehyde eventually catches up and strongly fixes molecules into place (Hopwood, 1972). The subsequent glutaraldehyde reaction is irreversible (Hopwood, 1970). Formaldehyde and glutaraldehyde may form a compound at alkaline pH (1,3-cyclo-hexanedione) which possesses its own crosslinking activity (Bloem, 1968). Others have found that adding the unsaturated aldehyde acrolein to this mixture accelerates fixative penetration, further increasing reaction rate (Mollenhauer and Totten, 1971).
Karnovsky's fixative (Karnovsky, 1965) and its more popular modified form (Ito and Karnovsky, 1968) combine glutaraldehyde with formaldehyde. Although glutaraldehyde has been used extensively in electron microscopic retinal studies, few retinal immunofluorescence studies have been published using Karnovsky's fixative or modifications of it (Castellarin et al., 1998; Cleary et al., 1980; Jan and Revel, 1974) and some light microscopic studies have used other combinations of glutaraldehyde and formaldehyde (Eldred et al., 1983; Marc, 1999; Margo and Lee, 1995; Ogata et al., 2012). Consistent with glutaraldehyde’s rapid crosslinking rate and wide range of functional group reactivity, and with a report that supplementing formaldehyde with glutaraldehyde improves cAMP retention (Ortez, 1980), we recently found repeatable differences in immunostaining of dark- and light-adapted adult rat retina with anti-cAMP antibodies following fixation in a mixture of 4% formaldehyde and 0.1% glutaraldehyde (Ogata et al., 2012). These retinae confirmed that light adaptation decreases outer retinal cAMP levels (Farber et al., 1981; Nir and Hall, 1974; Orr et al., 1976; Vaquero et al., 2001). Moreover, labeling of single sections with multiple primary antibodies showed that light adaptation concomitantly (1) decreased photoreceptor layer cAMP levels, (2) translocated arrestin from photoreceptor inner segments to the outer segment layer, (3) reduced transducin-like immunoreactivity in photoreceptor outer segments, (4) increased retinal ganglion cell cAMP levels, and (5) activated CaMKII in retinal ganglion cells, relative to tissue maintained in darkness (see Fig. 4 of Ogata et al., 2012). The compatibility of glutaraldehyde-containing fixatives with multiple state-dependent antigens was of critical importance, as this provided independent evidence that tissue was dark- vs light-adapted, and because identical experiments using formaldehyde alone were inconclusive.
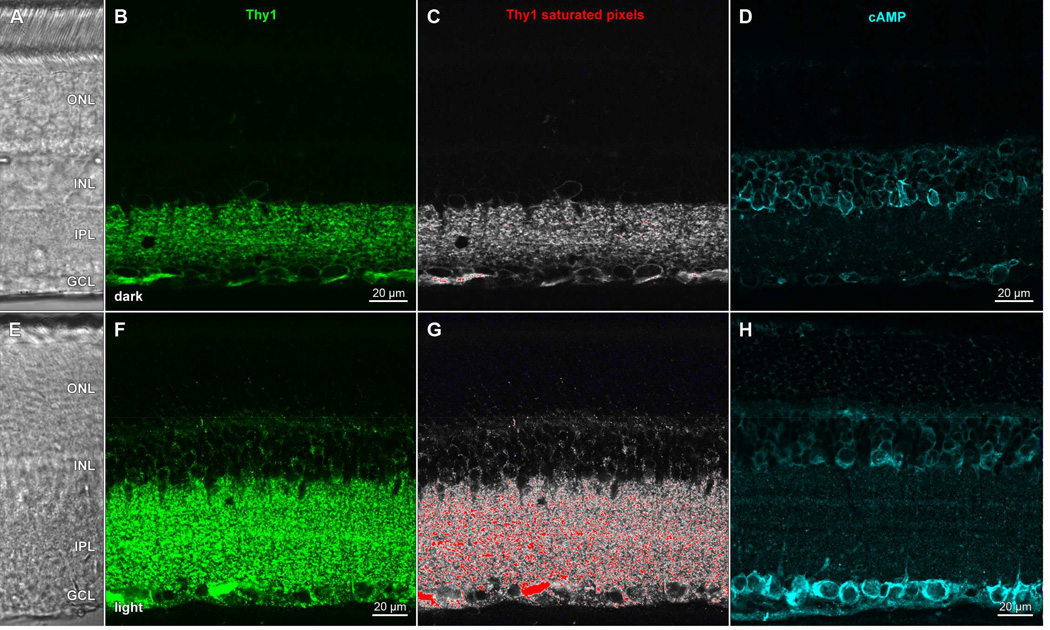
We have also found glutaraldehyde to be a useful fixative component when immunostaining for Thy1, a glycosylphosphatidylinositol (GPI)-anchored outer leaflet membrane glycoprotein. In formaldehyde-fixed adult rat retina, anti-Thy1 antibodies (clone OX-7; cat# MAB1406; Millipore) bound to retinal ganglion cell membranes, as evidenced by colocalization with staining by antibodies against a known membrane protein (HCN4) in somata whose nuclei bound antibodies against Brn3a (cf., Nadal-Nicolás et al., 2009). The Thy1 staining pattern circumscribed ganglion cell somata in single optical sections (Partida et al., 2012), consistent with a previous description of Thy1 distribution in rat retina (Barnstable and Dräger, 1984) and unlike the cytoplasmic staining found in subsequent studies. Fixation of dark-adapted retinae in formaldehyde yields a staining pattern identical to that found in Partida et al. (2012), provided the tissue is not permeabilized with detergents (Fig. 5A). However, most immunostaining protocols include the addition of detergents (e.g., Triton-X 100) to improve antibody penetration and reduce non-specific interactions (Juhl et al., 1984). Formaldehyde-fixed tissue exposed to detergent and immunostained for Thy1 expresses an altered punctate staining pattern (Fig. 5B). Addition of glutaraldehyde to the fixative solution confers resistance to the detergent-induced change in Thy1 staining pattern, even in tissue exposed to high concentrations of detergent (0.5% v/v Triton-X 100; Fig. 5C). Because Thy1 mRNA levels vary in a diurnal rhythm (Kamphuis et al., 2005), we tested whether Thy1 immunostaining differed in darkand light-adapted retinae. Curiously, we found no difference in formaldehyde-fixed tissue. By contrast, Thy1 immunostaining displayed at least two differences in tissue that was fixed in formaldehyde and glutaraldehyde, and characterized as dark- vs light-adapted by the cAMP-immunopositivity of their retinal ganglion cell layer somata (cf., Ogata et al., 2012). Dark-adapted retinae displayed Thy1-like immunofluorescence in thin, continuous bands around the perimeter of cells in the ganglion cell layer (GCL) and faint punctate fluorescence in the inner plexiform layer (IPL; Fig. 6B,C). Light-adapted retinal ganglion cells exhibited discontinuous punctate membrane staining (Fig. 6F,G). A similar effect also appeared in the IPL, where the size of puncta and overall signal intensity was increased in the light-adapted tissue. We interpret these differences as evidence of Thy1 antigen capping (Heneberg et al., 2006) in glutaraldehyde-fixed retinae after light adaptation, but not in darkness, even after exposure to detergent and divalent antibody. It remains to be seen whether the glutaraldehyde-dependent light-dark difference is due to crosslinking of the glycosylated portion of Thy1, or interactions elsewhere in its structure via glutaraldehyde-mediated stabilization of the plasma membrane, through direct binding to phospholipids or via the stabilization of cytoskeletal proteins that may conformationally change in a state-dependent manner (Chen et al., 2009; Ruppelt et al., 2007).
Figure 5.
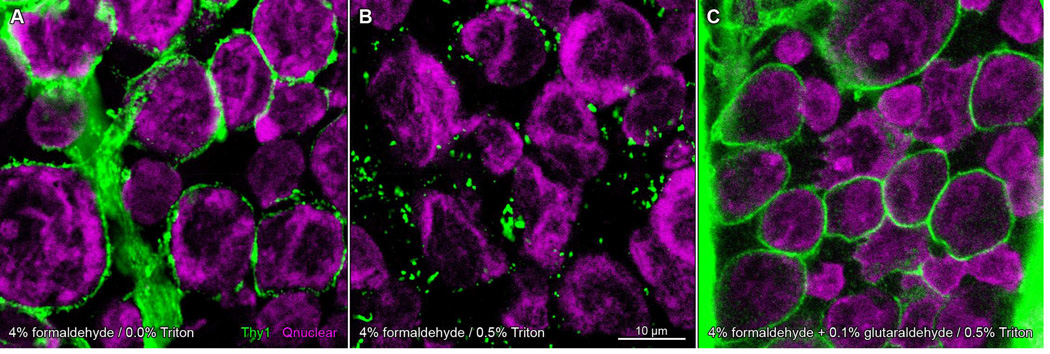
Glutaraldehyde fixation protects against detergent-induced aggregation of Thy1 in dark-adapted rat retinae. Whole-mounted retinae incubated in anti-Thy1 primary antibody and Alexa Fluor 488-conjugated anti-mouse secondary antibody (green), and imaged on a laser scanning confocal microscope. Nuclei counsterstained with Qnuclear Deep Red Stain (magenta). Panels show single optical sections through the ganglion cell layer of retina fixed in formaldehyde (A, B) or a mixture of formaldehyde and glutaraldehyde (C). Retinae in A–C from same animal (A, B from one eye; C from the opposite eye) and processed in parallel. Staining pattern in formaldehyde-fixed tissue circumscribes cell profiles (A). Treatment of formaldehyde-fixed tissue with Triton-X 100 results in a discontinuous punctate staining pattern (B). Addition of glutaraldehyde to fixative prevents the detergent-induced change in Thy1 staining (C). Scale bar in (B) is 10 µm and applies to (A–C).
Figure 6.
Light exposure and detergent treatment induce a change in Thy1 staining pattern and an elevation in cAMP levels in rat retina. Transretinal vibratome sections of dark- (AD) and light- (E–H) adapted retinae collected, fixed in a mixture of formaldehyde and glutaraldehyde, and processed side-by-side, as in Ogata et al., (2012); incubated in (B,F) anti-Thy1 primary antibody and Alexa Fluor 488-conjugated anti-mouse secondary antibody (green), or (D,H) anti-cAMP primary antibody and DyLight 549-conjugated antimouse secondary antibody (cyan). All staining buffers contained Triton-X 100 detergent. Panels show single optical sections (B,D,F,H) of fields under epifluorescence illumination imaged at the same settings (laser intensity, photomultiplier gain, pinhole diameter) on a laser scanning confocal microscope, or fields imaged under differential interference contrast optics (A,E). Thy1 data from (B,F) visualized with a pseudocolor table in which saturated pixels are assigned a red color (C, G). Acronyms positioned at retinal layers as in Fig. 1. Scale bar in (B) is 20 µm and applies to (A–C). Scale bar in F is 20 µm and applies to (E–G).
6.2. pH shifts
As mentioned in Section 4.4.2, the rate of formaldehyde crosslinking is influenced by pH (Highberger and Retzsch, 1939). Methylene bridge formation proceeds at a rate inversely proportional to hydrogen concentration; maximum crosslink formation rate is achieved at pH 11 while crosslinking is slowed dramatically at pH 6.5 (Berod et al., 1981). If tissue is first immersed in a relatively acidic primary fixative solution, followed by immersion in a more basic secondary fixative solution, the low pH in the primary fixative allows for uniform formaldehyde penetration before the basic secondary fixative initiates rapid crosslinking. This sequence (or a variant of brief initial fixation at pH 7.4 followed by overnight fixation at elevated pH) has been found to be effective in immunohistochemical studies of various retinae (Brandon, 1985a; Eldred et al., 1983; Kolb et al., 1987; Massey and Mills, 1999; Ogata et al., 2012; Stradleigh et al., 2011).
6.3. Sucrose supplementation
Structural characterization of retinal neurons relies on preservation of the cardinal features they display in vivo. Although the diameter, shape, and contour of dendrites and axons have been widely used to distinguish subtypes of retinal amacrine and ganglion cells, we have found that immersion in formaldehyde-based fixatives induces the formation of structural artifacts in ganglion cells in a process independent of fixation temperature, salinity, pH, and excitatory neurotransmitters. Regularly spaced bead-like swellings appear throughout the dendritic trees and along the proximal axons of ganglion cells within minutes of immersion in fixative, even in the absence of extracellular sodium or in the presence of glutamate receptor antagonists (Stradleigh et al., 2015). We found that addition of sucrose to fixation buffers prevents fixation-induced bead formation (Stradleigh et al., 2015). Previous studies have added sucrose to formaldehyde- and glutaraldehydebased fixatives (e.g., Blute et al., 1998; Marc and Liu, 1985; Stell and Lightfoot, 1975). However, we find that beading is prevented only if a minimum fraction of the diluent buffer osmolarity is due to sucrose. Hypertonic buffers in which more than 45% of buffer osmolarity is due to sucrose (i.e., sucrose fraction) provide protection from beading (Table 1). This holds true in buffers that are sodium-free (HEPESHIGH + SucroseHIGH; sucrose fraction = 46%), low in sodium (Low Na+ phosphate buffer (PB) + SucroseLOW; sucrose fraction = 58%), and at in vivo sodium concentrations (PBS + SucroseHIGH; sucrose fraction = 57%). Sodium-free buffers with sub-threshold sucrose fractions (HEPESHIGH + SucroseLOW; HEPESLOW; HEPESHIGH) do not confer protection against artifactual bead formation.
Table 1.
Protection from artifactual beading depends on sucrose fraction.
| Buffer | Protective? | Buffer Osmolarity |
Sucrose Osmolarity |
Sucrose Fraction |
|---|---|---|---|---|
| PB + SucroseLOW | Yes | 347 | 200 | 58% |
| PBS + SucroseHIGH | Yes | 700 | 400 | 57% |
| HEPESLOW + SucroseLOW | Yes | 403 | 200 | 50% |
| HEPESHIGH + SucroseHIGH | Yes | 879 | 400 | 46% |
| PBS + SucroseLOW | No | 500 | 200 | 40% |
| HEPESHIGH + SucroseLOW | No | 679 | 200 | 29% |
| PBS | No | 300 | 0 | 0% |
| HEPESLOW | No | 203 | 0 | 0% |
| HEPESHIGH | No | 479 | 0 | 0% |
| PB | No | 147 | 0 | 0% |
PBS: phosphate buffered saline; PB: low Na+ phosphate buffer
How sucrose protects ganglion cells against beading is unknown, and it is unclear whether other additives would grant similar protection. Osmotically induced water influx (Greenwood and Connolly, 2007) seems unlikely to explain bead formation in retinal neurons because images collected before and during exposure to fixatives show that the diameter of neurites connecting beads decrease during fixation (Stradleigh et al., 2015). However, organ transplant studies reveal that sucrose is more effective than the same concentrations of glucose or mannitol at preventing nephron necrosis in 48-hour cold storage of rat kidney (Andrews and Coffey, 1982). This may be attributable to osmotic effects on nephrons, as sucrose (423.30 g/mol) is significantly larger than glucose (180.16 g/mol) and mannitol (182.172 g/mol).
6.4. Tris as a quenching agent
Non-specific background staining caused by antibody binding to aldehydes not washed out after fixation can be visibly reduced by quenching crosslinker activity (Sutherland et al., 2008). Previous studies have used glycine and lysine to saturate unbound aldehydes prior to incubation in solutions containing primary antibodies (Sullivan et al., 1984). Alternatively, immersion of fixed tissue in a solution of sodium borohydride may reduce fixation-induced autofluorescence (Clancy and Cauller, 1998) and even restore some of the antigenicity lost following aldehyde fixation (Eldred et al., 1983; Fujiwara and Masuyama, 1995).
We found that sodium borohydride exposure disrupted morphology in lightly fixed, vibratome-sectioned retinae, and that glycine and lysine were ineffective at reducing nonspecific staining. However, we have found that slightly alkaline (pH 8) Tris buffer (2-amino- 2-hydroxymethyl-propane-1,3-diol) quenches efficaciously (Ogata et al., 2012). This is consistent with the presence, in each Tris molecule, of an amino group capable of binding free aldehydes and with previous reports that Tris is a stronger nucleophile than glycine, and quenches aldehyde activity to a significantly greater degree than glycine (Sutherland et al., 2008). We therefore use Tris in all buffer solutions to minimize residual aldehyde activity within fixed retinal tissue. Moreover, to facilitate Tris penetration into both flatmounted pieces of retina and 50-µm-thick vibratome sections, we supplement the Tris with Triton X-100, forming a Triton-Tris quenching and rinse solution that contains 100 mM Tris- HCl, 0.1% v/v Triton X-100, pH 8. We also include Tris-Triton in the solutions used to dilute primary and secondary antibodies.
6.5. Davidson's fluid
Davidson’s fluid and its modifications are gaining popularity as alternatives to aldehyde fixatives in retina research, having been used in rat (Latendresse et al., 2002; Stradleigh et al., 2011), rabbit (French et al., 2008; Pfeffer et al., 2009; Saenz-de-Viteri et al., 2014), and cat studies (Villalobos et al., 2013). Davidson’s fluid is primarily a fastacting coagulant fixative due to its acetic acid and ethanol content, and the inclusion of formaldehyde grants it a secondary crosslinking activity. It rapidly imparts structural rigidity and opacity to isolated retinae during fixation, improving the preservation of free-floating wholemount preparations (personal observations; Stradleigh et al., 2011). Davidson’s fluid and its modifications improve on the morphological preservation of formaldehyde (Chidlow et al., 2011) and Bouin’s fluid (French et al., 2008; Latendresse et al., 2002). But like other coagulant fixatives, Davidson’s fluid hardens retinae to the point that vibratome sectioning becomes difficult (personal observation). Tissue fixed in Davidson’s fluid is more commonly sectioned following embedding in paraffin (Latendresse et al., 2002).
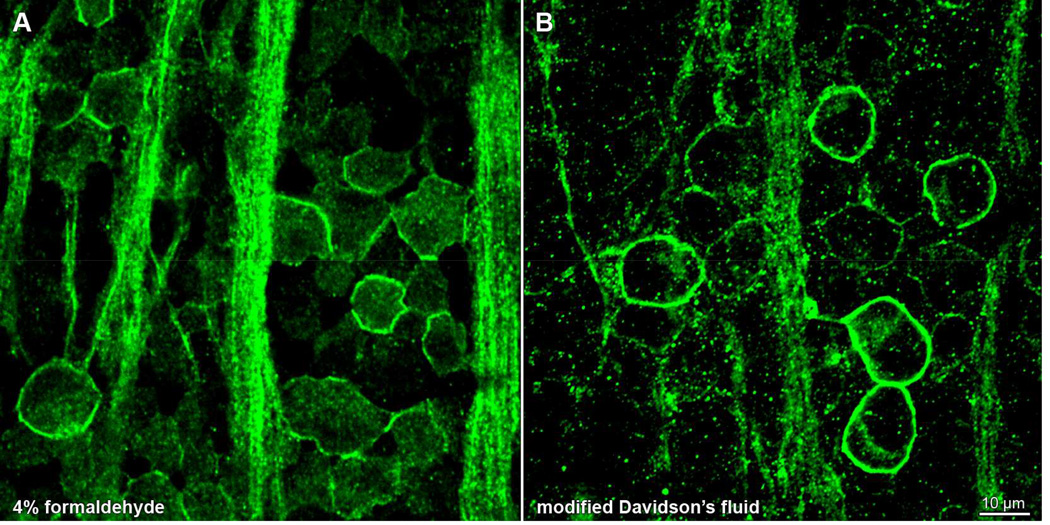
Davidson’s fluid is compatible with a wide range of antibodies commonly used in rat retina: Thy1 (Kwong et al., 2003), HCN pacemaker channels (Stradleigh et al., 2011), rhodopsin, GFAP, cleaved caspase-3, glutamine synthetase (McKay et al., 2009), Brn3a, tyrosine hydroxylase, parvalbumin, calbindin, choline acetyltransferase, and others (Chidlow et al., 2011). However, some small antigens (e.g., GABA, heat shock proteins) do not readily stain after fixation in Davidson’s fluid (Chidlow et al., 2011). Also, fixation in Davidson’s fluid may alter the staining pattern of some antibodies. Whole mounted rat retinae fixed in either modified Davidson’s fluid (Latendresse et al., 2002) or formaldehyde and stained with an antibody against the HCN4 pacemaker channel (clone N114/10; NeuroMab) stain the same number of cells in each field (Fig. 7). Whereas immunopositive cells show fluorescence around the cell membranes in both fixatives, and the formaldehyde-fixed cells also have a noticeable amount of HCN4 signal throughout the cytosol (Fig. 7A), the cells fixed in Davidson’s fluid have little cytosolic stain (Fig. 7B).
Figure 7.
Fixative type gives a differential staining pattern of HCN4 antibody in rat retina. Whole-mounted retinae (from opposite eyes of same animal) fixed in (A) 4% formaldehyde or (B) modified Davidson’s fluid, processed in parallel, and stained with anti-HCN4 primary antibody and Alexa Fluor 488-conjugated anti-mouse secondary antibody (green). Each panel shows a single optical section through the ganglion cell layer, collected on a laser scanning confocal microscope. Scale bar in (B) is 10 µm and applies to both panels.
7. Future Directions
Aldehyde fixatives have proven to be popular and reliable, and each particular aldehyde has its strengths and weaknesses. Formaldehyde, the most popular aldehyde fixative agent for immunohistochemistry, offers mostly satisfactory tissue preservation and immobilization of non-labile antigens in a wide range of applications. Its performance and reactivity can be fine-tuned by manipulating the conditions of fixation, such as solution pH and temperature. Formaldehyde remains a popular fixative despite drawbacks associated with its use; formaldehyde-fixed antigens may become overfixed, losing immunoreactivity in a dose-dependent manner. This dose-dependence also applies to background fluorescence and autofluorescence, which increase as fixation time increases. Formaldehyde's slow fixation and cytotoxic effects lead to morphological disruption, hindering anatomical analysis in thick tissue specimens. Formaldehyde has also proven incompatible with specific types of antigens, especially small state-dependent molecules.
As an alternative to formaldehyde, glutaraldehyde allows for faster fixation of statedependent antigens as well as improved preservation of tissue morphology. Glutaraldehyde use at high concentrations has drawbacks, most notably a significantly reduced antigen immunoreactivity with a corresponding increase in background autofluorescence. These effects are more severe, and proceed much more rapidly, than those seen in formaldehyde fixation. The addition of dilute glutaraldehyde to formaldehyde fixative solutions allows for faster and more complete fixation, and better preservation of labile state-dependent antigens, than formaldehyde alone. This highly efficient fixative works while avoiding the drawbacks of concentrated glutaraldehyde solutions.
Lastly, several modifications of routinely used protocols have been found to improve the intensity, subcellular distribution, and repeatability of immunostaining patterns obtained with at least some antibodies. No single protocol that we are aware of is optimal for use with all antibodies and preparations, and in agreement with studies of other tissues (Lorincz and Nusser, 2008), our experience has been that protocols yielding the best results must be empirically determined for each application. To date, few studies have provided systematic comparisons and detailed descriptions of the intensity and distribution of immunostaining obtained with antibodies in retinae fixed by different methods. Future studies may provide the information needed to list proteins that can be immunolabeled using each fixative, and fixation steps that facilitate immunostaining retinal wholemounts sections, selected cell types, and subcellular loci for specific proteins. For now, we can list protocols that permitted us to stain for three types of antigens (intracellularly expressed fluorescent label; integral membrane protein; and state-dependent signaling components; see Tables 2–4). Lists of antibodies, possible applications, and compatible tissues and species can also be found on the web (Marx, 2013). The development of protocols and methods that help identify the molecular phenotypes of cells, detect their lightresponsiveness, and test their cross-species relevance, clearly remains of keen interest.
Table 2.
Visualization of induced green fluorescent protein (GFP) and preservation of in vivo cell structure in ganglion cells of organotypically cultured rat and rabbit retina (Stradleigh et al., 2015)
| Antigen | green fluorescent protein | |
| Primary Antibody | Alexa Fluor 488-conjugated rabbit polyclonal anti-GFP (#A21311, Life Technologies) | |
| Fixative | PB + SucroseLOW (pH 7.4; Table 1) protectant (pH 7.4; Stradleigh et al., 2015) | |
| Buffer Recipe (10×, 100 mL) | sodium phosphate, monobasic, monohydrate (33.5 mM) | 4.62 g |
| sodium phosphate, dibasic, anhydrous (33.5 mM) | 4.76 g | |
| sucrose (200 mM) | 68.46 g | |
| water | bring to volume (100 mL) | |
| Fixative Recipe (10 mL) | 10× buffer stock | 1 mL |
| formalin (#F-1635, Sigma-Aldrich) | 1 mL | |
| water | 8 mL | |
. Culture retina and induce GFP expression (Koizumi et al, 2007; Greenberg et al., 2011; Stradleigh et al., 2015).
. Immerse membrane-bound retina in 10 mL formalin-free buffer, 30 min on ice.
. Immerse membrane-bound retina in 10 mL fixative, 18 hr at 4°C.
. Rinse and quench in Tris-buffered saline, 30 min at 4°C.
. Permeabilize in PBS (supplemented with 0.5% bovine serum albumin, 0.05% Triton X-100, 5% normal donkey serum), 18 hr at 4°C.
. Apply primary antibody in PBS, 18 hr at 4°C. Rin se in PBS.
. Apply secondary antibody in PBS, 18 hr at 4°C.
. Rinse in PBS and mount to slide.
Table 4.
Visualization of ion channel subunits in flat-mounted rat retina (Stradleigh et al., 2011)
| Antigen | HCN4 | |
| Primary Antibody | mouse monoclonal anti-HCN4 (clone N114/10, NeuroMab) | |
| Secondary Antibody | Alexa Fluor 488-conjugated donkey anti-mouse (#715-545-151, Jackson ImmunoResearch) | |
| Fixative | modified Davidson’s Fluid (mDF; Latendresse et al., 2002) | |
| Fixative Recipe (10 mL) | formalin (#F-1635, Sigma-Aldrich) | 3 mL |
| ethanol (100%) | 1.5 mL | |
| glacial acetic acid | 0.5 mL | |
| water | 5 mL | |
. Dissect whole retina in ice cold PBS, place vitreous side up on black nitrocellulose membrane filter (#HABG01300, Millipore), and flatten by applying suction.
. While applying suction, add 0.5 mL ice cold mDF dropwise to membrane-attached retina.
Immerse flat mount in 10 mL room temperature mDF, 30 min.
. Rinse and quench in Tris-buffered saline, 30 min at 4°C.
. Permeabilize in PBS (supplemented with 0.5% bovine serum albumin, 0.05% Triton X-100, 5% normal donkey serum), 18 hr at 4°C.
. Apply primary antibody in PBS, 18 hr at 4°C. Rin se in PBS.
. Apply secondary antibody in PBS, 18 hr at 4°C.
. Rinse in PBS and mount to slide.
Table 3.
Preservation of state-dependent antigens in vibratome sections of light- and dark adapted rat retina (Ogata et al., 2012).
| Antigens | cyclic adenosine monophosphate (cAMP) calcium/calmodulin-dependent protein kinase II, phosphorylated T286 (P-CaMKII) |
|
| Primary Antibodies | mouse monoclonal anti-cAMP (clone M486, Abcam) rabbit polyclonal anti-P-CaMKII (#3361, Cell Signaling) |
|
| Secondary Antibodies | Alexa Fluor 488-conjugated donkey anti-mouse (#715-545-151, Jackson ImmunoResearch) Cy3-conjugated donkey anti-rabbit (#711-166-152, Jackson ImmunoResearch) |
|
| Counterstain | Qnuclear Deep Red Stain (#Q10363, Life Technologies) | |
| Fixative | 4% formaldehyde, 0.1% glutaraldehyde, 200 mM sucrose, 1 mM EGTA in sodium borate (pH 10; 33.5 mM) | |
| Buffer Recipe (10×, 100 mL) | sodium tetraborate, decahydrate | 12.78 g |
| sucrose | 68.46 g | |
| EGTA | 0.38 g | |
| water | bring to volume (100 mL) | |
| Fixative Recipe (10 mL) | 10× buffer stock | 1 mL |
| formalin (#F-1635, Sigma-Aldrich) | 1 mL | |
| glutaraldehyde (50% solution; #18431, Ted Pella) | 20 µL | |
| water | bring to volume (10 mL) | |
. Light- or dark-adapt rat and collect eyes as in Ogata et al. (2012).
. Nick eye at limbus with a scalpel blade and immerse in fixative, 18 hr at 4°C.
. Dissect eye in Tris-buffered saline and isolate retina.
. Embed retina in 5% (w/v) low gelling temperature agarose (#32829, Affymetrix) diluted in Tris-buffered saline.
. Section retina on sagittal plane at 50 µm thickness with a vibrating microtome.
. Permeabilize and block sections in Tris-Triton solution (100 mM Tris-HCl, 0.1% Triton X-100, pH 8) supplemented with 5% normal donkey serum, 24 hr at 4°C.
. Apply primary antibody in Tris-Triton, 48 hr at 4°C. Rinse in Tris-Triton.
. Apply secondary antibody in Tris-Triton, 18 hr at 4°C. Rinse in Tris-Triton.
. Apply Qnuclear counterstain, 30 min at room temperature.
. Rinse in Tris-Triton and mount to slide.
Acknowledgements
The authors thank Drs. S.K. Fisher, W.D. Eldred, P.G. FitzGerald, D.W. Marshak, and B. Mulloney for comments on drafts of the text. While preparing this review and collecting the results presented in the figures, the authors were supported by the following grants from the National Institutes of Health (Bethesda, MD): EY008120 (A.T.I.), P30 EY012576 (J.S. Werner), R25 56765 (NIH-IMSD Fellowship to T.W.S.), T32 EY015387 (NIH-NEI Training Grant Fellowship to T.W.S.), and EY008120-17S1 (NIH Research Supplement to Promote Diversity in Health-Related Research to A.T.I.).
Abbreviations
- cAMP
3’-5’-cyclic adenosine monophosphate
- GPI
glycosylphosphatidylinositol
- HCN
hyperpolarization-activated cyclic nucleotide-gated
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- SNR
signal-to-noise ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CK, Perez JM, Hawthorne MN. Rod and cone densities in the Rhesus. Invest Ophthalmol. 1974;13:885–888. [PubMed] [Google Scholar]
- Ames A, 3rd, Isom JB, Nesbett FB. Effects of osmotic changes on water and electrolytes in nervous tissue. J Physiology. 1965;177:246–262. doi: 10.1113/jphysiol.1965.sp007590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammermüller J, Weiler R. Physiological and morphological characterization of OFF-center amacrine cells in the turtle retina. J Comp Neurol. 1988;273:137–148. doi: 10.1002/cne.902730202. [DOI] [PubMed] [Google Scholar]
- Anderson PJ. Purification and quantitation of glutaraldehyde and its effect on several enzyme activities in skeletal muscle. J Histochem Cytochem. 1967;15:652–661. doi: 10.1177/15.11.652. [DOI] [PubMed] [Google Scholar]
- Andrews PM, Coffey AK. Factors that improve the preservation of nephron morphology during cold-storage. Lab Invest. 1982;46:100–120. [PubMed] [Google Scholar]
- Avrameas S, Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969;6:53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Badea TC, Hua ZL, Smallwood PM, Williams J, Rotolo T, Ye X, Nathans J. New mouse lines for the analysis of neuronal morphology using CreERT)/loxP-directed sparse labeling. PLoS One. 2009;4:e7859. doi: 10.1371/journal.pone.0007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaton AJ, Vaury P, Baviera EE, Vuong PN, Galet BA. An EDTA-pressure cooker protocol. A high performance immunohistochemistry technique. Ann pathol. 1995;15:295. [PubMed] [Google Scholar]
- Bankfalvi AA, Piffko J, Ofner D, Dreier R, Bocker W, Werner K. Significance of wet autoclave pretreatment in immunohistochemistry. Pathol Oncol Res. 1996;2:71–77. doi: 10.1007/BF02893955. [DOI] [PubMed] [Google Scholar]
- Barnett CH, Cusick ET, Stockwell RA. A controlled-heat method of fixation. J Anat. 1966;100:937. doi: 10.3109/10520296609116314. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ, Dräger UC. Thy-1 antigen: a ganglion cell specific marker in rodent retina. Neurosci. 1984;11:847–855. doi: 10.1016/0306-4522(84)90195-7. [DOI] [PubMed] [Google Scholar]
- Barsony J, Marx SJ. Immunocytology on microwave-fixed cells reveals rapid and agonistspecific changes in subcellular accumulation patterns for cAMP or cGMP. Proc Natl Acad Sci U S A. 1990;87:1188–1192. doi: 10.1073/pnas.87.3.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battifora H, Kopinski M. The influence of protease digestion and duration of fixation on the immunostaining of keratins - a comparison of formalin and ethanol fixation. J Histochem Cytochem. 1986;34:1095–1100. doi: 10.1177/34.8.2426335. [DOI] [PubMed] [Google Scholar]
- Bee JA. Glycoconjugates of the avian eye: the development and maturation of the neural retina as visualized by lectin binding. Differentiation. 1982;23:128–140. doi: 10.1111/j.1432-0436.1982.tb01275.x. [DOI] [PubMed] [Google Scholar]
- Beisker W, Dolbeare F, Gray JW. An improved immunocytochemical procedure for high-sensitivity detection of incorporated bromodeoxyuridine. Cytometry. 1987;8:235–239. doi: 10.1002/cyto.990080218. [DOI] [PubMed] [Google Scholar]
- Bernstein SL, Koo JH, Slater BJ, Guo Y, Margolis FL. Analysis of optic nerve stroke by retinal Bex expression. Mol Vis. 2006;12:147–155. [PubMed] [Google Scholar]
- Berod A, Hartman BK, Pujol JF. Importance of fixation in immunohistochemistry - Use of formaldehyde solutions at variable pH for the localization of tyrosine-hydroxylase. J Histochem Cytochem. 1981;29:844–850. doi: 10.1177/29.7.6167611. [DOI] [PubMed] [Google Scholar]
- Blankenship T, Bradshaw L, Shibata B, FitzGerald P. Structural specializations emerging late in mouse lens fiber cell differentiation. Invest Ophth Vis Sci. 2007;48:3269–3276. doi: 10.1167/iovs.07-0109. [DOI] [PubMed] [Google Scholar]
- Bloem E. Thin layer chromatography of aldehydes. J Chromatography. 1968;35:108–110. doi: 10.1016/s0021-9673(01)82359-1. [DOI] [PubMed] [Google Scholar]
- Blom J, Giove T, Deshpande M, Eldred WD. Characterization of nitric oxide signaling pathways in the mouse retina. J Comp Neurol. 2012;520:4204–4217. doi: 10.1002/cne.23148. [DOI] [PubMed] [Google Scholar]
- Blute TA, Velasco P, Eldred WD. Functional localization of soluble guanylate cyclase in turtle retina: modulation of cGMP by nitric oxide donors. Vis Neurosci. 1998;15:485–498. doi: 10.1017/s0952523898153075. [DOI] [PubMed] [Google Scholar]
- Boiko T, Van Wart A, Caldwell JH, Levinson SR, Trimmer JS, Matthews G. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J Neurosci. 2003;23:2306–2313. doi: 10.1523/JNEUROSCI.23-06-02306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin S, Petrera F, Rosai J, Stanta G. DNA and RNA obtained from Bouin's fixed tissues. J Clin Pathol. 2005;58:313–316. doi: 10.1136/jcp.2004.016477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon ME, Kok LP. Theory and practice of combining coagulant fixation and microwave histoprocessing. Biotech Histochem. 2008;83:261–277. doi: 10.1080/10520290802553476. [DOI] [PubMed] [Google Scholar]
- Borovikov YS. The effect of glutaraldehyde and phalloidin on the conformation of F-actin. Gen Physiol Biophys. 1984;3:513–516. [PubMed] [Google Scholar]
- Bowes JH, Cater CW. Crosslinking of Collagen. J Appl Chem. 1965;15:296. [Google Scholar]
- Brandon C. Improved immunocytochemical staining through the use of Fab fragments of primary antibody, Fab-specific 2nd antibody, and Fab horseradish-peroxidase. J Histochem Cytochem. 1985a;33:715–719. doi: 10.1177/33.7.2409131. [DOI] [PubMed] [Google Scholar]
- Brandon C. Retinal GABA neurons: localization in vertebrate species using an antiserum to rabbit brain glutamate decarboxylase. Brain Res. 1985b;344:286–295. doi: 10.1016/0006-8993(85)90806-6. [DOI] [PubMed] [Google Scholar]
- Brandon C. Cholinergic neurons in the rabbit retina: immunocytochemical localization, and relationship to GABAergic and cholinesterase-containing neurons. Brain Res. 1987;401:385–391. doi: 10.1016/0006-8993(87)91426-0. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Dick O, Boeckers TM. The postsynaptic scaffold proteins ProSAP1/Shank2 and Homer1 are associated with glutamate receptor complexes at rat retinal synapses. J Comp Neurol. 2004;475:551–563. doi: 10.1002/cne.20194. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Fletcher EL, Garner CC, Gundelfinger ED, Wässle H. Differential expression of the presynaptic cytomatrix protein bassoon among ribbon synapses in the mammalian retina. Eur J Neurosci. 1999;11:3683–3693. doi: 10.1046/j.1460-9568.1999.00793.x. [DOI] [PubMed] [Google Scholar]
- Brecha N, Johnson D, Peichl L, Wässle H. Cholinergic amacrine cells of the rabbit retina contain glutamate-decarboxylase and gamma-aminobutyrate immunoreactivity. Proc Natl Acad Sci USA. 1988;85:6187–6191. doi: 10.1073/pnas.85.16.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecha N, Karten HJ, Laverack C. Enkephalin-containing amacrine cells in the avian retina: immunohistochemical localization. Proc Natl Acad Sci U S A. 1979;76:3010–3014. doi: 10.1073/pnas.76.6.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubenik GA, Brown GM, Uhlir I, Grota LJ. Immunohistological localization of Nacetylindolealkylamines in pineal gland, retina and cerebellum. Brain Res. 1974;81:233–242. doi: 10.1016/0006-8993(74)90938-x. [DOI] [PubMed] [Google Scholar]
- Bumsted K, Hendrickson A. Distribution and development of short-wavelength cones differ between Macaca monkey and human fovea. J Comp Neurol. 1999;403:502–516. [PubMed] [Google Scholar]
- Casini G, Brecha NC. Postnatal development of tyrosine hydroxylase immunoreactive amacrine cells in the rabbit retina: I. Morphological characterization. J Comp Neurol. 1992;326:283–301. doi: 10.1002/cne.903260210. [DOI] [PubMed] [Google Scholar]
- Castellarin AA, Sugino IK, Vargas JA, Parolini B, Lui GM, Zarbin MA. In vitro transplantation of fetal human retinal pigment epithelial cells onto human cadaver Bruch's membrane. Exp Eye Res. 1998;66:49–67. doi: 10.1006/exer.1997.0404. [DOI] [PubMed] [Google Scholar]
- Chaw YFM, Crane LE, Lange P, Shapiro R. Isolation and identification of cross-links from formaldehyde-treated nucleic-acids. Biochem. 1980;19:5525–5531. doi: 10.1021/bi00565a010. [DOI] [PubMed] [Google Scholar]
- Chen Y, Veracini L, Benistant C, Jacobson K. The transmembrane protein CBP plays a role in transiently anchoring small clusters of Thy-1, a GPI-anchored protein, to the cytoskeleton. J Cell Sci. 2009;122:3966–3972. doi: 10.1242/jcs.049346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidlow G, Daymon M, Wood JP, Casson RJ. Localization of a wide-ranging panel of antigens in the rat retina by immunohistochemistry: comparison of Davidson's solution and formalin as fixatives. J Histochem Cytochem. 2011;59:884–898. doi: 10.1369/0022155411418115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirita G, Chisalita D. Investigations of Reaction of Aldehydes with Functional Groups of Some Amino Acids of Collagen Structure. J Am Leather Chem As. 1971;66:369-&. [Google Scholar]
- Clancy B, Cauller LJ. Reduction of background autofluorescence in brain sections following immersion in sodium borohydride. J Neurosci Methods. 1998;83:97–102. doi: 10.1016/s0165-0270(98)00066-1. [DOI] [PubMed] [Google Scholar]
- Cleary PE, Minckler DS, Ryan SJ. Ultrastructure of traction retinal detachment in rhesus monkey eyes after a posterior penetrating ocular injury. Am J Ophthalmol. 1980;90:829–845. doi: 10.1016/s0002-9394(14)75198-0. [DOI] [PubMed] [Google Scholar]
- Contini M, Raviola E. GABAergic synapses made by a retinal dopaminergic neuron. Proc Natl Acad Sci U S A. 2003;100:1358–1363. doi: 10.1073/pnas.0337681100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs J, van der List D, Wang GY, Chalupa LM. Morphological properties of mouse retinal ganglion cells. Neurosci. 2006;140:123–136. doi: 10.1016/j.neuroscience.2006.02.079. [DOI] [PubMed] [Google Scholar]
- Coupland RE, Hopwood D. The mechanism of the differential staining reaction for adrenaline-and noreadrenaline-storing granules in tissues fixed in glutaraldehyde. J Anat. 1966;100:227–243. [PMC free article] [PubMed] [Google Scholar]
- Cristofanilli M, Akopian A. Calcium channel and glutamate receptor activities regulate actin organization in salamander retinal neurons. J Physiol. 2006;575:543–554. doi: 10.1113/jphysiol.2006.114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca N. Web Page: Retinal Microscopy; Universidad de Alicante. [Accessed March 19, 2015];2008 http://www.retinalmicroscopy.com/. [Google Scholar]
- Cuenca N, Fernandez-Sanchez L, Campello L, Maneu V, De la Villa P, Lax P, Pinilla I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res. 2014;43:17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- da Silva Filho M, Santos DV, Costa KM. A new low cost wide-field illumination method for photooxidation of intracellular fluorescent markers. PLoS One. 2013;8:e56512. doi: 10.1371/journal.pone.0056512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM. The dopaminergic amacrine cell. J Comp Neurol. 1990;301:461–489. doi: 10.1002/cne.903010310. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Peterson BB, Robinson FR, Gamlin PD. Fireworks in the primate retina: In vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003;37:15–27. doi: 10.1016/s0896-6273(02)01143-1. [DOI] [PubMed] [Google Scholar]
- Daniele LL, Adams RH, Durante DE, Pugh EN, Jr, Philp NJ. Novel distribution of junctional adhesion molecule-C in the neural retina and retinal pigment epithelium. J Comp Neurol. 2007;505:166–176. doi: 10.1002/cne.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marzo AM, Fedor HH, Gage WR, Rubin MA. Inadequate formalin fixation decreases reliability of p27 immunohistochemical staining: probing optimal fixation time using high-density tissue microarrays. Hum Pathol. 2002;33:756–760. doi: 10.1053/hupa.2002.126187. [DOI] [PubMed] [Google Scholar]
- de Vente J, Steinbusch HW, Schipper J. A new approach to immunocytochemistry of 3',5'-cyclic guanosine monophosphate: preparation, specificity, and initial application of a new antiserum against formaldehyde-fixed 3',5'-cyclic guanosine monophosphate. Neurosci. 1987;22:361–373. doi: 10.1016/0306-4522(87)90226-0. [DOI] [PubMed] [Google Scholar]
- Deerinck T. Web Page: First place, Olympus BioScapes International Digital Imaging Competition. [Accessed September 15, 2014];2006 http://www.olympusbioscapes.com/gallery/photographer/thomas-deerinck. [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- Doughty MJ, Bergmanson JP, Blocker Y. Shrinkage and distortion of the rabbit corneal endothelial cell mosaic caused by a high osmolality glutaraldehyde-formaldehyde fixative compared to glutaraldehyde. Tiss Cell. 1997;29:533–547. doi: 10.1016/s0040-8166(97)80054-7. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Ehinger B. Synaptic organization of the amine-containing interplexiform cells of the goldfish and Cebus monkey retinas. Science. 1975;188:270–273. doi: 10.1126/science.804181. [DOI] [PubMed] [Google Scholar]
- Duong H, Han M. A multispectral LED array for the reduction of background autofluorescence in brain tissue. J Neurosci Methods. 2013;220:46–54. doi: 10.1016/j.jneumeth.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldred WD, Zucker C, Karten HJ, Yazulla S. Comparison of fixation and penetration enhancement techniques for use in ultrastructural immunocytochemistry. J Histochem Cytochem. 1983;31:285–292. doi: 10.1177/31.2.6339606. [DOI] [PubMed] [Google Scholar]
- Elias RV, Sezate SS, Cao W, McGinnis JF. Temporal kinetics of the light/dark translocation and compartmentation of arrestin and alpha-transducin in mouse photoreceptor cells. Mol Vis. 2004;10:672–681. [PubMed] [Google Scholar]
- Eltoum I, Fredenburgh J, Myers RB, Grizzle WE. Introduction to the theory and practice of fixation of tissues. J Histotechnol. 2001;24:173–190. [Google Scholar]
- Eranko O, et al. Distribution of fluorescing islets, adrenaline and noradrenaline in the adrenal medulla of the cat. Acta Endocrinol. 1955a;18:180–188. doi: 10.1530/acta.0.0180180. [DOI] [PubMed] [Google Scholar]
- Eranko O, et al. Distribution of fluorescing islets, adrenaline and noradrenaline in the adrenal medulla of the hamster. Acta Endocrinol. 1955b;18:174–179. doi: 10.1530/acta.0.0180174. [DOI] [PubMed] [Google Scholar]
- Erickson PA, Fisher SK, Guerin CJ, Anderson DH, Kaska DD. Glial fibrillary acidic protein increases in Muller cells after retinal detachment. Exp Eye Res. 1987;44:37–48. doi: 10.1016/s0014-4835(87)80023-4. [DOI] [PubMed] [Google Scholar]
- Espina V, Mueller C, Edmiston K, Sciro M, Petricoin EF, Liotta LA. Tissue is alive: New technologies are needed to address the problems of protein biomarker pre-analytical variability. Proteomics. Clinical applications. 2009;3:874–882. doi: 10.1002/prca.200800001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers P, Uylings HB. Microwave-stimulated antigen retrieval is pH and temperature dependent. J Histochem Cytochem. 1994;42:1555–1563. doi: 10.1177/42.12.7983356. [DOI] [PubMed] [Google Scholar]
- Ezaki T. Antigen retrieval on formaldehyde-fixed paraffin sections: Its potential drawbacks and optimization for double immunostaining. Micron. 2000;31:639–649. doi: 10.1016/s0968-4328(99)00064-5. [DOI] [PubMed] [Google Scholar]
- Fahimi HD, Drochman P. Purification of glutaraldehyde - Its significance for preservation of acid phosphatase activity. J Histochem Cytochem. 1968;16:199–204. doi: 10.1177/16.3.199. [DOI] [PubMed] [Google Scholar]
- Falck B, Thieme G, Hillarp NA, Torp A. Fluorescence of catechol amines and related compounds condensed with formaldehyde. J Histochem Cytochem. 1962;10:348. doi: 10.1016/0361-9230(82)90113-7. [DOI] [PubMed] [Google Scholar]
- Falck B, Torp A. A fluorescence method for histochemical demonstration of noradrenalin in the adrenal medulla. Medicina experimentalis. 1961;5:428–432. [PubMed] [Google Scholar]
- Famiglietti EV. Starburst amacrine cells - Morphological constancy and systematic variation in the anisotropic field of rabbit retinal reurons. J Neurosci. 1985;5:562–577. doi: 10.1523/JNEUROSCI.05-02-00562.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV, Tumosa N. Immunocytochemical staining of cholinergic amacrine cells in rabbit retina. Brain Res. 1987;413:398–403. doi: 10.1016/0006-8993(87)91037-7. [DOI] [PubMed] [Google Scholar]
- Farber DB, Souza DW, Chase DG, Lolley RN. Cyclic nucleotides of cone-dominant retinas. Reduction of cyclic AMP levels by light and by cone degeneration. Invest Ophthalmol Vis Sci. 1981;20:24–31. [PubMed] [Google Scholar]
- Feder N, Sidman RL. Methods and principles of fixation by freeze-substitution. J Biophys Biochem Cytol. 1958;4:593–600. doi: 10.1083/jcb.4.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A. Web Page: Honorable mention, Nikon Small World Photomicrography Competition. [Accessed September 15, 2014];2008 http://www.nikonsmallworld.com/galleries/entry/2008-photomicrography-competition/25. [Google Scholar]
- Fisher SK. Web Page: Protocols, Retinal Cell Biology Lab; University of California Santa Barbara. [Accessed January 3, 2015];2013 https://labs.mcdb.ucsb.edu/fisher/steven/protocols.html. [Google Scholar]
- Fox CH, Johnson FB, Whiting J, Roller PP. Formaldehyde fixation. J Histochem Cytochem. 1985;33:845–853. doi: 10.1177/33.8.3894502. [DOI] [PubMed] [Google Scholar]
- Fraenkel-Conrat H, Olcott HS. The reaction of formaldehyde with proteins; cross-linking between amino and primary amide or guanidyl groups. J Am Chem Soc. 1948;70:2673–2684. doi: 10.1021/ja01188a018. [DOI] [PubMed] [Google Scholar]
- Free RB, Hazelwood LA, Cabrera DM, Spalding HN, Namkung Y, Rankin ML, Sibley DR. D1 and D2 dopamine receptor expression is regulated by direct interaction with the chaperone protein calnexin. J Biol Chem. 2007;282:21285–21300. doi: 10.1074/jbc.M701555200. [DOI] [PubMed] [Google Scholar]
- French J, Halliday J, Scott M, Adkins D, Liess C, Waterton JC, Stewart J. Retinal folding in the term rabbit fetus-Developmental abnormality or fixation artifact? Reprod Toxicol. 2008;26:262–266. doi: 10.1016/j.reprotox.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Masuyama Y. Monoclonal antibody against the glutaraldehyde-conjugated polyamine, spermine. Histochem Cell Biol. 1995;104:309–316. doi: 10.1007/BF01464327. [DOI] [PubMed] [Google Scholar]
- Gabriel R, Lesauter J, Banvolgyi T, Petrovics G, Silver R, Witkovsky P. AII amacrine neurons of the rat retina show diurnal and circadian rhythms of parvalbumin immunoreactivity. Cell Tiss Res. 2004;315:181–186. doi: 10.1007/s00441-003-0785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel R, Lesauter J, Silver R, Garcia-Espana A, Witkovsky P. Diurnal and circadian variation of protein kinase C immunoreactivity in the rat retina. J Comp Neurol. 2001;439:140–150. doi: 10.1002/cne.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage GJ, Kipke DR, Shain W. Whole animal perfusion fixation for rodents. J Vis Exp. 2012;30:3564. doi: 10.3791/3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbinur T, Obolensky A, Berenshtein E, Vinokur V, Chowers I, Chevion M, Banin E. Effect of para-aminobenzoic acid on the course of retinal degeneration in the rd10 mouse. J Ocular Pharmacol Ther. 2009;25:475–482. doi: 10.1089/jop.2009.0020. [DOI] [PubMed] [Google Scholar]
- Gallegos Ruiz MI, Floor K, Vos W, Grünberg K, Meijer GA, Rodriguez JA, Giaccone G. Epidermal growth factor receptor (EGFR) gene copy number detection in non-small-cell lung cancer; a comparison of fluorescence in situ hybridization and chromogenic in situ hybridization. Histopathol. 2007;51:631–637. doi: 10.1111/j.1365-2559.2007.02854.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Caballero T, Grabau D, Green AR, Gregory J, Schad A, Kohlwes E, Ellis IO, Watts S, Mollerup J. Determination of HER2 amplification in primary breast cancer using dual-colour chromogenic in situ hybridization is comparable to fluorescence in situ hybridization: a European multicentre study involving 168 specimens. Histopathol. 2010;56:472–480. doi: 10.1111/j.1365-2559.2010.03503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastinger MJ, O'Brien JJ, Larsen NB, Marshak DW. Histamine immunoreactive axons in the macaque retina. Invest Ophthalmol Vis Sci. 1999;40:487–495. [PMC free article] [PubMed] [Google Scholar]
- Goodyear MJ, Crewther SG, Murphy MJ, Giummarra L, Hazi A, Junghans BM, Crewther DP. Spatial and temporal dissociation of AQP4 and Kir4.1 expression during induction of refractive errors. Mol Vis. 2010;16:1610–1619. [PMC free article] [PubMed] [Google Scholar]
- Gown AM, Willingham MC. Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. J histochem cytochem. 2002;50:449–454. doi: 10.1177/002215540205000401. [DOI] [PubMed] [Google Scholar]
- Graham RC, Karnovsky MJ. Glomerular permeability - Ultrastructural cytochemical studies using peroxidases as protein tracers. J Exp Med. 1966;124:1123–1134. doi: 10.1084/jem.124.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg KP, Pham A, Werblin FS. Differential targeting of optical neuromodulators to ganglion cell soma and dendrites allows dynamic control of center-surround antagonism. Neuron. 2011;69:713–720. doi: 10.1016/j.neuron.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Greenwood SM, Connolly CN. Dendritic and mitochondrial changes during glutamate excitotoxicity. Neuropharmacol. 2007;53:891–898. doi: 10.1016/j.neuropharm.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Grillo TA, Ogunnaike PO, Faoye S. Effects of histological and electron microscopical fixatives on the insulin content of the rat pancreas. J Endocrinol. 1971;51:645–649. doi: 10.1677/joe.0.0510645. [DOI] [PubMed] [Google Scholar]
- Grunert U, Wässle H. Immunocytochemical localization of glycine receptors in the mammalian retina. J Comp Neurol. 1993;335:523–537. doi: 10.1002/cne.903350405. [DOI] [PubMed] [Google Scholar]
- Guo C, Hirano AA, Stella SL, Jr, Bitzer M, Brecha NC. Guinea pig horizontal cells express GABA, the GABA-synthesizing enzyme GAD 65, and the GABA vesicular transporter. J Comp Neurol. 2010;518:1647–1669. doi: 10.1002/cne.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase W, Friese W, Gordon RD, Müller H, Cook NJ. Immunological characterization and localization of the Na+/Ca2(+)-exchanger in bovine retina. J Neurosci. 1990;10:1486–1494. doi: 10.1523/JNEUROSCI.10-05-01486.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack I, Frech M, Dick O, Peichl L, Brandstatter JH. Heterogeneous distribution of AMPA glutamate receptor subunits at the photoreceptor synapses of rodent retina. Eur J Neurosci. 2001;13:15–24. [PubMed] [Google Scholar]
- Hageman GS, Johnson LV. Structure, composition and function of the retinal interphotoreceptor matrix. Progr Ret Res. 1991;10:207–249. [Google Scholar]
- Häggendal J, Malmfors T. Identification and cellular localization of the catecholamines in the retina and the choroid of the rabbit. Acta physiol Scand. 1965;64:58–66. doi: 10.1111/j.1748-1716.1965.tb04153.x. [DOI] [PubMed] [Google Scholar]
- Hamazaki S, Koshiba M, Habuchi T, Takahashi R, Sugiyama T. The Effect of Formalin Fixation on Restriction-Endonuclease Digestion of DNA and Pcr Amplification. Pathol Res Pract. 1993;189:553–557. doi: 10.1016/S0344-0338(11)80365-1. [DOI] [PubMed] [Google Scholar]
- Harahush BK, Green K, Webb R, Hart NS, Collin SP. Optimal preservation of the shark retina for ultrastructural analysis: an assessment of chemical, microwave, and high-pressure freezing fixation techniques. Micr Res Tech. 2012;75:1218–1228. doi: 10.1002/jemt.22052. [DOI] [PubMed] [Google Scholar]
- Hardy PM, Hughes GJ, Rydon HN. Formation of Quaternary Pyridinium Compounds by Action of Glutaraldehyde on Proteins. J Chem Soc Chem Comm. 1976:157–158. [Google Scholar]
- Hardy PM, Nicholls AC, Rydon HN. Nature of Glutaraldehyde in Aqueous Solution. J Chem Soc Chem Communications. 1969:565–566. [Google Scholar]
- Harrach B, Robenek H. Polyclonal antibodies against formaldehyde-modified apolipoprotein A-I. An approach to circumventing fixation-induced loss of antigenicity in immunocytochemistry. Arteriosclerosis. 1990;10:564–576. doi: 10.1161/01.atv.10.4.564. [DOI] [PubMed] [Google Scholar]
- Harries C, Tank L. Concerning the breakdown of cyclopentenes into semi aldehydes of glutaric acid or to glutaric dialdehyde. Ber Dtsch Chem Ges. 1908;41:1701–1711. [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Grunert U, Wässle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- Haverkamp S, Wässle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. J Neurosci. 2005;25:5438–5445. doi: 10.1523/JNEUROSCI.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida Y, Varela C, Ogata G, Partida GJ, Oi H, Stradleigh TW, Lee SC, Felipe Colado A, Ishida AT. Inhibition of adult rat retinal ganglion cells by D-1-type dopamine receptor activation. J Neurosci. 2009;29:15001–15016. doi: 10.1523/JNEUROSCI.3827-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander KG. Kinetic studies of formaldehyde binding in tissue. Biotech Histochem. 1994;69:177–179. doi: 10.3109/10520299409106282. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, Ryan M, Noble B, Wu JY. Colocalization of [3H]muscimol and antisera to GABA and glutamic acid decarboxylase within the same neurons in monkey retina. Brain Res. 1985;348:391–396. doi: 10.1016/0006-8993(85)90464-0. [DOI] [PubMed] [Google Scholar]
- Heneberg P, Lebduska P, Draberova L, Korb J, Draber P. Topography of plasma membrane microdomains and its consequences for mast cell signaling. Eur J Immunol. 2006;36:2795–2806. doi: 10.1002/eji.200636159. [DOI] [PubMed] [Google Scholar]
- Hermanson GT. Bioconjugate Techniques. 3rd edition ed. London: Academic Press; 2013. [Google Scholar]
- Highberger JH, Retzsch CE. The combination of formaldehyde with collagen. J Am Leath Chem Assoc. 1939;34:131. [Google Scholar]
- Hoffman EM, Schechter R, Miller KE. Fixative composition alters distributions of immunoreactivity for glutaminase and two markers of nociceptive neurons, Nav1.8 and TRPV1, in the rat dorsal root ganglion. J Histochem Cytochem. 2010;58:329–344. doi: 10.1369/jhc.2009.954008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D, et al. The effect of formaldehyde fixation and dehydration on ox adrenal medulla with respect to the chromaffin reaction and post-chroming. Histochemie. Histochemistry. Histochimie. 1967a;10:98–106. doi: 10.1007/BF00304378. [DOI] [PubMed] [Google Scholar]
- Hopwood D, et al. Some aspects of fixation with glutaraldehyde - a biochemical and histochemical comparison of effects of formaldehyde and glutaraldehyde fixation on various enzymes and glycogen with a note on penetration of glutaraldehyde into liver. J Anat. 1967b;101:83–92. [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. The effect of pH and various fixatives on isolated ox chromaffin granules with respect to the chromaffin reaction. J Anat. 1968;102:415–424. [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. Fixatives and fixation: a review. Histochem J. 1969;1:323–360. doi: 10.1007/BF01003278. [DOI] [PubMed] [Google Scholar]
- Hopwood D. The reactions between formaldehyde, glutaraldehyde and osmium tetroxide, and their fixation effects o bovine serum albumin and on tissue blocks. Histochemie. Histochemistry. Histochimie. 1970;24:50–64. doi: 10.1007/BF00310003. [DOI] [PubMed] [Google Scholar]
- Hopwood D. Theoretical and practical aspects of glutaraldehyde fixation. Histochem J. 1972;4:267–303. doi: 10.1007/BF01005005. [DOI] [PubMed] [Google Scholar]
- Hopwood D. Cell and tissue fixation, 1972–1982. Histochem J. 1985;17:389–442. doi: 10.1007/BF01003203. [DOI] [PubMed] [Google Scholar]
- Hornstein EP, Verweij J, Schnapf JL. Electrical coupling between red and green cones in primate retina. Nat Neurosci. 2004;7:745–750. doi: 10.1038/nn1274. [DOI] [PubMed] [Google Scholar]
- Hoshi H, Liu WL, Massey SC, Mills SL. ON inputs to the OFF layer: bipolar cells that break the stratification rules of the retina. J Neurosci. 2009;29:8875–8883. doi: 10.1523/JNEUROSCI.0912-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Wensel TG. Characterization of R9AP, a membrane anchor for the photoreceptor GTPase-accelerating protein, RGS9-1. Methods Enzymol. 2004;390:178–196. doi: 10.1016/S0076-6879(04)90012-2. [DOI] [PubMed] [Google Scholar]
- Hua C, Langlet C, Buferne M, Schmitt-Verhulst AM. Selective destruction by formaldehyde fixation of an H-2Kb serological determinant involving lysine 89 without loss of T-cell reactivity. Immunogenetics. 1985;21:227–234. doi: 10.1007/BF00375375. [DOI] [PubMed] [Google Scholar]
- Huang J, Honda W. CED: a conformational epitope database. BMC Immunol. 2006;7:7. doi: 10.1186/1471-2172-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Zhou D, Chase K, Gusella JF, Aronin N, DiFiglia M. Immunohistochemical localization of the D1 dopamine receptor in rat brain reveals its axonal transport, pre- and postsynaptic localization, and prevalence in the basal ganglia, limbic system, and thalamic reticular nucleus. Proc Natl Acad Sci U S A. 1992;89:11988–11992. doi: 10.1073/pnas.89.24.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Minassian H, More JD. Application of immunofluorescent staining on paraffin sections improved by trypsin digestion. Lab Invest. 1976;35:383–390. [PubMed] [Google Scholar]
- Huebner J. Antibody-antigen interactions and measurements of immunologic reactions. Immunology, Infection, and Immunity. 2004;(9) [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxlin KR, Goodchild AK. Retinal ganglion cells in the albino rat: revised morphological classification. J Comp Neurol. 1997;385:309–323. [PubMed] [Google Scholar]
- Ito S, Karnovsky MJ. Formaldehyde-glutaraldehyde fixatives containing trinitro compounds. J Cell Biol. 1968;39:A168-&. [Google Scholar]
- Ivanova E, Toychiev AH, Yee CW, Sagdullaev BT. Optimized protocol for retinal wholemount preparation for imaging and immunohistochemistry. J Vis Exp. 2013:e51018. doi: 10.3791/51018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Hammerman SB, Benz AM, Labruyere J, Zorumski CF, Olney JW. Comparison of rat retinal fixation techniques: chemical fixation and microwave irradiation. Exp Eye Res. 2000;70:191–198. doi: 10.1006/exer.1999.0779. [DOI] [PubMed] [Google Scholar]
- Jakobs TC, Koizumi A, Masland RH. The spatial distribution of glutamatergic inputs to dendrites of retinal ganglion cells. J Comp Neurol. 2008;510:221–236. doi: 10.1002/cne.21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan LY, Revel JP. Ultrastructural localization of rhodopsin in the vertebrate retina. J Cell Biol. 1974;62:257–273. doi: 10.1083/jcb.62.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen-Bienhold U, Dermietzel R, Weiler R. Distribution of connexin43 immunoreactivity in the retinas of different vertebrates. J Comp Neurol. 1998;396:310–321. [PubMed] [Google Scholar]
- Jasani B, Schmid KW. Significance of metallothionein overexpression in human tumours. Histopathol. 1997;31:211–214. doi: 10.1046/j.1365-2559.1997.2140848.x. [DOI] [PubMed] [Google Scholar]
- Job C, Lagnado L. Calcium and protein kinase C regulate the actin cytoskeleton in the synaptic terminal of retinal bipolar cells. J Cell Biol. 1998;143:1661–1672. doi: 10.1083/jcb.143.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GJ. Electron microscopy of frog photoreceptor outer segments after fixation with aldehydes. J Cell Sci. 1974;16:199–219. doi: 10.1242/jcs.16.1.199. [DOI] [PubMed] [Google Scholar]
- Juhl BR, Norgaard T, Bjerrum OJ. The effect of Tween 20 on indirect immunoperoxidase staining of blood group antigen A in human urothelium. J Histochem Cytochem. 1984;32:935–941. doi: 10.1177/32.9.6205049. [DOI] [PubMed] [Google Scholar]
- Kageyama GH, Meyer RL. Glutamate-immunoreactivity in the retina and optic tectum of goldfish. Brain Res. 1989;503:118–127. doi: 10.1016/0006-8993(89)91711-3. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, Cailotto C, Dijk F, Bergen A, Buijs RM. Circadian expression of clock genes and clock-controlled genes in the rat retina. Biochem Biophys Res Comm. 2005;330:18–26. doi: 10.1016/j.bbrc.2005.02.118. [DOI] [PubMed] [Google Scholar]
- Karma A, von Willebrand EO, Tommila PV, Paetau AE, Oskala PS, Immonen IJ. Primary intraocular lymphoma: improving the diagnostic procedure. Ophthalmol. 2007;114:1372–1377. doi: 10.1016/j.ophtha.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol. 1965;27:137. [Google Scholar]
- Kasukurthi R, Brenner MJ, Moore AM, Moradzadeh A, Ray WZ, Santosa KB, Mackinnon SE, Hunter DA. Transcardial perfusion versus immersion fixation for assessment of peripheral nerve regeneration. J Neurosci Methods. 2009;184:303–309. doi: 10.1016/j.jneumeth.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Keeley PW, Reese BE. Role of afferents in the differentiation of bipolar cells in the mouse retina. J Neurosci. 2010;30:1677–1685. doi: 10.1523/JNEUROSCI.5153-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser KT, Hughes TE, Whiting PJ, Lindstrom JM, Karten HJ. Cholinoceptive neurons in the retina of the chick: an immunohistochemical study of the nicotinic acetylcholine receptors. Vis Neurosci. 1988;1:349–366. doi: 10.1017/s0952523800004120. [DOI] [PubMed] [Google Scholar]
- Kiernan JA. Formaldehyde, formalin, paraformaldehyde and glutaraldehyde: What they are and what they do. Microscopy Today. 2000;00:8–12. [Google Scholar]
- Kiernan JA. A system for quantitative evaluation of fixatives for light microscopy using paraffin sections of kidney and brain. Biotech Histochem. 2009;84:1–10. doi: 10.1080/10520290802646619. [DOI] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]
- Kino M, Miayzaki T, Iwami T, Kohbara J. Retinal topography of ganglion cells in immature ocean sunfish, Mola mola. Environ Biol Fish. 2009;85:33–38. [Google Scholar]
- Klumpp DJ, Song EJ, Ito S, Sheng MH, Jan LY, Pinto LH. The Shaker-like potassium channels of the mouse rod bipolar cell and their contributions to the membrane current. J Neurosci. 1995;15:5004–5013. doi: 10.1523/JNEUROSCI.15-07-05004.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H, Cline C, Wang HH, Brecha N. Distribution and morphology of dopaminergic amacrine cells in the retina of the turtle (Pseudemys scripta elegans) J Neurocytol. 1987;16:577–588. doi: 10.1007/BF01637651. [DOI] [PubMed] [Google Scholar]
- Koopal SA, Coma MI, Tiebosch ATMG, Suurmeijer AJH. Low-temperature heating overnight in tris-HCl buffer pH 9 is a good alternative for antigen retrieval in formalin-fixed paraffin-embedded tissue. Appl Immunohistochem. 1998;6:228–233. [Google Scholar]
- Koulen P, Fletcher EL, Craven SE, Bredt DS, Wässle H. Immunocytochemical localization of the postsynaptic density protein PSD-95 in the mammalian retina. J Neurosci. 1998;18:10136–10149. doi: 10.1523/JNEUROSCI.18-23-10136.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouyama N, Marshak DW. Bipolar cells specific for blue cones in the macaque retina. J Neurosci. 1992;12:1233–1252. doi: 10.1523/JNEUROSCI.12-04-01233.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss R. New technique to demonstrate the network of blood capillaries of the human retina in their three-dimensional arrangement. Albrecht von Graefes Archiv f klin exp Ophthalmol. 1990;228:187–190. doi: 10.1007/BF00935731. [DOI] [PubMed] [Google Scholar]
- Krizaj D, Copenhagen DR. Compartmentalization of calcium extrusion mechanisms in the outer and inner segments of photoreceptors. Neuron. 1998;21:249–256. doi: 10.1016/s0896-6273(00)80531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuby J. In: Immunology. Freeman WH, editor. New York, NY: 1994. [Google Scholar]
- Kwong JM, Lam TT, Caprioli J. Hyperthermic pre-conditioning protects retinal neurons from N-methyl-D-aspartate (NMDA)-induced apoptosis in rat. Brain Res. 2003;970:119–130. doi: 10.1016/s0006-8993(03)02298-4. [DOI] [PubMed] [Google Scholar]
- Lamberts R, Goldsmith PC. Fixation, fine structure, and immunostaining for neuropeptides: perfusion versus immersion of the neuroendocrine hypothalamus. J Histochem Cytochem. 1986;34:389–398. doi: 10.1177/34.3.2419392. [DOI] [PubMed] [Google Scholar]
- Latendresse JR, Warbrittion AR, Jonassen H, Creasy DM. Fixation of testes and eyes using a modified Davidson's fluid: Comparison with Bouin's fluid and conventional Davidson's fluid. Toxicol Pathol. 2002;30:524–533. doi: 10.1080/01926230290105721. [DOI] [PubMed] [Google Scholar]
- Laties A. Photoreceptor morphology in freeze-dried retina. Cryobiol. 1966;2:314. [Google Scholar]
- Lee H, Brott BK, Kirkby LA, Adelson JD, Cheng S, Feller MB, Datwani A, Shatz CJ. Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature. 2014;509:195–200. doi: 10.1038/nature13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer SS. The crosslinking of actin and of tropomyosin by glutaraldehyde. Biochem Biophys Res Comm. 1972;48:967–976. doi: 10.1016/0006-291x(72)90703-6. [DOI] [PubMed] [Google Scholar]
- Li HB, Marshak DW, Dowling JE, Lam DM. Colocalization of immunoreactive substance P and neurotensin in amacrine cells of the goldfish retina. Brain Res. 1986;366:307–313. doi: 10.1016/0006-8993(86)91308-9. [DOI] [PubMed] [Google Scholar]
- Li W, DeVries SH. Separate blue and green cone networks in the mammalian retina. Nat Neurosci. 2004;7:751–756. doi: 10.1038/nn1275. [DOI] [PubMed] [Google Scholar]
- Liang H, Crewther SG, Crewther DP, Junghans BM. Structural and elemental evidence for edema in the retina, retinal pigment epithelium, and choroid during recovery from experimentally induced myopia. Invest Ophthalmol Vis Sci. 2004;45:2463–2474. doi: 10.1167/iovs.03-1009. [DOI] [PubMed] [Google Scholar]
- Light AC, Zhu Y, Shi J, Saszik S, Lindstrom S, Davidson L, Li X, Chiodo VA, Hauswirth WW, Li W, DeVries SH. Organizational motifs for ground squirrel cone bipolar cells. J Comp Neurol. 2012;520:2864–2887. doi: 10.1002/cne.23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Masland RH. Synaptic contacts between an identified type of ON cone bipolar cell and ganglion cells in the mouse retina. Eur J Neurosci. 2005;21:1257–1270. doi: 10.1111/j.1460-9568.2005.03967.x. [DOI] [PubMed] [Google Scholar]
- Lin B, Masland RH. Populations of wide-field amacrine cells in the mouse retina. J Comp Neurol. 2006;499:797–809. doi: 10.1002/cne.21126. [DOI] [PubMed] [Google Scholar]
- Lindstrom SH, Nacsa N, Blankenship T, Fitzgerald PG, Weller C, Vaney DI, Wilson M. Distribution and structure of efferent synapses in the chicken retina. Vis Neurosci. 2009;26:215–226. doi: 10.1017/S0952523809090063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kao WW. A novel protocol of whole mount electro-immunofluorescence staining. Mol Vis. 2009;15:505–517. [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Specificity of immunoreactions: the importance of testing specificity in each method. J Neurosci. 2008;28:9083–9086. doi: 10.1523/JNEUROSCI.2494-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedtke RR, Griffin SA, Conroy SS, Jin X, Pinto A, Sesack SR. Immunoblot and immunohistochemical comparison of murine monoclonal antibodies specific for the rat D1a and D1b dopamine receptor subtypes. J Neuroimmunol. 1999;101:170–187. doi: 10.1016/s0165-5728(99)00142-3. [DOI] [PubMed] [Google Scholar]
- Luftig RB, McMillan PN. The importance of adequate fixation in preservation of membrane ultrastructure. Internatl Rev Cytol. Suppl. 1981;12:309–325. doi: 10.1016/b978-0-12-364373-5.50017-2. [DOI] [PubMed] [Google Scholar]
- Macri J, Martin PR, Grunert U. Distribution of the alpha1 subunit of the GABA(A) receptor on midget and parasol ganglion cells in the retina of the common marmoset Callithrix jacchus. Vis Neurosci. 2000;17:437–448. doi: 10.1017/s0952523800173109. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Wässle H, Jusuf PR, Haverkamp S. Mirror-symmetrical populations of wide-field amacrine cells of the macaque monkey retina. J Comp Neurol. 2008;508:13–27. doi: 10.1002/cne.21666. [DOI] [PubMed] [Google Scholar]
- Man YG, Tavassoli FA. A simple epitope retrieval method without the use of microwave oven or enzyme digestion. Appl Immunohistochem. 1996;4:139–141. [Google Scholar]
- Mani SK, Fienberg AA, O'Callaghan JP, Snyder GL, Allen PB, Dash PK, Moore AN, Mitchell AJ, Bibb J, Greengard P, O'Malley BW. Requirement for DARPP-32 in progesterone-facilitated sexual receptivity in female rats and mice. Science. 2000;287:1053–1056. doi: 10.1126/science.287.5455.1053. [DOI] [PubMed] [Google Scholar]
- Manoonkitiwongsa PS, Schultz RL. Proper nomenclature of formaldehyde and paraformaldehyde fixatives for histochemistry. Histochem J. 2002;34:365–367. [PubMed] [Google Scholar]
- Marc RE. Mapping glutamatergic drive in the vertebrate retina with a channel-permeant organic cation. J Comp Neurol. 1999;407:47–64. doi: 10.1002/(sici)1096-9861(19990428)407:1<47::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Marc RE. Functional neuroanatomy of the retina, Albert and Jakobiec’s principles and practice of ophthalmology. 3rd ed. New York: Elsevier; 2008. pp. 1565–1592. [Google Scholar]
- Marc RE. Web Page: Marc Lab Protocols; University of Utah. [Accessed January 3, 2015];2014 http://prometheus.med.utah.edu/~marclab/marclab_09_tools-protocols.html. [Google Scholar]
- Marc RE, Jones BW. Molecular phenotyping of retinal ganglion cells. J Neurosci. 2002;22:413–427. doi: 10.1523/JNEUROSCI.22-02-00413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Anderson JR, Sigulinsky C, Lauritzen S. Retinal connectomics: towards complete, accurate networks. Prog Retin Eye Res. 2013;37:141–162. doi: 10.1016/j.preteyeres.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Liu WL. (3H) glycine-accumulating neurons of the human retina. J Comp Neurol. 1985;232:241–260. doi: 10.1002/cne.902320209. [DOI] [PubMed] [Google Scholar]
- Margo CE, Lee A. Fixation of whole eyes: the role of fixative osmolarity in the production of tissue artifact. Albrecht von Graefes Archiv fur klin exp Ophthalmol. 1995;233:366–370. doi: 10.1007/BF00200486. [DOI] [PubMed] [Google Scholar]
- Marx V. Finding the right antibody for the job. Nat Methods. 2013;10:703–707. doi: 10.1038/nmeth.2570. [DOI] [PubMed] [Google Scholar]
- Masland RH. Neuronal diversity in the retina. Curr Op Neurobiol. 2001;11:431–436. doi: 10.1016/s0959-4388(00)00230-0. [DOI] [PubMed] [Google Scholar]
- Masland RH, Mills JW. Autoradiographic identification of acetylcholine in the rabbit retina. J Cell Biol. 1979;83:159–178. doi: 10.1083/jcb.83.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SC, Mills SL. A calbindin-immunoreactive cone bipolar cell type in the rabbit retina. J Comp Neurol. 1996;366:15–33. doi: 10.1002/(SICI)1096-9861(19960226)366:1<15::AID-CNE2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Massey SC, Mills SL. Antibody to calretinin stains AII amacrine cells in the rabbit retina: double-label and confocal analyses. J Comp Neurol. 1999;411:3–18. [PubMed] [Google Scholar]
- McKay JS, Steele SJ, Ahmed G, Johnson E, Ratcliffe K. An antibody panel for immunohistochemical analysis of the retina in Davidson's-fixed, paraffin-embedded eyes of rats. Exp Tox Pathol. 2009;61:91–100. doi: 10.1016/j.etp.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Meissner DH, Schwarz H. Improved cryoprotection and freeze-substitution of embryonic quail retina: a TEM study on ultrastructural preservation. J Electr Micr Tech. 1990;14:348–356. doi: 10.1002/jemt.1060140410. [DOI] [PubMed] [Google Scholar]
- Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC. Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotech. 2004;37:790–796. 798–802. doi: 10.2144/04375RV01. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Distribution and coverage of A- and B-type horizontal cells stained with Neurobiotin in the rabbit retina. Vis Neurosci. 1994;11:549–560. doi: 10.1017/s0952523800002455. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Stratification of the inner plexiform layer in the mammalian retina. In: Werner J, Chalupa L, editors. The New Visual Neurosciences. MIT Press; 2014. [Google Scholar]
- Mills SL, O'Brien JJ, Li W, O'Brien J, Massey SC. Rod pathways in the mammalian retina use connexin 36. J Comp Neurol. 2001;436:336–350. [PMC free article] [PubMed] [Google Scholar]
- Mobius W, Cooper B, Kaufmann WA, Imig C, Ruhwedel T, Snaidero N, Saab AS, Varoqueaux F. Electron microscopy of the mouse central nervous system. Meth Cell Biol. 2010;96:475–512. doi: 10.1016/S0091-679X(10)96020-2. [DOI] [PubMed] [Google Scholar]
- Molday RS, Molday LL, Dose A, Clark-Lewis I, Illing M, Cook NJ, Eismann E, Kaupp UB. The cGMP-gated channel of the rod photoreceptor cell characterization and orientation of the amino terminus. J Biol Chem. 1991;266:21917–21922. [PubMed] [Google Scholar]
- Mollenhauer HH, Totten C. Studies on seeds. I. Fixation of seeds. J Cell Biol. 1971;48:387–394. doi: 10.1083/jcb.48.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsan P, Puzo G, Mazarguil H. Mechanism of glutaraldehyde-protein bond formation. Biochimie. 1975;57:1281–1292. [PubMed] [Google Scholar]
- Moran J, Hurtado S, Pasantes-Morales H. Similar properties of taurine release induced by potassium and hyposmolarity in the rat retina. Exp Eye Res. 1991;53:347–352. doi: 10.1016/0014-4835(91)90240-f. [DOI] [PubMed] [Google Scholar]
- Morgan JL, Dhingra A, Vardi N, Wong ROL. Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat Neurosci. 2006;9:85–92. doi: 10.1038/nn1615. [DOI] [PubMed] [Google Scholar]
- Morgans CW. Localization of the alpha(1F) calcium channel subunit in the rat retina. Invest Ophthalmol Vis Sci. 2001;42:2414–2418. [PubMed] [Google Scholar]
- Morgans CW, El Far O, Berntson A, Wässle H, Taylor WR. Calcium extrusion from mammalian photoreceptor terminals. J Neurosci. 1998;18:2467–2474. doi: 10.1523/JNEUROSCI.18-07-02467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosiman VL, Patterson BK, Canterero L, Goolsby CL. Reducing cellular autofluorescence in flow cytometry: an in situ method. Cytometry. 1997;30:151–156. [PubMed] [Google Scholar]
- Müller F, Scholten A, Ivanova E, Haverkamp S, Kremmer E, Kaupp UB. HCN channels are expressed differentially in retinal bipolar cells and concentrated at synaptic terminals. Eur J Neurosci. 2003;17:2084–2096. doi: 10.1046/j.1460-9568.2003.02634.x. [DOI] [PubMed] [Google Scholar]
- Nadal-Nicolás FM, Jiménez-López M, Sobrado-Calvo P, Nieto-López L, Cánovas-Martínez I, Salinas-Navarro M, Vidal-Sanz M, Agudo M. Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest Ophthalmol Vis Sci. 2009;50:3860–3868. doi: 10.1167/iovs.08-3267. [DOI] [PubMed] [Google Scholar]
- Nagle BW, Okamoto C, Taggart B, Burnside B. The teleost cone cytoskeleton. Localization of actin, microtubules, and intermediate filaments. Invest Ophthalmol Vis Sci. 1986;27:689–701. [PubMed] [Google Scholar]
- Nakagawa T, Izawa K, Yagi S, Shibukawa A, Tanaka H, Tashima T, Imai M. Development of Effective Cross-Linking Method for Bioactive Substance - Enzyme Immobilization Using Glutaraldehyde Oligomers. Chem Pharm Bull. 1989;37:2463–2466. doi: 10.1248/cpb.37.2463. [DOI] [PubMed] [Google Scholar]
- Nakane PK, Pierce GB., Jr Enzyme-labeled antibodies: preparation and application for the localization of antigens. J Histochem Cytochem. 1966;14:929–931. doi: 10.1177/14.12.929. [DOI] [PubMed] [Google Scholar]
- Negishi K, Hayashi T, Nakamura T, Drujan BD. Histochemical studies on catecholaminergic cells in the carp retina. Neurochem Res. 1979;4:473–482. doi: 10.1007/BF00964642. [DOI] [PubMed] [Google Scholar]
- Nelson R, Famiglietti EV, Jr, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978;41:472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- Neumann M, Gabel D. Simple method for reduction of autofluorescence in fluorescence microscopy. J Histochem Cytochem. 2002;50:437–439. doi: 10.1177/002215540205000315. [DOI] [PubMed] [Google Scholar]
- NIOSH. Web Page: Picric acid, NIOSH Pocket Guide to Chemical Hazards; Centers for Disease Control and Prevention. [Accessed January 5, 2015];2011 http://www.cdc.gov/niosh/npg/npgd0515.html.
- NIOSH. Web Page: Picric acid, Immediately Dangerous to Life of Health Concentrations (IDLH); Centers for Disease Control and Prevention. [Accessed January 5, 2015];2014 http://www.cdc.gov/niosh/idlh/88891.html.
- Nir I, Hall MO. The ultrastructure of lipid-depleted rod photoreceptor membranes. J Cell Biol. 1974;63:587–598. doi: 10.1083/jcb.63.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff AB, Novikoff PM, Davis C, Quintana N. Diffusion artifacts in 3,3'- diaminobenzidine cytochemistry. J Histochem Cytochem. 1972;20:745-&. doi: 10.1177/20.9.745. [DOI] [PubMed] [Google Scholar]
- O'Brien BJ, Isayama T, Richardson R, Berson DM. Intrinsic physiological properties of cat retinal ganglion cells. J Physiol. 2002;538:787–802. doi: 10.1113/jphysiol.2001.013009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JJ, Chen X, Macleish PR, O'Brien J, Massey SC. Photoreceptor coupling mediated by connexin36 in the primate retina. J Neurosci. 2012;32:4675–4687. doi: 10.1523/JNEUROSCI.4749-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JJ, Li W, Pan F, Keung J, O'Brien J, Massey SC. Coupling between A-type horizontal cells is mediated by connexin 50 gap junctions in the rabbit retina. J Neurosci. 2006;26:11624–11636. doi: 10.1523/JNEUROSCI.2296-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata G, Stradleigh TW, Partida GJ, Ishida AT. Dopamine and full-field illumination activate D1 and D2-D5-type receptors in adult rat retinal ganglion cells. J Comp Neurol. 2012;520:4032–4049. doi: 10.1002/cne.23159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HT, Lowry OH, Cohen AI, Ferrendelli JA. Distribution of 3':5'-cyclic AMP and 3':5'-cyclic GMP in rabbit retina in vivo: selective effects of dark and light adaptation and ischemia. Proc Natl Acad Sci U S A. 1976;73:4442–4445. doi: 10.1073/pnas.73.12.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortez RA. Residual cyclic nucleotide associated with tissues after exposure to aqueous buffer analogous to that used in immunocytochemistry. J Cyclic Nucl Res. 1980;6:93–104. [PubMed] [Google Scholar]
- Ortez RA, Sikes RW, Sperling HG. Immunohistochemical localization of cyclic GMP in goldfish retina. J Histochem Cytochem. 1980;28:263–270. doi: 10.1177/28.3.6243683. [DOI] [PubMed] [Google Scholar]
- Ottesen M, Svensson B. Modification of papain by treatment with glutaraldehyde under reducing and non-reducing conditions. Comptes-rendus des travaux du Laboratoire Carlsberg. 1971;38:171–185. [PubMed] [Google Scholar]
- Partida GJ, Lee SC, Haft-Candell L, Nichols GS, Ishida AT. DARPP-32-like immunoreactivity in AII amacrine cells of rat retina. J Comp Neurol. 2004;480:251–263. doi: 10.1002/cne.20330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partida GJ, Stradleigh TW, Ogata G, Godzdanker I, Ishida AT. Thy1 associates with the cation channel subunit HCN4 in adult rat retina. Invest Ophth Vis Sci. 2012;53:1696–1703. doi: 10.1167/iovs.11-9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L. Alpha and delta ganglion cells in the rat retina. J Comp Neurol. 1989;286:120–139. doi: 10.1002/cne.902860108. [DOI] [PubMed] [Google Scholar]
- Petrides A, Trexler EB. Differential output of the high-sensitivity rod photoreceptor: AII amacrine pathway. J Comp Neurol. 2008;507:1653–1662. doi: 10.1002/cne.21617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer BA, Bernstein SA, Bartels SP. Preservation of structure and immunoreactivity at the vitreoretinal interface of the rabbit eye. Albrecht v Graefes Archiv fur klin exp Ophthalmol. 2009;247:193–205. doi: 10.1007/s00417-008-0991-4. [DOI] [PubMed] [Google Scholar]
- Philp NJ, Chang W, Long K. Light-stimulated protein movement in rod photoreceptor cells of the rat retina. FEBS letters. 1987;225:127–132. doi: 10.1016/0014-5793(87)81144-4. [DOI] [PubMed] [Google Scholar]
- Piccolino M, Neyton J, Gerschenfeld HM. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984;4:2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pileri SA, Roncador G, Ceccarelli C, Piccioli M, Briskomatis A, Sabattini E, Ascani S, Santini D, Piccaluga PP, Leone O, Damiani S, Ercolessi C, Sandri F, Pieri F, Leoncini L, Falini B. Antigen retrieval techniques in immunohistochemistry: comparison of different methods. J Pathol. 1997;183:116–123. doi: 10.1002/(SICI)1096-9896(199709)183:1<116::AID-PATH1087>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Pinheiro P, Lockner P. Standardized heat fixation for blood smears. Stain Technol. 1963;38:227–229. doi: 10.3109/10520296309061183. [DOI] [PubMed] [Google Scholar]
- Polyak S. The Retina. University of Chicago Press; 1941. [Google Scholar]
- Pow DV. Immunocytochemical detection of amino acid neurotransmitters in paraformaldehyde-fixed tissues. Meth Molec Biol. 1997;72:103–123. doi: 10.1385/0-89603-394-5:103. [DOI] [PubMed] [Google Scholar]
- Pow DV, Wright LL, Vaney DI. The immunocytochemical detection of amino-acid neurotransmitters in paraformaldehyde-fixed tissues. J Neurosci Meth. 1995;56:115–123. doi: 10.1016/0165-0270(94)00113-u. [DOI] [PubMed] [Google Scholar]
- Prochniewicz-Nakayama E, Yanagida T. The effect of crosslinking of thin filament with glutaraldehyde on the contractility of muscle fiber. J Biochem. 1982;92:1269–1277. doi: 10.1093/oxfordjournals.jbchem.a134045. [DOI] [PubMed] [Google Scholar]
- Quiocho FA, Richards FM. Enzymic behavior of carboxypeptidase-A in solid state. Biochem. 1966;5:4062–4076. [Google Scholar]
- Ramón y Cajal S. La rétine des vertébrés. La Cellule. 1893;9:119–257. [Google Scholar]
- Rasband MN, Trimmer JS, Peles E, Levinson SR, Shrager P. K+ channel distribution and clustering in developing and hypomyelinated axons of the optic nerve. J Neurocytol. 1999;28:319–331. doi: 10.1023/a:1007057512576. [DOI] [PubMed] [Google Scholar]
- Rasmussen KE. Fixation in aldehydes. A study on the influence of the fixative, buffer, and osmolarity upon the fixation of the rat retina. J Ultrastr Res. 1974;46:87–102. doi: 10.1016/s0022-5320(74)80024-9. [DOI] [PubMed] [Google Scholar]
- Reale E, Luciano L. Fixation with aldehydes. Their usefulness for histological and histochemical studies in light and electron microscopy. Histochemie. Histochemistry. Histochimie. 1970;23:144–170. doi: 10.1007/BF00305848. [DOI] [PubMed] [Google Scholar]
- Reyes R, Lauritzen I, Lesage F, Ettaiche M, Fosset M, Lazdunski M. Immunolocalization of the arachidonic acid and mechanosensitive baseline traak potassium channel in the nervous system. Neurosci. 2000;95:893–901. doi: 10.1016/s0306-4522(99)00484-4. [DOI] [PubMed] [Google Scholar]
- Rhodes KJ, Trimmer JS. Antibodies as valuable neuroscience research tools versus reagents of mass distraction. J Neurosci. 2006;26:8017–8020. doi: 10.1523/JNEUROSCI.2728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards FM, Knowles JR. Glutaraldehyde as a protein cross-linkage reagent. J Molec Biol. 1968;37:231–233. doi: 10.1016/0022-2836(68)90086-7. [DOI] [PubMed] [Google Scholar]
- Riepe RE, Norenburg MD. Muller cell localization of glutamine-synthetase in rat retina. Nature. 1977;268:654–655. doi: 10.1038/268654a0. [DOI] [PubMed] [Google Scholar]
- Riggs JL, Seiwald RJ, Burckhalter JH, Downs CM, Metcalf TG. Isothiocyanate compounds as fluorescent labeling agents for immune serum. Am J Pathol. 1958;34:1081–1097. [PMC free article] [PubMed] [Google Scholar]
- Robertson EA, Schultz RL. The impurities in commercial glutaraldehyde and their effect on the fixation of brain. J Ultrastr Res. 1970;30:275–287. doi: 10.1016/s0022-5320(70)80063-6. [DOI] [PubMed] [Google Scholar]
- Rockhill RL, Daly FJ, MacNeil MA, Brown SP, Masland RH. The diversity of ganglion cells in a mammalian retina. J Neurosci. 2002;22:3831–3843. doi: 10.1523/JNEUROSCI.22-09-03831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger J, Symonds AC, Springbett J, Shen WY, Bartlett CA, Rakoczy PE, Beazley LD, Dunlop SA. Eph/ephrin expression in the adult rat visual system following localized retinal lesions: localized and transneuronal up-regulation in the retina and superior colliculus. Eur J Neurosci. 2005;22:1840–1852. doi: 10.1111/j.1460-9568.2005.04381.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez AR, de Sevilla Müller LP, Brecha NC. The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J Comp Neurol. 2014;522:1411–1143. doi: 10.1002/cne.23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhlich P, van Veen T, Szél A. Two different visual pigments in one retinal cone cell. Neuron. 1994;13:1159–1166. doi: 10.1016/0896-6273(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Rohrenbeck J, Wässle H, Boycott BB. Horizontal cells in the monkey retina: Immunocytochemical staining with antibodies against calcium binding proteins. Eur J Neurosci. 1989;1:407–420. doi: 10.1111/j.1460-9568.1989.tb00349.x. [DOI] [PubMed] [Google Scholar]
- Rosillo JC, Olivera-Bravo S, Casanova G, Garcia-Verdugo JM, Fernandez AS. Olfacto-retinalis pathway in Austrolebias charrua fishes: a neuronal tracer study. Neurosci. 2013;253:304–315. doi: 10.1016/j.neuroscience.2013.08.047. [DOI] [PubMed] [Google Scholar]
- Rost FW, Ewen SW. New methods for the histochemical demonstration of catecholamines, tryptamines, histamine and other arylethylamines by acid- and aldehyde-induced fluorescence. Histochem J. 1971;3:207–212. doi: 10.1007/BF01002565. [DOI] [PubMed] [Google Scholar]
- Ruppelt A, Mosenden R, Gronholm M, Aandahl EM, Tobin D, Carlson CR, Abrahamsen H, Herberg FW, Carpen O, Tasken K. Inhibition of T cell activation by cyclic adenosine 5'-monophosphate requires lipid raft targeting of protein kinase A type I by the A-kinase anchoring protein ezrin. J Immunol. 2007;179:5159–5168. doi: 10.4049/jimmunol.179.8.5159. [DOI] [PubMed] [Google Scholar]
- Ryskamp DA, Witkovsky P, Barabas P, Huang W, Koehler C, Akimov NP, Lee SH, Chauhan S, Xing W, Renteria RC, Liedtke W, Krizaj D. The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J Neurosci. 2011;31:7089–7101. doi: 10.1523/JNEUROSCI.0359-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DD, Bensch K, Barrnett RJ. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz-de-Viteri M, Heras-Mulero H, Fernandez-Robredo P, Recalde S, Hernandez M, Reiter N, Moreno-Orduna M, Garcia-Layana A. Oxidative stress and histological changes in a model of retinal phototoxicity in rabbits. Oxidative medicine and cellular longevity. 2014;2014:637137. doi: 10.1155/2014/637137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez A, Andreu FJ, Segui MA, Bare ML, Fernandez S, Dinares C, Rey M. HER-2 gene amplification by chromogenic in situ hybridisation (CISH) compared with fluorescence in situ hybridisation (FISH) in breast cancer-A study of two hundred cases. Breast. 2006;15:519–527. doi: 10.1016/j.breast.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Saper CB. A guide to the perplexed on the specificity of antibodies. J Histochem Cytochem. 2009;57:1–5. doi: 10.1369/jhc.2008.952770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol. 2003;465:161–163. doi: 10.1002/cne.10858. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Kirsch J, Grunert U, Greferath U, Fritschy JM, Mohler H, Betz H, Wässle H. Colocalization of gephyrin and GABAA-receptor subunits in the rat retina. J Comp Neurol. 1995;357:1–14. doi: 10.1002/cne.903570102. [DOI] [PubMed] [Google Scholar]
- Schmidt MJ, Schmidt DE, Robison GA. Cyclic adenosine monophosphate in brain areas: microwave irradiation as a means of tissue fixation. Science. 1971;173:1142–1143. doi: 10.1126/science.173.4002.1142. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. Distribution and immunoreactivity of glia in the retina of the rabbit. J Comp Neurol. 1985;240:128–142. doi: 10.1002/cne.902400203. [DOI] [PubMed] [Google Scholar]
- Seydewitz V, Rothermel A, Fuhrmann S, Schneider A, DeGrip WJ, Layer PG, Hofmann HD. Expression of CNTF receptor-alpha in chick violet-sensitive cones with unique morphologic properties. Invest Ophthalmol Vis Sci. 2004;45:655–661. doi: 10.1167/iovs.03-0182. [DOI] [PubMed] [Google Scholar]
- Shehab SA, Cronly-Dillon JR, Nona SN, Stafford CA. Preferential histochemical staining of protoplasmic and fibrous astrocytes in rat CNS with GFAP antibodies using different fixatives. Brain Res. 1990;518:347–352. doi: 10.1016/0006-8993(90)90996-o. [DOI] [PubMed] [Google Scholar]
- Shi SR, Chaiwun B, Young L, Imam A, Cote RJ, Taylor CR. Antigen retrieval using pH 3.5 glycine-HCl buffer or urea solution for immunohistochemical localization of Ki-67. Biotechnic Histochem. 1994;69:213–215. doi: 10.3109/10520299409106289. [DOI] [PubMed] [Google Scholar]
- Shi SR, Cote RJ, Hawes D, Thu S, Shi Y, Young LL, Taylor CR. Calcium-induced modification of protein conformation demonstrated by immunohistochemistry: What is the signal? J Histochem Cytochem. 1999;47:463–470. doi: 10.1177/002215549904700404. [DOI] [PubMed] [Google Scholar]
- Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Siegert S, Scherf BG, Del Punta K, Didkovsky N, Heintz N, Roska B. Genetic address book for retinal cell types. Nat Neurosci. 2009;12:1197–1204. doi: 10.1038/nn.2370. [DOI] [PubMed] [Google Scholar]
- Smith MT, Redick JA, Baron J. Quantitative immunohistochemistry: a comparison of microdensitometric analysis of unlabeled antibody peroxidase-antiperoxidase staining and of microfluorometric analysis of indirect fluorescent antibody staining for nicotinamide adenosine dinucleotide phosphate (NADPH)-cytochrome c (P-450) reductase in rat liver. J Histochem Cytochem. 1983;31:1183–1189. doi: 10.1177/31.10.6411804. [DOI] [PubMed] [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- Sompuram SR, Vani K, Hafer LJ, Bogen SA. Antibodies immunoreactive with formalin-fixed tissue antigens recognize linear protein epitopes. Am J Clin Pathol. 2006;125:82–90. [PubMed] [Google Scholar]
- Sompuram SR, Vani K, Messana E, Bogen SA. A molecular mechanism of formalin fixation and antigen retrieval. Am J Clin Pathol. 2004;121:190–199. doi: 10.1309/BRN7-CTX1-E84N-WWPL. [DOI] [PubMed] [Google Scholar]
- Stahl WL, Baskin DG. Immunocytochemical localization of Na+,K+ adenosinetriphosphatase in the rat retina. J Histochem Cytochem. 1984;32:248–250. doi: 10.1177/32.2.6319483. [DOI] [PubMed] [Google Scholar]
- Stefanini M, De Martino C, Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967;216:173–174. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Reid M, Lacy PL. The distribution of rods and cones in the retina of the cat (Felis domesticus) J Comp Neurol. 1973;148:229–248. doi: 10.1002/cne.901480209. [DOI] [PubMed] [Google Scholar]
- Stell WK. The morphological organization of the vertebrate retina. In: Fuortes M, editor. Handbook of Sensory Physiology. Berlin: Springer; 1972. p. 111. [Google Scholar]
- Stell WK, Lightfoot DO. Color-specific interconnections of cones and horizontal cells in the retina of the goldfish. J Comp Neurol. 1975;159:473–502. doi: 10.1002/cne.901590404. [DOI] [PubMed] [Google Scholar]
- Sternberger LA. The unlabeled antibody method. Hormone receptor, Golgi-like and dual color immunocytochemistry. J Histochem Cytochem. 1979;27:1658–1659. doi: 10.1177/27.12.230258. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Villasmil ML, Frampton MW. Changes in fluorescence intensity of selected leukocyte surface markers following fixation. Cytom Part A. 2007;71A:379–385. doi: 10.1002/cyto.a.20392. [DOI] [PubMed] [Google Scholar]
- Stradleigh TW, Greenberg KP, Partida GJ, Pham A, Ishida AT. Moniliform deformation of retinal ganglion cells by formaldehyde-based fixatives. J Comp Neurol. 2015;523:545–564. doi: 10.1002/cne.23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stradleigh TW, Ogata G, Partida GJ, Oi H, Greenberg KP, Krempely KS, Ishida AT. Colocalization of hHyperpolarization-activated, cyclic nucleotide-gated channel subunits in rat retinal ganglion cells. J Comp Neurol. 2011;519:2546–2573. doi: 10.1002/cne.22638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SJ, Daukas G, Zigmond SH. Asymmetric distribution of the chemotactic peptide receptor on polymorphonuclear leukocytes. J Cell Biol. 1984;99:1461–1467. doi: 10.1083/jcb.99.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland BW, Toews J, Kast J. Utility of formaldehyde cross-linking and mass spectrometry in the study of protein-protein interactions. J Mass Spectr. 2008;43:699–715. doi: 10.1002/jms.1415. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Oishi Y, Oyaizu T, Tsubura A, Morii S. Proliferating cell nuclear antigen (Pcna) immunohistochemistry - Influence of tissue fixation, processing and effects of antigen retrieval. Micron. 1993;24:385–388. [Google Scholar]
- Tanito M, Nishiyama A, Tanaka T, Masutani H, Nakamura H, Yodoi J, Ohira A. Change of redox status and modulation by thiol replenishment in retinal photooxidative damage. Invest Ophthalmol Vis Sci. 2002;43:2392–2400. [PubMed] [Google Scholar]
- Tauchi M, Masland RH. The shape and arrangement of the cholinergic neurons in the rabbit retina. Proc R Soc Lond 223B. 1984:101–119. doi: 10.1098/rspb.1984.0085. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Morgans C. Localization and properties of voltage-gated calcium channels in cone photoreceptors of Tupaia belangeri. Vis neurosci. 1998;15:541–552. doi: 10.1017/s0952523898153142. [DOI] [PubMed] [Google Scholar]
- Terada N, Ohno N, Ohguro H, Li Z, Ohno S. Immunohistochemical detection of phosphorylated rhodopsin in light-exposed retina of living mouse with in vivo cryotechnique. J Histochem Cytochem. 2006;54:479–486. doi: 10.1369/jhc.5A6844.2006. [DOI] [PubMed] [Google Scholar]
- Terada N, Ohno N, Saitoh S, Saitoh Y, Ohno S. Immunoreactivity of glutamate in mouse retina inner segment of photoreceptors with in vivo cryotechnique. J Histochem Cytochem. 2009;57:883–888. doi: 10.1369/jhc.2009.953851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Chauhan BC, LeBlanc RP, Wax MB. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest Ophthalmol Vis Sci. 2003;44:3025–3033. doi: 10.1167/iovs.02-1136. [DOI] [PubMed] [Google Scholar]
- Tezel G, Wax MB. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol. 2004;122:1348–1356. doi: 10.1001/archopht.122.9.1348. [DOI] [PubMed] [Google Scholar]
- Thermo Web Page: Antibody Production (Immunogen Preparation); Pierce Protein Biology Products. [Accessed September 9, 2014];2015 http://www.piercenet.com/method/antibody-production-immunogen-preparation. [Google Scholar]
- Thiessen G, Thiessen H, Dowidat HJ, Luciano L, Reale E. The diffusin of 59Felabelled hemoglobin, an artefact of the fixation with glutaraldehyde. Histochemie. Histochemistry. Histochimie. 1970;23:171–175. doi: 10.1007/BF00305849. [DOI] [PubMed] [Google Scholar]
- Tkatchenko AV. Whole-mount BrdU staining of proliferating cells by DNase treatment: application to postnatal mammalian retina. Biotech. 2006;40:29–30. 32. doi: 10.2144/000112094. [DOI] [PubMed] [Google Scholar]
- Tomimatsu Y, Jansen EF, Gaffield W, Olson AC. Physical chemical observations on alpha-chymotrypsin glutaraldehyde system during formation of an insoluble derivative. J Colloid Interf Sci. 1971;36:51-&. [Google Scholar]
- Trexler EB, Li W, Mills SL, Massey SC. Coupling from AII amacrine cells to ON cone bipolar cells is bidirectional. J Comp Neurol. 2001;437:408–422. doi: 10.1002/cne.1292. [DOI] [PubMed] [Google Scholar]
- Usukura J. Rapid freezing and subsequent preparation methods in retinal cell biology. In: Hargrave P, editor. Methods in Neurosciences. San Diego, CA: Academic Press; 1993. pp. 37–53. [Google Scholar]
- Van Hook MJ, Wong KY, Berson DM. Dopaminergic modulation of ganglion-cell photoreceptors in rat. Eur J Neurosci. 2012;35:507–518. doi: 10.1111/j.1460-9568.2011.07975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart A, Trimmer JS, Matthews G. Polarized distribution of ion channels within microdomains of the axon initial segment. J Comp Neurol. 2007;500:339–352. doi: 10.1002/cne.21173. [DOI] [PubMed] [Google Scholar]
- Vaney DI. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. Neurosci Lett. 1991;125:187–190. doi: 10.1016/0304-3940(91)90024-n. [DOI] [PubMed] [Google Scholar]
- Vanha-Perttula T, Grimley PM. Loss of proteins and other macromolecules during preparation of cell cultures for high resolution autoradiography. Quantitation by a micromethod. J Histochem Cytochem. 1970;18:565–573. doi: 10.1177/18.8.565. [DOI] [PubMed] [Google Scholar]
- Vani K, Bogen SA, Sompuram SR. A high throughput combinatorial library technique for identifying formalin-sensitive epitopes. J Immunol Methods. 2006;317:80–89. doi: 10.1016/j.jim.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero CF, Pignatelli A, Partida GJ, Ishida AT. A dopamine- and protein kinase Adependent mechanism for network adaptation in retinal ganglion cells. J Neurosci. 2001;21:8624–8635. doi: 10.1523/JNEUROSCI.21-21-08624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero CF, Velasco A, de la Villa P. Protein kinase C localization in the synaptic terminal of rod bipolar cells. Neuroreport. 1996;7:2176–2180. doi: 10.1097/00001756-199609020-00024. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Erickson PA, Fisher SK. Glial Fibrillary Acidic Protein (GFAP) Immunoreactivity in Rabbit Retina - Effect of Fixation. Exp Eye Res. 1990;50:385–392. doi: 10.1016/0014-4835(90)90139-l. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Lasater EM. Distribution of F-actin in bipolar and horizontal cells of bass retinas. Am J Physiol. 1990;259:C205–C214. doi: 10.1152/ajpcell.1990.259.2.C205. [DOI] [PubMed] [Google Scholar]
- Verhoeff FH. The Condition of the Ocular Structures Immediately after Removal of the Lens in Capsule, as Determined by Microscopic Examination. Trans Am Ophthalmol Soc. 1931;29:184–192. [PMC free article] [PubMed] [Google Scholar]
- Villalobos J, Nayagam DA, Allen PJ, McKelvie P, Luu CD, Ayton LN, Freemantle AL, McPhedran M, Basa M, McGowan CC, Shepherd RK, Williams CE. A wide-field suprachoroidal retinal prosthesis is stable and well tolerated following chronic implantation. Invest Ophthalmol Vis Sci. 2013;54:3751–3762. doi: 10.1167/iovs.12-10843. [DOI] [PubMed] [Google Scholar]
- Villegas GM. Ultrastructure of the Human Retina. J Anat. 1964;98:501–513. [PMC free article] [PubMed] [Google Scholar]
- Voigt T, Wässle H. Dopaminergic innervation of A II amacrine cells in mammalian retina. J Neurosci. 1987;7:4115–4128. doi: 10.1523/JNEUROSCI.07-12-04115.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi B, Abrams J, Paul DL, Bloomfield SA. Morphology and tracer coupling pattern of alpha ganglion cells in the mouse retina. J Comp Neurol. 2005;492:66–77. doi: 10.1002/cne.20700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi B, Chheda S, Bloomfield SA. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J Comp Neurol. 2009;512:664–687. doi: 10.1002/cne.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner HJ, Luo BG, Ariano MA, Sibley DR, Stell WK. Localization of D2 dopamine receptors in vertebrate retinae with anti-peptide antibodies. J Comp Neurol. 1993;331:469–481. doi: 10.1002/cne.903310404. [DOI] [PubMed] [Google Scholar]
- Walls GL. The Vertebrate Eye and Its Adaptive Radiation. New York: Hafner Publishing; 1963. [Google Scholar]
- Walt DR, Agayn VI. The Chemistry of Enzyme and Protein Immobilization with Glutaraldehyde. Trac-Trend Anal Chem. 1994;13:425–430. [Google Scholar]
- Wan CP, Park CS, Lau BH. A rapid and simple microfluorometric phagocytosis assay. J Immunol Methods. 1993;162:1–7. doi: 10.1016/0022-1759(93)90400-2. [DOI] [PubMed] [Google Scholar]
- Wang JH, Tu JI. Modification of glycogen phosphorylase b by glutaraldehyde. Preparation and isolation of enzyme derivatives with enhanced stability. Biochem. 1969;8:4403–4410. doi: 10.1021/bi00839a027. [DOI] [PubMed] [Google Scholar]
- Wässle H. Parallel processing in the mammalian retina. Nature Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Wässle H, Dacey DM, Haun T, Haverkamp S, Grunert U, Boycott BB. The mosaic of horizontal cells in the macaque monkey retina: with a comment on biplexiform ganglion cells. Vis Neurosci. 2000;17:591–608. doi: 10.1017/s0952523800174097. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grunert U, Rohrenbeck J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. J Comp Neurol. 1993;332:407–420. doi: 10.1002/cne.903320403. [DOI] [PubMed] [Google Scholar]
- Wässle H, Puller C, Müller F, Haverkamp S. Cone Contacts, Mosaics, and Territories of Bipolar Cells in the Mouse Retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster HD, Ames A, 3rd, Nesbett FB. A quantitative morphological study of osmotically induced swelling and shrinkage in nervous tissue. Tiss Cell. 1969;1:201–216. doi: 10.1016/s0040-8166(69)80022-4. [DOI] [PubMed] [Google Scholar]
- Wendt KD, Jensen CA, Tindall R, Katz ML. Comparison of conventional and microwave-assisted processing of mouse retinas for transmission electron microscopy. J Micr. 2004;214:80–88. doi: 10.1111/j.0022-2720.2004.01310.x. [DOI] [PubMed] [Google Scholar]
- Wenk P. Fixatives: Coagulative vs Non-Coagulative or Is It Additive vs Non-Additive? Histologic. 2006;39:4. [Google Scholar]
- Werner M, Chott A, Fabiano A, Battifora H. Effect of formalin tissue fixation and processing on immunohistochemistry. Am J Surg Pathol. 2000;24:1016–1019. doi: 10.1097/00000478-200007000-00014. [DOI] [PubMed] [Google Scholar]
- Wiemelt AP, Engleka MJ, Skorupa AF, McMorris FA. Immunochemical visualization and quantitation of cyclic AMP in single cells. J Biol Chem. 1997;272:31489–31495. doi: 10.1074/jbc.272.50.31489. [DOI] [PubMed] [Google Scholar]
- Wikler KC, Rakic P. Distribution of photoreceptor subtypes in the retina of diurnal and nocturnal primates. J Neurosci. 1990;10:3390–3401. doi: 10.1523/JNEUROSCI.10-10-03390.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, Svenningsson P, Yan L, Bateup H, Silver R. Cellular localization and function of DARPP-32 in the rodent retina. Eur J Neurosci. 2007;25:3233–3242. doi: 10.1111/j.1460-9568.2007.05571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, Veisenberger E, Haycock JW, Akopian A, Garcia-Espana A, Meller E. Activity-dependent phosphorylation of tyrosine hydroxylase in dopaminergic neurons of the rat retina. J Neurosci. 2004;24:4242–4249. doi: 10.1523/JNEUROSCI.5436-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollner DA, Catterall WA. Localization of sodium channels in axon hillocks and initial segments of retinal ganglion cells. Proc Natl Acad Sci U S A. 1986;83:8424–8428. doi: 10.1073/pnas.83.21.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Ivanova E, Zhang Y, Pan ZH. rAAV-mediated subcellular targeting of optogenetic tools in retinal ganglion cells in vivo. PLoS One. 2013;8:e66332. doi: 10.1371/journal.pone.0066332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Vasudeva V, Vardi N, Sterling P, Freed MA. Different types of ganglion cell share a synaptic pattern. J Comp Neurol. 2008;507:1871–1878. doi: 10.1002/cne.21644. [DOI] [PubMed] [Google Scholar]
- Yamada T, Marshak D, Basinger S, Walsh J, Morley J, Stell W. Somatostatin-like immunoreactivity in the retina. Proc Natl Acad Sci U S A. 1980;77:1691–1695. doi: 10.1073/pnas.77.3.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S, Okada Y. Mechanisms of heat-induced antigen retrieval: analyses in vitro employing SDS-PAGE and immunohistochemistry. J Histochem Cytochem. 2005;53:13–21. doi: 10.1177/002215540505300103. [DOI] [PubMed] [Google Scholar]
- Yanez J, Anadon R. Are the dopaminergic cells of the lamprey retina interplexiform cells? A dopamine, tyrosine hydroxylase and dopamine beta-hydroxylase immunocytochemical study. Neurosci Lett. 1994;165:63–66. doi: 10.1016/0304-3940(94)90710-2. [DOI] [PubMed] [Google Scholar]
- Yoshiki A, Sakakura T, Kusakabe M. The mouse chimera during intrauterine stages: immunohistochemical analysis with the C3H strain-specific antibody. J Histochem Cytochem. 1993;41:1583–1590. doi: 10.1177/41.10.8245417. [DOI] [PubMed] [Google Scholar]
- Young HM, Vaney DI. Rod-signal interneurons in the rabbit retina: 1. Rod bipolar cells. J Comp Neurol. 1991;310:139–153. doi: 10.1002/cne.903100202. [DOI] [PubMed] [Google Scholar]
- Zafra F, Aragón C, Olivares L, Danbolt NC, Giménez C, Storm-Mathisen J. Glycine transporters are differentially expressed among CNS cells. J Neurosci. 1995;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li W, Hoshi H, Mills SL, Massey SC. Stratification of alpha ganglion cells and ON/OFF directionally selective ganglion cells in the rabbit retina. Vis Neurosci. 2005;22:535–549. doi: 10.1017/S0952523805224148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XM, Li AM, Brown B, Weiss ER, Osawa S, Craft CM. Mouse cone arrestin expression pattern: Light induced translocation in cone photoreceptors. Mol Vis. 2002;8:462–471. [PubMed] [Google Scholar]
- Zucker CL. Localization of gephyrin and glycine receptor subunit immunoreactivity in the rabbit retina. Vis Neurosci. 1998;15:389–395. doi: 10.1017/s0952523898152173. [DOI] [PubMed] [Google Scholar]