Abstract
Objective
The present study explored the cross-sectional and predictive effect of drive for thinness and/or negative affect scores on the development of self-reported anorexia nervosa (AN) and bulimia nervosa (BN).
Method
K-means were used to cluster the Eating Disorder Inventory-Drive for Thinness (DT) and Child Behavior Checklist Anxious/Depressed (A/D) scores from 615 unrelated female twins at age 16–17. Logistic regressions were used to assess the effect of these clusters on self-reported eating disorder diagnosis at ages 16–17 (n=565) and 19–20 (n=451).
Results
DT and A/D scores were grouped into four clusters: Mild (scores lower than 90th percentile on both scales), DT (higher scores only on DT), A/D (higher scores only on A/D), and DT-A/D (higher scores on both the DT and A/D scales). DT and DT-A/D clusters at age 16–17 were associated cross-sectionally with AN and both cross-sectionally and longitudinally with BN. The DT-A/D cluster had the highest prevalence of AN at follow-up compared with all other clusters. Similarly, an interaction was observed between DT and A/D that predicted risk for AN.
Discussion
Having elevated DT and A/D scores may increase risk for eating disorder symptomatology above and beyond a high score on either alone. Findings suggest that cluster modeling based on DT and A/D may be useful to inform novel and useful intervention strategies for AN and BN in adolescents.
Keywords: Drive for thinness, anxiety, depression, negative affect, subtyping, eating disorders, anorexia, bulimia, risk
Eating disorders are psychiatric syndromes that typically onset in adolescence and afflict predominantly girls and women.1 Prospective studies have identified several risk factors that increase the probability of developing an eating disorder such as high drive for thinness (DT) and related constructs, such as weight concerns and dieting, and negative affect, or internalizing symptoms, such as depression and anxiety.2–7 DT is characteristic of individuals with fear of weight gain who diet to prevent it, but also of those who seek to attain an unhealthily low body weight as seen in many individuals with anorexia nervosa (AN) or bulimia nervosa (BN).8–10 Negative affect, a temperamental disposition towards experiencing high levels of negative emotions such as anxiety and sadness, although not a defining feature of AN or BN as presently recognized in the DSM-5 (APA, 2013),11 is frequently associated with eating pathology.3–4 Studies that have prospectively explored both dimensions as independent constructs generally find that DT is significantly associated with bulimic pathology whereas negative affect is not when both DT and negative affect are considered together.12, 13 However, despite DT and negative affect being potential risk factors for eating pathology, the interaction between these risk factors in the development of AN or BN pathology during adolescence remains largely unexplored.
In contrast, a qualitative approach based on identifying differing subtypes along two related variables, dietary restraint and depression, has suggested a multiplicative effect. A dietary restraint-depressive subtype has been associated with increased psychopathology and functional impairment, increased treatment seeking, poorer response to multiple forms of treatment,14, 15, 18 greater persistence of binge eating,16 and a lower likelihood of recovery17 in adults14–17 and adolescents18 with bulimic disorders compared with a dietary restraint only cluster. Similarly, in a diverse community sample of girls aged 10, a combined dietary restraint-negative affect cluster predicted binge eating at ages 12 and 14 in comparison with a subtype characterized by very little dietary restraint and negative affect.19 Although studies suggest an interaction between DT and negative affect in risk for eating disorders, the longitudinal, multiplicative impact ranging the peak period of risk, late adolescence to young adulthood, remains largely unexplored.
Furthermore, if the ultimate aim of studying the predictive effects of these two potent risks factors is to provide better prevention and intervention efforts, using qualitative methodology, such as cluster/partition modeling, in conjunction with standard quantitative methods, can aid in identifying homogenous groups of adolescents who may benefit from targeted strategies. To date, a majority of eating disorder prevention programs have focused on reducing DT or related dietary restraint,20–22 and only a few have addressed negative affect,23, 24 with only limited success.4, 25 One reason for this lack of success may be the fact that prevention interventions are not developed to address both DT and negative affect, despite the fact that DT and negative affect tend to co-occur.3, 4 This is important considering that a meta-analysis of prevention programs has shown that interventions are more successful for individuals who present with the relevant risk factors targeted.25 Thus, the effect of high DT and/or negative affect during adolescence on the onset and maintenance of eating disorders may be most usefully explored using two models: 1) a traditional or quantitative approach including these continuous variables (DT and negative affect) and their interaction and; 2) a qualitative approach based on clustering individuals along these dimensions. The first model allows us to answer which of the three continuous factors (DT, negative affect, or their interaction) is more strongly predictive of eating pathology over this risk period. The second model allows the detection of the number of individuals at higher risk across three different trajectories (primarily DT, primarily negative affect, or both) as well as allowing for the comparison of the frequency of occurrence of AN or BN between the individuals at risk across the three different trajectories with those with low scores on both dimensions acting as a control group.
Finally, it is also unclear whether those who present with only negative affect (e.g., anxious/depressive symptoms) represent a different etiological path to the development of specific eating disorder pathology (AN vs. BN) than those who present with DT. This is an important consideration given that history of depression,5 depressive symptoms,6 and anxiety2 consistently predict eating disorder onset. For example, we previously demonstrated that four clusters based on DT and depressive symptoms best explain differences in eating disorder and comorbid pathology in a large clinical group of adult women with AN and BN.10 These four clusters were characterized by either low scores on both DT and depression (“Mild”), high DT only (“DT”), high depression with moderate levels of DT (“Depressive-ModDT”) and high scores on both DT and depression (“DT-depressive”). Overall, eating and comorbid psychopathology were lowest in the Mild group and highest in the DT-depressive group. Moreover, a majority of AN cases were found in the Depressive-ModDT cluster compared with the DT or DT-depressive subtypes whereas more BN cases were observed in the DT and DT-depressive clusters,10 which is in agreement with findings from previous studies.16–18 Although it is unclear whether the high depression with moderate levels of DT subtype represents a path to AN development, is a consequence of the disorder, or a combination of both, these observations do suggest that a cluster combining DT and depressive symptoms may play an additive role in risk of AN such that those exhibiting both may be at even greater risk.
To date, the concurrent and predictive validity of this four-cluster model, based on similar constructs, on the development and onset of eating disorders during adolescence remains unexplored. Given that adolescence is the peak risk period of eating disorder onset, this inquisition is imperative. Thus, the main objectives of the present study were to explore the cross-sectional and predictive effect of DT and negative affect at ages 16–17 on the development of self-reported AN and BN at ages 16–17 and 19–20 using both a qualitative approach based on four clusters with different levels of DT and negative affect and a quantitative approach based on the dimensions of DT and negative affect and an interactive term.
Methods
Participants
Participants were drawn from the Swedish Twin study of Child and Adolescent Development (TChAD), which includes all twins born in Sweden between May 1985 and December 1986.26 Twins and their parents were identified via the Swedish Medical Birth Register and invited to participate in TChAD via mailed self-report questionnaires. Four assessment waves have been completed, and we include information from Waves 3 and 4, which is when the primary measures of interest for the present study were assessed.
Only unrelated twin females (one per family) from the 958 who completed the relevant measures at Wave 3 (when participants were 16–17 years old) were included. Of these 958 females, 37.9% were monozygotic (MZ) and 26.6% dizygotic (DZ) same-sex twins, 28.6% opposite-sex, and 6.9% unknown. To create this sample of unrelated females, we randomly selected one female from each MZ (n= 181) and DZ (n=127) pair, all the females from opposite-sex pairs (n=274), and those whose co-twin did not participate (n=33). This resulted in a final sample of 615 unrelated females. Of these, 73.3% responded to Wave 4 questionnaires (when participants were 19–20 years old).
Ethical approval was obtained from the Regional Ethics Review Board in Stockholm and the University of North Carolina Institutional Review Board.
Measures
Eating Disorder Inventory- Drive for Thinness
The 7-item Drive for Thinness subscale of the Eating Disorder Inventory–II27 (EDI) was used to assess DT. This scale measures excessive concern with dieting and weight, fear of fatness, and the pursuit of thinness. The Swedish version of the EDI has shown good psychometric properties,28 and for the present sample, the Cronbach's alpha for DT was 0.84.
Eating Disorder Inventory-Bulimia
The 7-item Bulimia subscale of the EDI was used for validation purposes of self-reported BN, since it measures episodes of binge eating and purging. For the present sample, the Cronbach’s alpha was 0.74.
Child Behavior Checklist Anxious/Depressed Scale
The Child Behavior Checklist (CBCL)29 is a 118-item checklist for children 4–18 years of age that measures parents’ perceptions of the child’s behavior and yields 8 subscales. For this study, only the 14-item Anxious/Depressed (A/D) scale was used as a measure of negative affect, such as being fearful or sad. The CBCL is extensively used and has good psychometric properties,30 and for the present sample, the Cronbach's alpha of the CBCL- A/D subscale was 0.87.
Eating Disorder Diagnosis
To assess eating disorder diagnosis, participants were asked if “they have ever had any of the following diseases or health problems: 1) Anorexia, 2) Bulimia,” with a dichotomous response format that included 'Yes' or 'No' as the options. Of the final sample of 615 girls included in the present report, 92% responded to this item at Wave 3 and 73% at Wave 4 (Table 1), with 67% of the sample responding to this question at both waves.
Table 1.
Differences between the four clusters based on EDI-Drive for Thinness (DT) and CBCL-Anxious/Depressed (A/D) scores in self-reported history of anorexia nervosa (AN) or bulimia nervosa (BN) at Waves 3 and 4 among unrelated females with a twin sister or brother (n=615) from the Swedish Twin Study of Child and Adolescent Development cohort.
| Mean (SD) for quantitative criteria and percentage for binary |
Comparison between clusters |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 615 unrelated girls aged 16–17 | Mild (1) (n=471;76.6%) |
DT (2) (n=69;11.2%) |
A/D (3) (n=62;10.1%) |
DT-A/D (4) (n=13; 2.1%) |
p-value/ 1Test Value |
2Contrasts (p<.05) |
||||
| EDI – Drive for Thinness | 1.29 | (1.61) | 11.10 | (3.82) | 1.66 | (2.01) | 14.69 | (3.75) | <.001/228.6 | 1=3<2<4 |
| CBCL – Anxious-Depressive | 1.01 | (1.32) | 1.38 | (1.55) | 9.34 | (3.79) | 11.38 | (3.77) | <.001/220.0 | 1=2<3=4 |
| Wave 3 (16–17 years old) cross-sectional eating disorders | ||||||||||
| History of AN (1.9%; n=11/565) | 0.9% 4/431 |
4.5% 3/66 |
3.4% 2/58 |
20.0% 2/10 |
<.001/22.4 | 1<2,4 | ||||
| History of BN (1.2%; n=7/564) | 0.7% 3/430 |
4.6% 3/65 |
0.0% 0/58 |
9.1% 1/11 |
<.01/13.3 | 1<2,4 | ||||
| Wave 4 (19–20 years old) prospective eating disorders | ||||||||||
| History of AN (4.4%; n=20/451) | 3.4% 12/348 |
2.1% 1/47 |
2.3% 1/44 |
50.0% 6/12 |
<.001/60.6 | 1,2,3<4 | ||||
| History of BN (2.7%; n=12/451) | 1.1% 4/351 |
8.9% 4/45 |
4.5% 2/44 |
18.2% 2/11 |
<.001/20.7 | 1<2,4 | ||||
Abbreviations: EDI: Eating Disorders Inventory; CBCL: Child Behavior Checklist
Test values: Kruskal Wallis and Pearson chi-squared tests (3 degrees of freedom)
Contrasts: Mann Whitney U
Statistical Analysis
Cluster analyses for subtyping along EDI-DT and CBCL-A/D
The EDI-DT and CBCL-A/D scales were submitted to K-means cluster analysis. An iterative procedure wherein participants are repeatedly assigned to cluster membership on the basis of their smallest Euclidean distance to each subsequent cluster centroid was employed. The distributions for EDI-DT and CBCL-A/D scores were positively skewed. However, they were not log transformed because raw EDI-DT scores are typically used to maximize differences and variability between the groups.31
The K-means portioning model was chosen, since it easily allows establishing, a priori, the number of groups to be tested, looking for the most homogeneous groups. A four-cluster solution was specified in the model since four distinct groups along the dimensions of DT and A/D have shown clinical validity in terms of eating pathology and severity previously, and our main objective was to examine if these four clusters are associated with the development of an eating disorder in community adolescents.7 The differences between the clusters on each of the relevant continuous variables, DT and A/D, were analyzed with Kruskal-Wallis and U de Mann-Whitney tests. For the self-report categorical measures of history of AN or BN, differences were examined by using chi-squared tests (Pearson’s and Fisher’s exact tests).
Logistic regression analyses were then performed to assess the independent continuous variables of interest (DT, A/D and their interaction, DTxA/D interaction) as well as the clusters as predictors for the development of self-reported diagnosis of AN or BN, adjusting by presence of reported AN and/or BN at ages 16–17.
Results
Cluster analysis
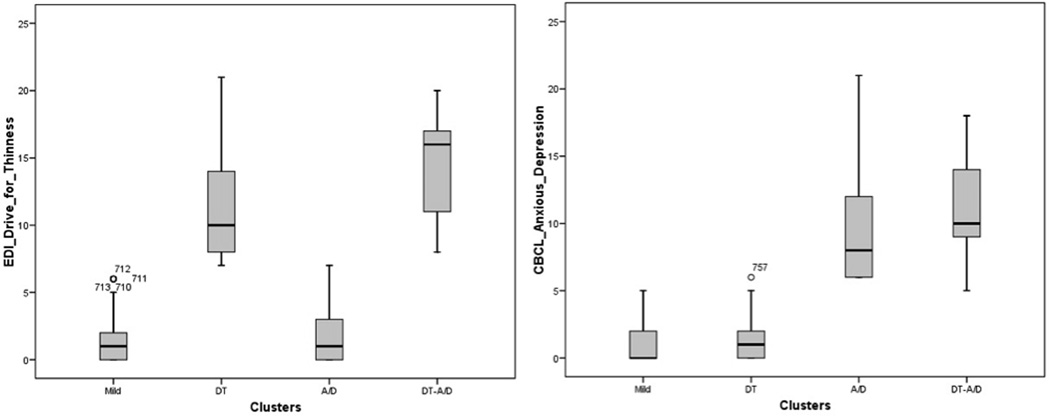
The K-means cluster analysis yielded four groups with nearly mutually exclusive range levels of DT and/or A/D corresponding to the 90th percentile (Figure 1). The largest group from this adolescent community sample had low scores on both EDI-DT and CBCL-A/D (“Mild” group; 76.6%). The other three groups were defined by high scores on DT (above 7) and/or on A/D (above 5), representing the top 10%. The high DT only group comprised 11.2% of the sample (“DT”) and the high A/D only group comprised 10.1% of the sample (“A/D”). Those with high scores on both DT and negative affect represented 2.1% of the sample (“DT-A/D”).
Figure 1.
The four K-means clusters with nearly “mutually exclusive” range levels of drive or thinness (left) and/or Child Behavior Checklist-Anxious/Depressed (right): lower than 90th percentile on both scales (Mild), higher only on DT (DT) or A/D (A/D), and on both (DT-A/D).
Comparisons between the four clusters based on DT and A/D on self-reported AN or BN
Overall, the frequencies of self-reported AN and BN were 1.9% and 1.2% at age 16–17 and 4.4% and 2.7% at age 19–20, respectively (Table 1). The prevalence of AN and BN were different across the clusters at both ages 16–17 and 19–20 (Table 1). At age 16–17, both the DT and DT-A/D clusters showed a higher prevalence of AN and BN compared with the Mild cluster. At age 19–20, there was a higher prevalence of AN in the DT-A/D cluster compared with all other clusters as well as a higher prevalence of BN in the DT-A/D and DT clusters compared with the Mild cluster.
Logistic regressions including history of self-reported eating disorder at age 16–17 as a covariate and the Mild cluster as the reference category showed that the multivariate model for AN at age 19–20 was significant (X2=38.4; df=4; p< .001), accounting for 27.5% of the variance in AN development (Nagelkerke R2 = .28). Specifically, the DT-A/D cluster at age 16–17 predicted risk for AN at age 19–20 (B = 3.5 p <.001). Similarly, the multivariate model for BN at age 19–20 was also significant (X2=17.7; df=4; p< .001), accounting for 19.2% of the variance (Nagelkerke R2 = .19), such that the risk for BN was significantly predicted by the two DT clusters, DT-A/D (B = 2.6, p<.05) and DT (B = 1.8, p<.05).
We completed two additional analyses in an effort to confirm findings. First, to test the potential impact of attrition on our results, analyses were repeated including only those females who participated in both Wave 3 and 4 (n=412). A Fischer’s exact test showed that attrition was not significantly associated with any of the four clusters such that significant findings observed in the full sample were all observed in the reduced sample (Table 2). Furthermore, logistic regressions including history of self-reported eating disorder at age 16–17 as a covariate and the Mild cluster as the reference category showed that the multivariate model for AN at age 19–20 was significant (X2=28.5; df=4; p< .001), accounting for 21.4% of the variance in AN development (Nagelkerke R2 = .214). Again, the DT-A/D cluster at age 16–17 predicted risk for AN at age 19–20 (B = 3.16 p <.001). The multivariate model for BN at age 19–20 also remained significant (X2 =19.6; df=4; p< .001; Nagelkerke R2 = .228), and the associations of DT (B = 2.10) and DT-AD (B = 2.97) versus Mild clusters with self-reported BN at age 19–20 remained significant (p< .05).
Table 2.
Differences between the four clusters based on EDI-Drive for Thinness (DT) and CBCL-Anxious/Depressed (A/D) scores in self-reported history of anorexia nervosa (AN) or bulimia nervosa (BN) among unrelated females who participated in both Waves 3 and 4 (n=412)
| Mean (SD) for quantitative criteria and percentage for binary |
Comparison between clusters |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 412 unrelated girls aged 16–17 | Mild (1) 77% (n=320) |
DT (2) 10.4% (n=43) |
A/D (3) 9.9% (n=41) |
DT-A/D (4) 1.9% (n=8) |
p-value/ 1Test Value |
2Contrasts (p<.05) |
||||
| EDI – Drive for Thinness | 1.19 | (1.52) | 11.16 | (3.59) | 1.54 | (1.96) | 15.75 | (3.5) | <.001/466.5 | 1=3<2<4 |
| CBCL – Anxious-Depressive | 0.94 | (1.24) | 1.35 | (1.53) | 9.32 | (3.64) | 10.63 | (4.34) | <.001/343.4 | 1=2<3=4 |
| Wave 3 (16–17 years old) cross-sectional eating disorders | ||||||||||
| History of AN (2.4%; n=10/412) | 1.3% (n=4) |
4.7% (n=2) |
4.9% (n=2) |
25% (n=2) |
<.001/22.4 | 1<2,3,4 | ||||
| History of BN (1.2%; n=5/412) | 0.6% (n=2) |
4.7% (n=2) |
0.0% (n=0) |
12.5% (n=1) |
<.01/14.17 | 1<2,4 | ||||
| Wave 4 (19–20 years old) prospective eating disorders | ||||||||||
| History of AN (4.4%; n=18/412) | 3.8% (n=12) |
2.3% (n=1) |
2.4% (n=1) |
50.0% (n=4) |
<.001/40.9 | 1,2,3<4 | ||||
| History of BN (2.4%; n=10/412) | 0.9% (n=3) |
9.3% (n=4) |
2.4% (n=1) |
25.0% (n=2) |
<.001/28.8 | 1<2,4 | ||||
Abbreviations: EDI: Eating Disorders Inventory; CBCL: Child Behavior Checklist
Test values: Kruskal Wallis and Pearson chi-squared tests (3 degrees of freedom)
Contrasts: Mann Whitney U
Second, we repeated analyses with a replication sample—those female twins not initially randomly selected to be included in the primary sample (n=343). Confirming initial findings, the DT-AD cluster showed the highest prevalence of AN and BN compared with all other clusters. Although this difference was not statistically significant in the replication sample, likely due to the smaller size of the sample, the fact that results remained in the same direction provides confidence in our initial findings.
Comparisons between risk factors (DT, A/D, DTxA/D) on self-reported history of AN or BN
Logistic regressions using DT and A/D in their continuous format and including an interaction term (DTxA/D) confirmed cluster findings. The overall model was significant for AN (X2=30.7; df=4; p< .001), accounting for 22.2% of the variance (Nagelkerke R2 = .222), and BN (X2=17.6; df=4; p< .001), accounting for 19.1% of the variance (Nagelkerke R2 = .19), at age 19–20. More specifically, DTxA/D at age 16–17 predicted risk for AN at age 19–20 (B = 1.0, p = .017), whereas DT at age 16–17 predicted risk for BN at age 19–20 (B = 1.1, p = .047). These results were maintained when controlling for history of any eating disorder (AN and/or BN) at age 16–17 (B = 1.0, p = .02 for AN; B = 1.1, p = .05 for BN).
Results remained consistent when analyses were completed with only those female twins that participated at both waves. Controlling for any eating disorder during adolescence, the model was significant for AN (X2 = 22.2; df=4; p <. 01; Nagelkerke R2 = .17), with DTxA/D predicting AN (B = 1.0, p = .05), as well as for BN (X2 = 18.76; df=4; p = .01; Nagelkerke R2 = .23), with DT predicting risk for BN (B=1.2, p =. 047) at age 19–20.
Finally, we again repeated analyses in the replication sample of those unrelated female twins that were not included in the primary sample. The overall models were significant for AN (X2=20.8; df=4; p< .001), accounting for 21.6% of the variance (Nagelkerke R2 = .216), and BN (X2=13.2; df=4; p=.01), accounting for 22.9% of the variance (Nagelkerke R2 = .23) at age 19–20. Similarly, DT at age 16–17 continued to significantly predict risk for BN in young adulthood (B = 1.20, p = .03); however DTxA/D at age 16–17 was not significantly associated with AN at follow-up (B = 1.01, p = .22). Despite DTxA/D no longer remaining significant in the replication sample, findings were in the same direction suggesting that the lack of statistical significance may be attributable to the smaller sample size of the replication sample.
Discussion
The current four-cluster solution based on low and/or high levels of DT and negative affect in a large sample of unrelated female Swedish twins from the community included, as expected, a larger number of individuals with low DT and low A/D and a smaller proportion of individuals with high DT and high A/D than typically observed in clinical samples. However, as predicted, the cluster subtypes differed in the frequency of self-reported histories of AN or BN both concurrently and prospectively. Individuals scoring high on DT only were more likely to have AN at age 16–17 and BN at both ages compared with those with low scores on both factors. Perhaps most importantly, the risk of developing AN or BN at age 16–17 and 19–20 was substantially increased in those individuals with high scores on both DT and A/D (DT-A/D) in comparison with those in the Mild cluster. The confluence of these two risk factors (DT-A/D) further increased the odds of having suffered from AN by age 19–20 compared with individuals scoring high on only one of the risk factors. Thus, results suggest the presence of both DT and A/D at age 16–17 is a pernicious combination that markedly increases risk for the development or maintenance of an eating disorder both cross-sectionally and prospectively. However, when using a multivariate approach including the three dimensions (DT, A/D, and DTxA/D), while this combination also predicted the development of AN at ages 19–20, it was only DT that longitudinally predicted the development of BN.
Although the relatively small number of self-reported eating disorders in the present community sample of females limits the generalizability of these findings to clinical samples of females with an eating disorder diagnosis, our results are consistent with previous research. 14–18 The present DT-A/D cluster signals a higher prevalence of self-reported AN or BN cross-sectionally and longitudinally, which fits with the dietary restraint-depressive clusters that have been consistently associated with worse psychopathology and prospectively with less abstinence of binge eating and/or vomiting after treatment,14, 15, 18 or poorer course of the disease.16, 17 Similarly, the DT cluster was also associated cross-sectionally and prospectively with BN, which is consistent with previous cluster analysis studies based on the dietary restraint-depressive subtyping scheme. 14–18
The relevance of these findings are considerable for both prevention and treatment. In terms of prevention, many efforts target DT and the related construct body dissatisfaction. However, this may not be enough, especially for those high-risk individuals who have high levels of both DT and negative affect. Individuals who score high on DT only might be expected to benefit most from a prevention intervention strategy that focuses on reduction of fear of weight gain and accompanying restrictive dietary patterns such as Stice’s cognitive based-dissonance training.32 Indeed, young women with high baseline thin-ideal internalization scores experienced greater dissonance and behavioral change in such interventions.32 Individuals who primarily score high on negative affect or score high on both negative effect and DT, are at increased risk for eating disorders via a different pathway, thus may not respond to such interventions.33 In fact, unaddressed negative affect in individuals with weight concerns undergoing a preventive dissonance program can increase the likelihood of developing an eating disorder over time (20%) compared with those who have low negative affect (5%).34
In terms of treatment, the use of cluster subtypes may be helpful as they have been associated with differential outcomes in clinical samples, suggesting they represent different paths in the course of eating disorders. The confluence of DT and negative affect versus DT only are associated with poorer response to treatment in adolescent and adult patients with bulimic disorders,14, 15, 18 increased dropout rate,35 higher treatment seeking and lower likelihood of recovery over 3 to 5 year follow-up.16, 17 Capturing these two dimensions accurately may assist with designing interventions that appropriately target to enhance outcome.
Our results must be considered within the context of the study’s limitations. First is our use of self-reported AN and BN diagnosis. However, independent samples t-tests suggest that our self-reported eating disorder diagnoses function similarly to interview based diagnoses. As would be expected, self-reported AN at age 16–17 and young adulthood was characterized by significantly higher scores on the DT and A/D scales and the DTxA/D interactive term and had a significantly lower body mass index (BMI) during late adolescence. Self-reported BN at age 16–17 and young adulthood was similarly characterized by significantly higher scores on the EDI bulimia subscale and the DTxA/D interactive term as well as higher A/D scores and BMI in young adulthood (all p’s < .05). Further, self-report eating disorder items such as these have been used in similar, large-scale population based studies where determining clinical diagnoses via interview is not feasible36 and have been shown to reliably detect a lifetime AN diagnosis.37 The overall rates of self-reported AN and BN by ages 19–20 (6.2%) in our sample also approximated estimates of eating disorders from other young female populations from the USA, Australia, and other European countries.38–41 Although we did observe a higher prevalence of AN in comparison with BN, this pattern has been observed previously in Europe.42–43 For example, in a large Dutch population aged 18–64, the lifetime prevalence for AN was double that of BN.43 One potential explanation for the increased frequency of AN in comparison with BN is that symptoms of BN may be less outwardly visible, which could contribute to a lower degree of service utilization and therefore diagnosis.44 For example, in a community sample of Finnish adolescents, Keski-Rahkonen and colleagues observed that over half the cases of AN in their sample were not detected within the healthcare system;45 however, this pattern may also represent an artifact of our self-report diagnoses. Despite this, it is likely that our measure is representative of individuals with eating disorder pathology, and we consider it unlikely that, given the age of our sample, a participant would respond in the affirmative to these questions had she not actually suffered from the disorder or associated symptomatology; however greater details regarding actual “caseness” are clearly desirable. Finally, as our sample comprised unrelated twins from Sweden, results might not generalize to a non-twin sample or to participants from other ancestry groups.
In summary, cluster modeling is useful to detect homogeneous subsets of community adolescent females with a combination of both risk factors DT and A/D (or just DT), who are at increased risk for developing or maintaining AN and BN during early adulthood. However, further research remains to determine whether the use of other methods such as latent profile analysis and clinically validated eating disorder diagnoses would support present findings. The presence of DT and A/D appears to be a pernicious combination that, in particular, increases risk for AN. The verification and validation of these cluster-derived profiles may assist with tailoring both prevention and treatment interventions to accurately target symptoms relevant to the development of eating pathology. Furthermore, these four clusters may represent different developmental patterns of eating disorders characterized by differences in underlying biological and environmental processes that should be targeted in a timely fashion to lower risk.
Acknowledgements
Dr. Bulik is a consultant for Shire Pharmaceuticals. Dr. Baker was partially supported by National Institute of Health grant T32MH076694 (PI: Bulik). This project was also supported by funds from the Swedish Council for Working Life and Social Research and the Swedish Research Council provided to Dr. Lichtenstein.
Footnotes
Disclosure of Conflicts:
None of the other authors report conflict of interest.
References
- 1.Fairburn CG, Harrison PJ. Eating disorders. Lancet. 2003;361:407–416. doi: 10.1016/S0140-6736(03)12378-1. [DOI] [PubMed] [Google Scholar]
- 2.Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Agras WS. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychol Bull. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- 3.Stice E. Risk and maintenance factors for eating pathology: A meta-analytic review. Psychol Bull. 2002;128:825–848. doi: 10.1037/0033-2909.128.5.825. [DOI] [PubMed] [Google Scholar]
- 4.Stice E, Ng J, Shaw H. Risk factors and prodromal eating pathology. J Child Psychol Psychiatry. 2010;51:518–525. doi: 10.1111/j.1469-7610.2010.02212.x. [DOI] [PubMed] [Google Scholar]
- 5.Jacobi C, Fittig E, Bryson SW, Wilfley D, Kraemer HC, Taylor CB. Who is really at risk? Identifying risk factors for subthreshold and full syndrome eating disorders in a high-risk sample. Psychol Med. 2011;41:1939–1949. doi: 10.1017/S0033291710002631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stice E, Marti CN, Durant S. Risk factors for onset of eating disorders: evidence of multiple risk pathways from an 8-year prospective study. Behav Res Ther. 2011;49:622–627. doi: 10.1016/j.brat.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohde P, Stice E, Marti CN. Development and predictive effects of eating disorder risk factors during adolescence: Implications for prevention efforts. Int J Eat Disord. 2014 doi: 10.1002/eat.22270. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chernyak Y, Lowe MR. Motivations for dieting: Drive for thinness is different from drive for objective thinness. J Abnorm Psychol. 2010;119:276–281. doi: 10.1037/a0018398. [DOI] [PubMed] [Google Scholar]
- 9.Becker AE, Thomas JJ, Pike KM. Should non-fat-phobic anorexia nervosa be included in DSM-V? Int J Eat Disord. 2009;42:620–635. doi: 10.1002/eat.20727. [DOI] [PubMed] [Google Scholar]
- 10.Peñas-Lledó E, Fernandez-Aranda F, Jimenez-Murcia S, Granero R, Penelo E, Soto A, et al. Subtyping eating disordered patients along drive for thinness and depression. Behav Res Ther. 2009;47:513–519. doi: 10.1016/j.brat.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 12.Killen JD, Taylor CB, Hayward C, Haydel KF, Wilson DM, Hammer L, et al. Weight concerns influence the development of eating disorders: a 4-year prospective study. J Consult Clin Psychol. 1996;64:936–940. doi: 10.1037//0022-006x.64.5.936. [DOI] [PubMed] [Google Scholar]
- 13.Stice E, Killen JD, Hayward C, Taylor CB. Age of onset for binge eating and purging during late adolescence: a 4-year survival analysis. J Abnorm Psychol. 1998;107:671–675. doi: 10.1037//0021-843x.107.4.671. [DOI] [PubMed] [Google Scholar]
- 14.Stice E, Agras WS. Subtyping bulimic women along dietary restraint and negative affect dimensions. J Consult Clin Psychol. 1999;67:460–469. doi: 10.1037//0022-006x.67.4.460. [DOI] [PubMed] [Google Scholar]
- 15.Stice E, Agras WS, Telch CF, Halmi KA, Mitchell JE, Wilson T. Subtyping binge eating-disordered women along dieting and negative affect dimensions. Int J Eat Disord. 2001;30:11–27. doi: 10.1002/eat.1050. [DOI] [PubMed] [Google Scholar]
- 16.Stice E, Fairburn CG. Dietary and dietary-depressive subtypes of bulimia nervosa show differential symptom presentation, social impairment, comorbidity, and course of illness. J Consult Clin Psychol. 2003;71:1090–1094. doi: 10.1037/0022-006X.71.6.1090. [DOI] [PubMed] [Google Scholar]
- 17.Stice E, Bohon C, Marti CN, Fischer K. Subtyping women with bulimia nervosa along dietary and negative affect dimensions: Further evidence of reliability and validity. J Consult Clin Psychol. 2008;76:1022–1033. doi: 10.1037/a0013887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen EY, Le Grange D. Subtyping adolescents with bulimia nervosa. Behav Res Ther. 2007;45:2813–2820. doi: 10.1016/j.brat.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen EY, McCloskey MS, Keenan KE. Subtyping dietary restraint and negative affect in a longitudinal community sample of girls. Int J Eat Disord. 2009;42:275–283. doi: 10.1002/eat.20661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McVey GL, Lieberman M, Voorberg N, Wardrope D, Blackmore E. School-based peer support groups: A new approach to the prevention of disordered eating. Eat Disord. 2003;11:169–185. doi: 10.1080/10640260390218297. [DOI] [PubMed] [Google Scholar]
- 21.Neumark-Sztainer D, Butler R, Palti H. Dieting and binge eating: Which dieters are at risk? J Am Diet Assoc. 1995;95:586–589. doi: 10.1016/S0002-8223(95)00160-3. [DOI] [PubMed] [Google Scholar]
- 22.Stewart DA, Carter JC, Drinkwater J, Hainsworth J, Fairburn CG. Modification of eating attitudes and behavior in adolescent girls: A controlled study. Int J Eat Disord. 2001;29:107–118. doi: 10.1002/1098-108x(200103)29:2<107::aid-eat1000>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Burton E, Stice E, Bearman SK, Rohde P. Experimental test of the affect-regulation theory of bulimic symptoms and substance use: A randomized trial. The Int J Eat Disord. 2007;40:27–36. doi: 10.1002/eat.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stice E, Rohde P, Seeley JR, Gau JM. Brief cognitive-behavioral depression prevention program for high-risk adolescents outperforms two alternative interventions: A randomized efficacy trial. J Consult Clin Psychol. 2008;76:595–606. doi: 10.1037/a0012645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw H, Stice E, Becker CB. Preventing eating disorders. Child Adolesc Psychiatr Clin N Am. 2009;18:199–207. doi: 10.1016/j.chc.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lichtenstein P, Tuvblad C, Larsson H, Carlstrom E. The Swedish twin study of Child and adolescent development: The TCHAD-study. Twin Res Hum Genet. 2007;10:67–73. doi: 10.1375/twin.10.1.67. [DOI] [PubMed] [Google Scholar]
- 27.Garner D. Eating Disorders Inventory-2: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 28.Nevonen L, Clinton D, Norring C. Validating the EDI-2 in three Swedish female samples: Eating disorders patients, psychiatric outpatients and normal controls. Nord J Psychiatry. 2006;60:44–50. doi: 10.1080/08039480500504537. [DOI] [PubMed] [Google Scholar]
- 29.Achenbach TM. Manual for Child Behavior Checklist/4–18 and 1991 Profile. Burlington, VT: University of Vermont, Dept. of Psychiatry; 1991. [Google Scholar]
- 30.McConaughy SH. Advances in empirically based assessment of children’s behavioral and emotional problems. School Psychology Review. 1993;22:285–307. [Google Scholar]
- 31.Stoddard AM. Standardization of measures prior to cluster analysis. Biometrics. 1979;35:765–773. [PubMed] [Google Scholar]
- 32.Stice E, Marti N, Shaw H, O’Neil K. General and program-specific moderators of two eating disorder prevention programs. Int J Eat Disord. 2008;41:611–617. doi: 10.1002/eat.20524. [DOI] [PubMed] [Google Scholar]
- 33.Peñas-Lledó E, Agüera Z, Sánchez I, Gunnard K, Jiménez-Murcia S, Fernández-Aranda F. Differences in cognitive behavioral therapy dropout rates between bulimia nervosa subtypes based on drive for thinness and depression. Psychother Psychosom. 2013;82:125–126. doi: 10.1159/000339620. [DOI] [PubMed] [Google Scholar]
- 34.Stice E, Rohde P, Gau J, Shaw H. Effect of a dissonance-based prevention program on risk for eating disorder onset in the context of eating disorder risk factors. Prev Sci. 2012;13:129–139. doi: 10.1007/s11121-011-0251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrard I, Crepin C, Ceschi G, Golay A, Van der Linden M. Relations between pure dietary and dietary-negative affect subtypes and impulsivity and reinforcement sensitivity in binge eating individuals. Eat Behav. 2012;13:13–19. doi: 10.1016/j.eatbeh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Micali N, Stahl D, Treasure J, Simonoff E. Childhood psychopathology in children of women with eating disorders: understanding risk mechanisms. J Child Psychol Psychiatry. 2014;55:124–134. doi: 10.1111/jcpp.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keski-Rahkonen A, Sihvola E, Raevuori AH, Kaukoranta J, Bulik CM, Hoek HW, Rissanen A, Kaprio J. Reliability of self-reported eating disorders: optimizing population screening. Int J Eat Disord. 2005;39:754–762. doi: 10.1002/eat.20277. [DOI] [PubMed] [Google Scholar]
- 38.Favaro A, Ferrara S, Santonastaso P. The spectrum of eating disorders in young women: A prevalence study in a general population sample. Psychosom Med. 2003;65:701–708. doi: 10.1097/01.psy.0000073871.67679.d8. [DOI] [PubMed] [Google Scholar]
- 39.Patton GC, Coffey C, Carlin JB, Sanci L, Sawyer S. Prognosis of adolescent partial syndromes of eating disorder. Br J Psychiatry. 2008;192:294–299. doi: 10.1192/bjp.bp.106.031112. [DOI] [PubMed] [Google Scholar]
- 40.Stice E, Marti CN, Shaw H, Jaconis M. An 8-year longitudinal study of the natural history of threshold, subthreshold, and partial eating disorders from a community sample of adolescents. J Abnorm Psychol. 2009;118:587–597. doi: 10.1037/a0016481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kjelsås E, Bjørnstrøm C, Götestam KG. Prevalence of eating disorders in female and male adolescents (14–15 years) Eat Behav. 2004;5:13–25. doi: 10.1016/S1471-0153(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 42.Götestam KG, Eriksen L, Heggestad T, Nielsen S. Prevalence of eating disorders in Norwegian general hospitals 1990–1994: admissions per year and seasonality. Int J Eat Disord. 1998;23:57–64. doi: 10.1002/(sici)1098-108x(199801)23:1<57::aid-eat7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 43.Bijl RV, Ravelli A, van Zessen G. Prevalence of psychiatric disorder in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Soc Psychiatry Psychiatr Epidemiol. 1998;33:587–595. doi: 10.1007/s001270050098. [DOI] [PubMed] [Google Scholar]
- 44.Fairburn CG, Harrison PJ. Eating disorders. Lancet. 2003;361:407–416. doi: 10.1016/S0140-6736(03)12378-1. [DOI] [PubMed] [Google Scholar]
- 45.Keski-Rahkonen A, Hoek HW, Susser ES, Linna MS, Sihvola E, Raevuori A, Bulik CM, Kaprio J, Rissanen A. Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry. 2007;164:1259–1265. doi: 10.1176/appi.ajp.2007.06081388. [DOI] [PubMed] [Google Scholar]