Abstract
Spectral domain optical coherence tomography (SD-OCT) three-dimensional (3D) imaging in choroidal metastasis, due to uterine endometrial carcinoma, is reported for the first time. SD-OCT revealed a dome-shaped elevation of the retina. Neurosensory detachment with splitting of the retinal layers, at some places, was observed. 3D SD-OCT allows evaluation of secondary retinal pigment epithelial and intraretinal changes due to metastatic choroidal lesion.
Background
Intraocular metastasis is now considered the most common malignancy of the eye.1 The frequency of choroidal metastasis in patients with cancer is estimated to be approximately 2−7%.2 3 While any portion of the eye can be involved by metastatic disease, the most common tissue involved is the highly vascular choroid.1–3 There seems to be no predilection for metastasis to preferentially affect the right or left eye.3 In each affected eye, more than one metastasis may be noted. Most choroidal metastases occur posterior to the equator of the fundus, in the macular or paramacular regions. The most common primary sites of cancer are from the breast followed by the lung and the gastrointestinal tract. Diagnosis of ocular metastases is based primarily on clinical findings supplemented by imaging studies. Spectral domain optical coherence tomography (SD-OCT) three-dimensional (3D) imaging, of the retina, in choroidal metastasis, due to uterine endometrial carcinoma, is reported for the first time.
Case presentation
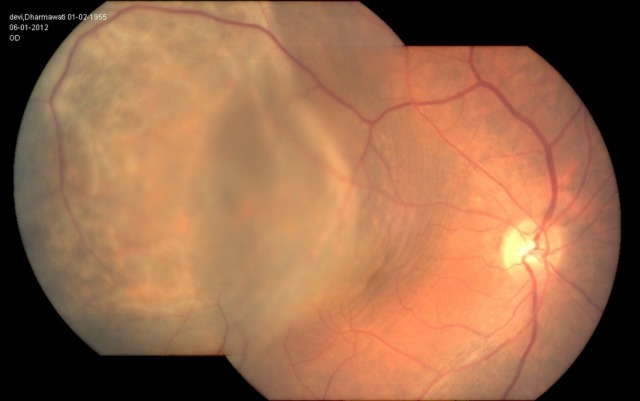
The authors confirm adherence to the tenets of the Declaration of Helsinki. A written informed voluntary consent was taken. A 56-year-old woman presented at our tertiary care centre with the complaints of gradually progressive painless diminution of vision in the right eye since 1 month. On detailed history it was found that the patient was a treated case of uterine endometrial carcinoma stage IV (FIGO classification). She had undergone hysterectomy and radiotherapy for the same 1 year back. The histopathological type was adenocarcinoma with squamous differentiation. Currently she was not taking any treatment. Mammography was normal. Chest x-ray was unremarkable. On examination, the vision in the right eye was counting finger 1 m and in the left eye was 20/20. Left eye examination was unremarkable. Detailed right-eye examination was undertaken. Intraocular pressure was within normal limits. The anterior segment examination was within normal limits. Fundus examination of the right eye revealed a solid, flat, plaque-like, mottled, yellow-brown lesion involving the macula and extending beyond the equator with associated serous retinal detachment in the same region (figure 1). The optic nerve head was within normal limits. Fluorescein angiography revealed hyper-fluorescence in the early arteriovenous phase with progressive and more intense staining in the late phase. On B-scan ultrasound of the eye, there was an acoustically solid convex mass. A-scan ultrasound shows moderate internal reflectivity.
Figure 1.
Fundus photograph of the right eye showing a solid, flat, plaque-like, mottled, yellow-brown lesion involving the macula and extending beyond the equator with associated serous retinal detachment in the same region.
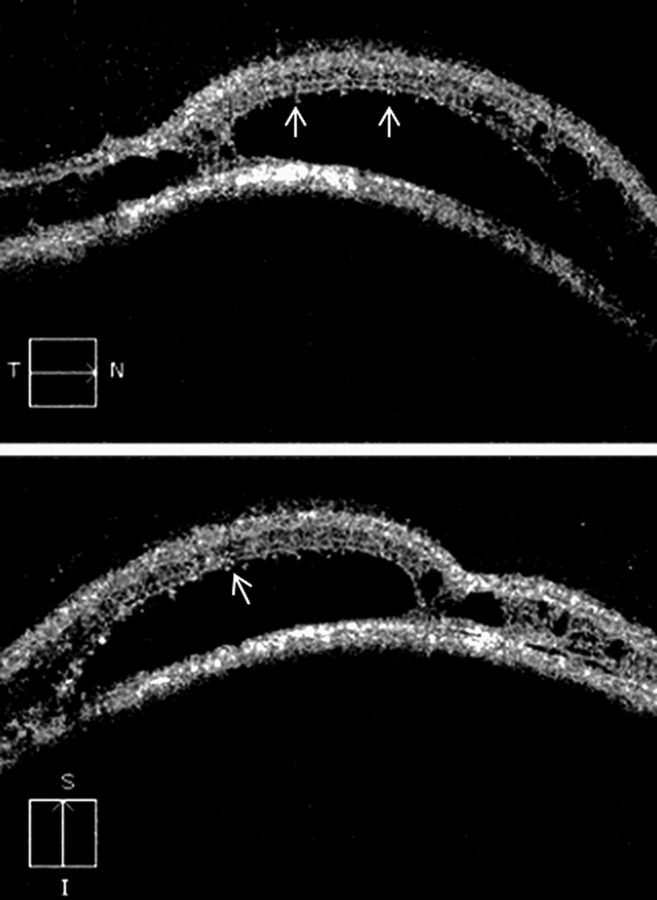
SD-OCT (Cirrus high-definition OCT (Carl Zeiss Meditec Inc.), California, USA) over the tumour mass revealed a dome-shaped retinal elevation, due to underlying choroidal mass. Hyper-reflective retinal pigment epithelium (RPE) was also noted. Optical shadowing was observed beyond the RPE. Neurosensory detachment with splitting of the retinal layers, at some places, was observed as hypo reflective areas (figure 2). Large cystoid spaces were noticed that were localised between the inner and outer retina, specifically in the outer plexiform layer. These cystoid spaces were not of uniform thickness, and there were multiple bridging strands crossing in the cystoid spaces. Occasional tiny pockets of cystoid changes were seen in the inner retina, probably in the inner plexiform layer. In areas of neurosensory detachment, outer photoreceptor layer (OPL) showed presence of granularity which might be phagocytosed OPL cells. No vitreoretinal traction was observed. The cube average thickness was 406 μm with a cube volume of 14.6 mm3. On 3D SD-OCT, retinal elevation was observed. Splitting of the retinal layers was also clearly visualised. The patient died within a month of presentation.
Figure 2.
Spectral domain optical coherence tomography, of the retina, over the tumour mass shows a dome-shaped retinal elevation with hyper-reflective retinal pigment epithelium. Neurosensory detachment with splitting of the retinal layers, at some places, is observed. Large cystoid spaces are noticed that are localised in the outer plexiform layer. These cystoid spaces are not of uniform thickness. There are multiple bridging strands crossing in the cystoid spaces. Occasional tiny pockets of cystoid changes are seen in the inner retina. In areas of neurosensory detachment, outer photoreceptor layer shows the presence of granularity (arrow).
Discussion
Choroidal metastasis has been frequently described in breast and lung cancers. However, uterine endometrial carcinoma presenting with choroidal metastasis is very rare and only few cases have been reported so far.4 5 Ferry and Font6 speculated that the distribution of tumours within the choroid may be related to its vascular characteristics. There is abundant supply of posterior ciliary arteries to the choroid, especially the posterior choroid. The numerous posterior ciliary vessels allow a greater flow of tumour emboli to the posterior uvea as compared with the fewer anterior ciliary vessels supplying the anterior uvea. Metastatic disease involving the uvea should be considered particularly when the patient has a known primary malignancy.
Optical coherence tomography features for choroidal metastasis following breast and lung carcinoma have been reported by Arevalo et al.7 They found anterior displacement of the photoreceptor layer by subretinal fluid overlying a hyper-reflective thickened RPE−choriocapillaris complex. Subretinal deposits with several degrees of hyper-reflectivity were seen. Loss of normal retinal architecture with intraretinal splitting within the neurosensory retina was also reported.
First ever SD-OCT 3D imaging of retina over choroidal metastatic lesion, due to uterine endometrial carcinoma, revealed hyperplastic RPE and phagocytosed OPL cells due to the chronicity of the underlying lesion abutting the retina. Neurosensory detachment at some places with splitting of the retinal layers was also observed. Spatial understanding of splitting of retinal layers was revealed characteristically on 3D imaging. These findings suggest different developmental mechanisms. Neurosensory detachment resulted from effusion of fluid from underlying choroidal lesion. However, splitting of retinal layers might have resulted at places where the choroidal lesion tightly abutted the retina. Here, the shearing force of effusing fluid did not create a cleavage plane between the RPE and neurosensory retina and retinal degeneration resulted in splitting of the retinal layers instead.
Learning points.
Three-dimensional spectral domain optical coherence tomography allows evaluation of secondary retinal pigment epithelial and intraretinal changes due to metastatic choroidal lesion.
However, some limitations result from the choroidal location of the metastasis.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Shields J, Shields C. Intraocular tumors: a text and atlas. 4th edn Philadelphia, PA: WB Saunders Co, 1992, 208–38. [Google Scholar]
- 2.Bloch R, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol 1971;85:673–5. [DOI] [PubMed] [Google Scholar]
- 3.Eliassi-Rad B, Albert D, Green W. Frequency of ocular metastases in patients dying of cancer in eye bank populations. Br J Ophthalmol 1996;80:125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence S, Netland P, Morris W, et al. Uterine papillary serous carcinoma metastatic to the choroid. Retin Cases Brief Rep 2010;4:62–4. [DOI] [PubMed] [Google Scholar]
- 5.Cormio G, Martino R, Loizzi V, et al. A rare case of choroidal metastasis presented after conservative management of endometrial cancer. Int J Gynecol Cancer 2006;16:2044–8. [DOI] [PubMed] [Google Scholar]
- 6.Ferry A, Font R. Carcinoma metastatic to the eye and orbit. I: a clinicopathologic study of 227 cases. Arch Ophthalmol 1974;92:276–86. [DOI] [PubMed] [Google Scholar]
- 7.Arevalo JF, Fernandez CF, Garcia RA. Optical coherence tomography characteristics of choroidal metastasis. Ophthalmology 2005;112:1612–19. [DOI] [PubMed] [Google Scholar]