Abstract
The processes underpinning post-developmental neurogenesis in the mammalian brain continue to be defined. Such processes involve the proliferation of neural stem cells and neural progenitor cells (NPCs), neuronal migration, differentiation and integration into a network of functional synapses within the brain. Both intrinsic (cell signalling cascades) and extrinsic (neurotrophins, neurotransmitters, cytokines, hormones) signalling molecules are intimately associated with adult neurogenesis and largely dictate the proliferative activity and differentiation capacity of neural cells. Cannabinoids are a unique class of chemical compounds incorporating plant-derived cannabinoids (the active components of Cannabis sativa), the endogenous cannabinoids and synthetic cannabinoid ligands, and these compounds are becoming increasingly recognized for their roles in neural developmental processes. Indeed, cannabinoids have clear modulatory roles in adult neurogenesis, probably through activation of both CB1 and CB2 receptors. In recent years, a large body of literature has deciphered the signalling networks involved in cannabinoid-mediated regulation of neurogenesis. This timely review summarizes the evidence that the cannabinoid system is intricately associated with neuronal differentiation and maturation of NPCs and highlights intrinsic/extrinsic signalling mechanisms that are cannabinoid targets. Overall, these findings identify the central role of the cannabinoid system in adult neurogenesis in the hippocampus and the lateral ventricles and hence provide insight into the processes underlying post-developmental neurogenesis in the mammalian brain.
Tables of Links
| LIGANDS | |
|---|---|
| 2-AG | Glutamate |
| 5-HT | HU-210 |
| Δ9-THC | HU-308 |
| AA | IGF-1 |
| ACEA | IL-1β |
| Adenosine | IL-6 |
| AM251 | IL-10 |
| AM630 | JWH-133 |
| AM1241 | NGF |
| Anandamide (AEA) | Noradrenaline |
| BDNF | SR141716A |
| Cannabidiol | SR144528 |
| Cannabinol | TNF-α |
| Dopamine | URB597 |
| FGF-2 | VEGF |
| GABA | WIN55,212-2 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,c,d,eAlexander et al., 2013a,b,c,d,e,,,,).
Introduction
For decades, the true plasticity of the mammalian CNS was underestimated and the adult brain was long considered to be a post-mitotic organ incapable of self-regeneration. However, pioneering work in the 1960s by Joseph Altman and colleagues challenged this long-standing dogma (Altman and Das, 1965). In this groundbreaking publication, Altman provided the first evidence that new neurons were generated in the adult rat hippocampus. Subsequent experiments demonstrated that adult neurogenesis was not specific to the hippocampus, with the adult olfactory bulb identified as another brain region where new neurons are added to existing circuitry throughout life (Altman, 1969). In spite of this work, the concept of post-developmental neurogenesis in the mammalian brain was subject to contemporary scepticism; currently, however, the phenomenon of adult neurogenesis is widely studied and research in the intervening years has confirmed adult neurogenesis in the murine hippocampus (Cameron et al., 1993; Kempermann et al., 1997), while the lateral ventricles (Lois and Alvarez-Buylla, 1993), regions adjacent to the ventricles (such as striatum and septum), as well as the thalamus and hypothalamus (Pencea et al., 2001) have been shown to be capable of generating new neurons during adulthood. In the human brain, evidence continues to mount to support the absence of neurogenesis in the adult human neocortex (Rakic, 2006). However, adult neurogenesis has been described in the hippocampus (Eriksson et al., 1998), the lateral ventricles (Sanai et al., 2004) and more recently in the striatum (Ernst et al., 2014).
Cannabinoids incorporate the active components of the hemp plant Cannabis sativa (the plant-derived cannabinoids), the endogenous cannabinoids (endocannabinoids) produced in humans and animals and the synthetic cannabinoid compounds. The cannabinoid system is linked with all aspects of human physiology and elicits diverse effects by activating the G protein-coupled cannabinoid receptors (CB) type 1 (CB1) and type 2 (CB2) subtypes, the expression of which has been localized on glia, immune cells and neurons throughout the CNS (Downer, 2011). A body of data indicates that cannabinoid ligands control cell genesis in the adult brain, regulating cell proliferation and overall neurogenesis in the mammalian brain (Kochman et al., 2006; Mackowiak et al., 2007). Furthermore, neural progenitor cells (NPCs) express a functional endocannabinoid system (Aguado et al., 2005; Compagnucci et al., 2013) and are producers of endogenous cannabinoids (Butti et al., 2012). Such findings, alongside a number of knockout studies targeting enzymes involved in the biosynthesis and degradation of endocannabinoids (Aguado et al., 2005; Gao et al., 2010), in addition to CB1 (Jin et al., 2004) and CB2 receptors (Palazuelos et al., 2006), place the cannabinoid system as a key player in the processes underlying adult neurogenesis.
Adult neurogenesis
Adult neurogenesis can be loosely divided into four stages: proliferation of neural stem cells (NSCs) and NPCs, migration, neuronal differentiation and finally integration into functional synaptic networks. The two regions in which adult neurogenesis has been most extensively studied are the dentate gyrus of the hippocampus and the lateral ventricles. NSCs in the dentate gyrus reside predominantly in the subgranular zone (SGZ) where four types (type I, type IIa, type IIb and type III) have been characterized based upon proliferation rate, protein expression and morphology. In the murine forebrain, all newborn neurons are derived from type I NPCs, these cells possess a glial-like radial process, although are predominantly unipolar/bipolar in contrast to multipolar astrocytes, express glial fibrillary acidic protein (GFAP) and the intermediate filament protein nestin (Garcia et al., 2004). Type I NSCs are characterized by a low rate of proliferation (Ahn and Joyner, 2005). In contrast, type IIa cells are non-radial, do not express GFAP and exhibit a considerably higher proliferation rate compared with the relatively quiescent type I cells. Type IIa cells maintain nestin expression and both cell types are positive for the Sox gene family (Suh et al., 2007). Type IIb cells maintain important properties of stem cells as they uphold expression of nestin and Sox, but begin to express markers of neuronal committed progenitors, in particular the microtubule-associated protein doublecortin (DCX). If local conditions are favourable, type IIb cells can mature to the nestin negative/DCX positive early neuronal type III cell (Kronenberg et al., 2003).
In lateral ventricles, the subventricular zone (SVZ) contains the majority of ventricular NSCs and is one of the key regions of the brain where neurogenesis occurs throughout adulthood (Curtis et al., 2007). Three cell types have been discovered in the SVZ: type B cells much resemble type I cells in the SGZ; they are GFAP positive, possess a radial process and have a relatively low proliferation rate. Type C cells in the SVZ are reminiscent of type II cells in the SGZ as they are GFAP negative, non-radial and highly proliferative. Both cell types express nestin and Sox (Doetsch et al., 1997). Type A cells represent a population of neuroblasts which migrate at a rate of 30 000 per day along the rostral migratory stream (RMS) to the olfactory bulb (Alvarez-Buylla et al., 2001).
NSCs in both the dentate gyrus and the lateral ventricles have the capacity to produce cells that differentiate to neurons, astrocytes and oligodendrocytes (Gage, 2000). Neuroblasts originating in the SVZ primarily differentiate into olfactory bulb interneurons (Luskin, 1993). Under the right conditions, NSCs in the dentate gyrus can migrate to the granular cell layer and give rise to granular cells that integrate into the hippocampal circuitry forming glutamatergic synapses with granular neurons, interneurons and pyramidal cells in cornu ammonis region 3 (Toni et al., 2008). It has been suggested that these new born granular cells begin to resemble mature neurons, with regard to both their morphology and electrophysiological properties after approximately 4 weeks, although the maturation process continues for several months (Suh et al., 2009). In the young adult rat hippocampus, approximately 9000 new cells are generated each day with 50% of these cells expressing neuronal markers within 5–12 days. Although survival rate is low, it has been estimated that each month the number of new granular cells generated equates to about 6% of the total granular cell number (Cameron and McKay, 2001).
Extrinsic signals in adult neurogenesis
NSC/NPCs are highly sensitive to their microenvironment (i.e. their stem cell niche) and extrinsic signalling molecules largely dictate the proliferative activity and differentiation capacity of these cells. The functions of neurotrophic factors as extrinsic signalling molecules in adult neurogenesis continues to be unravelled, with strong evidence indicating that Trk receptors (and p75NTR co-receptor) are abundant on dividing progenitor cells in the adult primate SVZ/SGZ (Tonchev et al., 2007), with a body of literature indicating that brain-derived neurotrophic factor (BDNF) is a central player in adult neurogenesis. A common method of labelling proliferating cells in the dentate gyrus is to administer the thymidine analogue 5-bromo-2'deoxyuridine (BrdU), which incorporates into the DNA of cells during the S-phase of the cell cycle thus allowing the post-mortem identification of cells that have undergone proliferation. Intrahippocampal infusion of BDNF has been shown to increase the number of cells positive for BrdU and the neuron-specific protein neuronal nuclei in adult rats (Scharfman et al., 2005), while dentate gyrus-specific BDNF RNA interference reduces net neurogenesis in rats by impairing the survival of immature neurons (Taliaz et al., 2010). Similarly, NPC-specific deletion of the high-affinity BDNF receptor TrkB in mice compromises dendritic development and the survival capacity of immature neurons (Bergami et al., 2008), while BDNF-TrkB signalling has been shown to be imperative for hippocampal NSC proliferation in mice (Li et al., 2008). Of note, two other neurotrophic factors have been implicated in the regulation of adult neurogenesis; nerve-growth factor (NGF) has been shown to increase cell proliferation (Birch and Kelly, 2013) and immature neuron survival (Frielingsdorf et al., 2007) in the rat dentate gyrus, while VEGF has also been shown to induce cell proliferation (Jin et al., 2002) and promote immature neuron survival (Schanzer et al., 2004) in the SVZ and SGZ of the adult rat.
In addition to neurotrophic factors, data indicate that several growth factors, including insulin-like growth factor-1 (IGF-1) and FGF-2 are extrinsic factors involved in the regulation of adult neurogenesis. Indeed, s.c. or intraventricular infusion of IGF-1 enhances neurogenesis in the adult rat hippocampus (Aberg et al., 2000), while data from Zhao et al. (2007) demonstrate that conditional deletion of FGFR1 impairs the proliferation of NPCs in the dentate of adult mice (Zhao et al., 2007).
Neurotransmitters are also important regulators of neurogenesis in the adult brain. In particular, Bolteus and Bordey (2004) demonstrated that GABA has a direct effect on migrating neuroblasts in the adult mouse SVZ (Bolteus and Bordey, 2004), while many other studies have delineated the role of GABA in the regulation of NSC proliferative activity, fate decision and synaptic integration of immature neurons (Pallotto and Deprez, 2014). Similarly, glutamate can influence both proliferation and survival of NPCs; activation of the NMDA glutamate receptor has an inhibitory effect on cell proliferation and net neurogenesis in the rat (Cameron et al., 1995) and, in a somewhat paradoxical fashion, induction of LTP at the perforant path-dentate gyrus pathway in rats increases proliferation and survival of NPCs/immature neurons via a NMDA receptor-dependent mechanism (Bruel-Jungerman et al., 2006). Furthermore, the NMDA receptor has been shown to regulate survival of neuroblasts migrating from the mouse SVZ (Platel et al., 2010). Taken together, this suggests a complex role for glutamate in neurogenesis regulation. Additionally, monoamine neurotransmitters such as 5-HT, noradrenaline and dopamine have also been identified as neurogenic modulators, either via direct links in the case of dopamine (Van Kampen et al., 2004) or due to the fact that antidepressants and antipsychotics targeting these systems can affect neurogenesis (Dranovsky and Hen, 2006).
The immune system can also heavily influence the fate of NSCs/NPCs with the antiproliferative and antineuronal differentiative effects of inflammatory cytokines such as IL-6, IL-1β and TNF-α (Kohman and Rhodes, 2013). Elsewhere, the pro-neurogenic effects of the anti-inflammatory cytokine IL-10 have been demonstrated in the amyloid precursor protein/presenilin protein 1 transgenic mouse (Kiyota et al., 2012). Importantly, a body of data indicates that cross-talk may exist between inflammatory mediators (particularly TNF-α) and NSCs/NPCs that may have important consequences for neural development and repair in disease states. Indeed, central administration of TNF-α to rats increases BrdU incorporation in SVZ cells (Wu et al., 2000), while inhibiting endogenous TNF-α signalling regulates the proliferative capacity of mouse neural precursor cells (Rubio-Araiz et al., 2008). In support of this, clear evidence indicates that this cytokine is up-regulated in the mouse brain during demyelination and remyelination, enhancing the proliferative capacity of oligodendrocyte progenitor cells (Arnett et al., 2001). Furthermore, Katakowski et al. (2007) have shown that TNF-α-converting enzyme proteolysis promotes stroke-induced SVZ progenitor cell neurogenesis in rats (Katakowski et al., 2007), indicating that TNF-α signalling may intricately impact neural development and brain repair, particularly in stroke pathogenesis.
Finally, several hormones including thyroid hormones (Remaud et al., 2014), glucocorticoids and, perhaps more speculatively, oxytocin (Schoenfeld and Gould, 2012) have been linked to neurogenesis regulation.
Intrinsic signals in adult neurogenesis
A large body of research has delineated the multiple mechanisms regulating events associated with adult neurogenesis, including cell proliferation, differentiation, maturation, migration and integration of neural cells into neuronal networks (Gage, 2000). Furthermore, through studies predominantly performed in rodents, the complexity of the cellular and molecular signalling processes regulating neurogenesis in the mammalian brain continues to be deciphered. It is now clear that key intrinsic signalling pathways involving Sonic Hedgehog (Shh), Wnt, bone morphogenetic protein (BMP), Notch and transcription factors are intimately associated with adult neurogenesis (Faigle and Song, 2013).
Shh is a signalling glycoprotein which acts through the patched 1 (Ptc1)–smoothened (Smo) receptor complex to activate intricate signal transduction pathways involved in the development of the CNS (Ruiz i Altaba et al., 2002). Indeed, Ptc and Smo are expressed in the adult hippocampus (Traiffort et al., 1998) and conditional deletion of Smo reduces the proliferation of progenitor cells in the postnatal hippocampus and SVZ (Machold et al., 2003). In support of this, pharmacological inhibition of Shh signalling has been shown to reduce granule cell proliferation in the adult rat dentate gyrus (Lai et al., 2003). More recent evidence also indicates that Shh signalling mediates cellular migration in the adult mouse mammalian brain (Balordi and Fishell, 2007), indicating the multifaceted role of Shh signalling in neurogenesis.
The Wnt signalling pathway is a long-standing player in the regulation of adult neurogenesis (McMahon and Bradley, 1990). Wnt ligands are a family of glycoproteins that play a role in the maturation of neurons, remodelling of axons and the maintenance of adult tissue homeostasis (Clevers and Nusse, 2012). Indeed, Wnt signalling, via β-catenin, mediates cellular differentiation in adult-derived mouse hippocampal progenitor cells (Lie et al., 2005) and data elsewhere indicates that Wnt-mediated neurogenesis requires NeuroD1 in adult mouse hippocampal NPCs (Gao et al., 2009). Overall, loss of function of Wnt signalling is strongly associated with determining the development of CNS disorders (De Ferrari and Inestrosa, 2000; Lovestone et al., 2007).
BMPs are members of the TGF-β superfamily and consist of at least 20 growth factors that act as key regulators of axonal growth in a number of neuronal populations (Hegarty et al., 2013). Indeed, clear evidence indicates that BMPs act as potent inhibitors of neuronal differentiation in the adult mouse SVZ (Lim et al., 2000), while Mira et al. (2010) have demonstrated that inhibition of BMP signalling in adult mouse SGZ neural precursor cells differentially regulates neurogenesis.
The components of the Notch signalling pathway are expressed in the SVZ and SGZ of the adult mammalian brain and data indicates that this pathway, through the inhibition of proneural genes, is a key regulator of neurogenesis in the CNS (Irvin et al., 2004). Indeed, Notch signalling is associated with reducing the adult mouse neural progenitor pool (Hitoshi et al., 2002) and promoting the self-renewal of nestin-expressing cells in the adult mouse SGZ (Ables et al., 2010). Interestingly, recent evidence indicates that cross-talk between Notch and EGFR signalling exist, with downstream consequences on NSCs/NPCs in the adult mouse SVZ (Aguirre et al., 2010). Furthermore, Notch 1 knockout mice demonstrate a reduction in dendritic trees associated with granule cells in the mouse dentate gyrus (Ables et al., 2010), highlighting the intrinsic role of Notch signalling in an array of neurodevelopmental cellular processes.
Recently, several transcription factors have been highlighted for their role in adult neurogenesis. In addition to the long-standing role of cAMP response element-binding protein (CREB) in regulating cell development (Finkbeiner et al., 1997), more recent data indicate that CREB phosphorylation robustly enhances progenitor cell proliferation and controls the survival of new neurons in the adult mouse hippocampus in vivo (Jagasia et al., 2009). Interestingly, overexpression of Ascl1 transcription factor regulates the fate of oligodendrocytes in the mouse SGZ in vivo (Jessberger et al., 2008) and both the orphan nuclear receptor Tlx (Zhang et al., 2008) and Sox2 gene family (Ferri et al., 2004) are central in regulating NSC proliferation in the mouse hippocampus. In support of this data indicating that transcription factors are strongly linked to neural differentiation in the rodent brain in vivo, further evidence has identified that Tbr2 (Hodge et al., 2012) and distal-less (Brill et al., 2008) are also associated with neural differentiation in the mouse dentate gyrus and olfactory bulb respectively.
Cannabinoids
The Cannabis plant has been utilized by humans in several capacities for thousands of years and Western medicine has recognized its therapeutic potential since the late 1800s (Reynolds, 1890). Today, this potential is still recognized (Robson, 2014) and the properties of the endocannabinoid system continue to be deciphered.
The CB1 receptor was first described and cloned in the early 1990s (Matsuda et al., 1990; Gerard et al., 1991); it was found to be abundantly expressed throughout the CNS, and, in particular, in areas associated with learning and memory including the hippocampus (Herkenham et al., 1990). A second cannabinoid receptor, the CB2 receptor, was also cloned in the 1990s (Munro et al., 1993) where it was initially thought to be localized to the periphery; however, its expression in the CNS has been demonstrated (Gong et al., 2006). Shortly after the identification of these receptors [receptor nomenclature follows (Alexander et al., 2013a)], their endogenous ligands, known as endocannabinoids, were discovered. The two endocannabinoids that have been studied in most detail are N-arachidonoylethanolamide (also known as anandamide; AEA) (Devane et al., 1992) and 2-arachidonoylglycerol (2-AG) (Mechoulam et al., 1995). AEA is a phospholipid-derived molecule that is an agonist at the CB1 and CB2 receptor; it is detectable peripherally in the plasma and throughout the mammalian brain; in particular. it is found at high concentrations in the hippocampus, cerebellum and cortex (Felder and Glass, 1998). AEA is rapidly synthesized in neurons following depolarization and subsequent Ca2+ influx (Dimarzo et al., 1994). 2-AG, similar to AEA, is synthesized in an activity-dependent manner, is ubiquitously found in the CNS and is both a CB1 and CB2 receptor agonist; however, the concentration of 2-AG is up to 1000 times that of AEA (Sugiura et al., 1995). In neuronal signaling, endocannabinoids function as retrograde neurotransmitters; they are synthesized and released by a postsynaptic neuron and activate receptors on presynaptic neurons (Wilson and Nicoll, 2001). Deactivation of endocannabinoids occurs through specific enzymatic reactions. Fatty acid amide hydrolase (FAAH) is an intracellular membrane-bound enzyme that degrades fatty acid amides and it is responsible for inactivating AEA by catalyzing its breakdown to arachidonic acid (AA) and ethanolamine (Cravatt et al., 1996). Deactivation of 2-AG is primarily achieved by the enzyme monoacylglycerol lipase again producing AA (Dinh et al., 2002).
In addition to endogenous cannabinoid receptor ligands, other classes of cannabinoids have been identified. The identification of Cannabis plant-derived cannabinoids, or phytocannabinoids, including cannabinol, cannabidiol (CBD) and the main psychoactive component of the plant Δ9-tetrahydrocannabinol (THC), preceded the discovery of endocannabinoids by several decades (Mechoulam et al., 2014). To date, it has been suggested that there is over 100 phytocannabinoids and novel cannabinoids continue to be isolated from the C. sativa plant (Radwan et al., 2009). Moreover, many synthetic agonists, inverse agonists and antagonists of the cannabinoid receptors have been produced. The synthetic cannabinoids HU-210 and R-(+)-WIN55212 show a high affinity for both the CB1 and CB2 receptor (Rinaldi-Carmona et al., 1994), while selective agonists have also been identified including the CB1 selective agonist arachidonyl-2′-chloroethylamide (ACEA) (Hillard et al., 1999) and the CB2 selective agonist JWH-133 (Huffman et al., 1999). Other synthetic ligands that bind cannbinoid receptors but evoke inhibitory effects include SR141716A and SR144528 which exert CB1 and CB2 selectivity respectively (Rinaldi-Carmona et al., 1994; 1998,), as well as the high-affinity CB1 ligand AM251 (Gatley et al., 1996) and the high-affinity CB2 ligand AM630 (Ross et al., 1999). Several lines of evidence suggest that theses ligands not only result in receptor antagonism but also inverse agonism (Pertwee, 2005).
In vivo effect of cannabinoids on adult neurogenesis
In addition to the various neurogenesis regulators discussed earlier, there is considerable evidence to suggest that both exogenous and endogenous cannabinoids can control cell genesis in the adult brain, although the effects can vary considerably according to the cannabinoid, dose and duration of administration (see Table 2014). What appears to be a common characteristic of both synthetic (Mackowiak et al., 2007) and plant-derived (Kochman et al., 2006) cannabinoids is that acute administration has no effect on cell proliferation or overall neurogenesis in the hippocampus; however, chronic administration of exogenous cannabinoids has been shown to affect the process. For example, chronic treatment with the potent synthetic cannabinoid HU-210, a drug that has a high affinity for both CB1 and CB2 receptors, enhances both proliferation and survival of cells in the rat dentate gyrus (Jiang et al., 2005). Similarly, chronic administration of the CB2 selective agonist HU-308 also exhibits proliferative-enhancing affects (Palazuelos et al., 2012), raising the possibility that these effects may be mediated, at least in part, by CB2 receptor signalling. This is supported by evidence that a number of BrdU+ cells in the dentate gyrus are reduced in CB2-deficient mice (Palazuelos et al., 2006). In contrast to this, chronic administration of another synthetic CB1/CB2 agonist WIN55,212-2 to rats during adulthood was found to have no effect on the number of immature neurons in the dentate gyrus, however, interestingly, administration during adolescence decreased the number of immature neurons, an affect that is attributed to selective suppression of dorsal but not ventral hippocampal neurogenesis (Abboussi et al., 2014). Further contrasting effects are observed in the aged brain where WIN55,212-2 administration partially restored age-related deficits in hippocampal neurogenesis in rats (Marchalant et al., 2009), suggesting a unique temporal role for cannabinoid receptors in the regulation of neurogenesis throughout the lifespan. The effects of the phytocannabinoid Δ9-THC appear to be dose- and/or time-dependent; 3 weeks of oral administration of a weekly escalating dose of Δ9-THC was found to have no effect on cell proliferation in the mouse dentate gyrus (Kochman et al., 2006), whereas, 6 weeks of oral administration of a static dose of Δ9-THC has been shown to decrease cell proliferation without having an effect on overall neurogenesis in mice (Wolf et al., 2010). Interestingly, the study by Wolf et al. (2010) found that chronic administration of another phytocannabinoid CBD also decreased proliferation but, strikingly, and perhaps appearing somewhat counterintuitive, is that CBD induced a substantial increase in net neurogenesis by a CB1 receptor-dependent mechanism (Wolf et al., 2010). These data are supported by evidence that repeated administration of CBD to wild-type mice increases hippocampal NPC proliferation via CB1 receptors, which may underlie the anxiolytic effect of CBD in chronically stressed animals (Campos et al., 2013).
Table 1.
Literature assessing the in vivo effects of cannabinoids in neurogenesis
| Treatment | Measurement | Observation | Reference |
|---|---|---|---|
| HU-210 | Cell proliferation in the dentate gyrus in adult rats | Enhanced | Jiang et al. (2005) |
| HU-308 | Hippocampal progenitor proliferation in adult mice | Enhanced | Palazuelos et al. (2012) |
| WIN55,212-2 | Dorsal hippocampal neurogenesis during adolescence | Reduced | Abboussi et al. (2014) |
| WIN55,212-2 | Age-related deficits in hippocampal neurogenesis | Partial restoration | Marchalant et al. (2009) |
| Δ9-THC/CBD | Precursor cell proliferation in the dentate gyrus | Reduced | Wolf et al. (2010) |
| CBD | Cell survival in the dentate gyrus | Enhanced | Wolf et al. (2010) |
| CBD | Number of BrdU+ cells colocalized with NeuN+ cells in hippocampus | Enhanced | Campos et al. (2013) |
| DAGL inhibitor | Cell proliferation in the adult SVZ | Reduced | Goncalves et al. (2008) |
| URB597/AEA/WIN55,212-2 | Adult hippocampal NPC proliferation | Enhanced | Aguado et al. (2005) |
| WIN55,212-2/JWH-133/URB597 | Progenitor cell proliferation in the SVZ | Enhanced | Goncalves et al. (2008) |
| AM251 | Cell proliferation in the SGZ | Enhanced | Hill et al. (2006) |
| AM251 | Cell proliferation in the SGZ | Enhanced at 24 h/ reduced at 48 h | Wolf et al. (2010) |
| FAAH−/− | Cell proliferation in the dentate gyrus of adult mice | Enhanced | Aguado et al. (2005) |
| DAGLα−/− | Cell proliferation and number of DCX+ neurons in the hippocampus | Reduced | Gao et al. (2010) |
| DAGLβ−/− | Cell proliferation in the hippocampus | Reduced | Gao et al. (2010) |
| CB1−/− | Cell proliferation in the dentate gyrus and SVZ | Reduced | Jin et al. (2004) |
| Kim et al. (2006) | |||
| CB1−/− | Number of BrdU+ cells colocalized with S100β+ cells in the SGZ and granule cell layer of the dentate gyrus | Reduced | Aguado et al. (2006) |
| CB1−/− | Number of BrdU+ cells colocalized with NeuN+ cells in the SGZ and granule cell layer of the dentate gyrus | Enhanced | Aguado et al. (2006) |
| CB1−/− | Kainic acid-induced hippocampal NPC proliferation | Reduced | Aguado et al. (2007) |
| CB1−/− | Cortical thickness | Reduced at P2 | Diaz-Alonso et al. (2012) |
| SR141716A | Cell proliferation in the SVZ | Enhanced | Jin et al. (2004) |
| JTE-907/AM630 | Cell proliferation in the SVZ | Reduced | Goncalves et al. (2008) |
| CB2−/− | Number of BrdU+ cells in dentate gyrus | Reduced | Palazuelos et al. (2006) |
JTE-907 and AM630 are CB2 receptor antagonists. NeuN, neuronal nuclei.
The CB1 receptor inverse agonist AM251 is often used to oppose the effects of endocannabinoids at the receptor and acute administration of this drug increases cell proliferation in the SGZ 24 h post-treatment (Hill et al., 2006; Wolf et al., 2010). However, this increase reverts to a decrease from 48 h onwards (Wolf et al., 2010), again suggesting a complex temporal role for cannabinoid signalling in NSC fate. Chronically, the same inverse agonist was found to have no effect (Rivera et al., 2011); however, it has been shown to block the proliferative-enhancing effects of aerobic exercise (Hill et al., 2010). This raises the possibility that endocannabinoid signalling via the CB1 receptor may not be important for basal regulation of NPCs, but rather is essential for mediating the effects of exercise, which is a well-established, potent neurogenesis stimulator (van Praag, 2009). Another drug used to inhibit endocannabinoid activity, the CB1 and transient receptor potential cation channel subfamily V member 1 (TRPV1) antagonist SR141716A, has been shown to increase cell proliferation in the dentate gyrus and the lateral ventricles of mice (Jin et al., 2004). This effect was observed in both wild-type and CB1, but not TRPV1, knockout mice. Furthermore, Aguado et al. (2006) have observed reduced astrogliogenesis and increased neurogenesis in CB1-deficient mice (Aguado et al., 2006). These findings illustrate that multiple receptors are responsible for the effects of cannabinoids on neurogenesis, which may account for the complexity of the results observed.
Studies utilizing gene knockdown technology to limit the activity of the endocannabinoid system have provided compelling evidence linking cannabinoids and neurogenesis in the adult brain. Knockdown of the enzyme responsible for AEA hydrolysis, FAAH, increases cell proliferation in the dentate gyrus of adult mice (Aguado et al., 2005), while Goncalves et al. (2008) have demonstrated that chronic inhibition of the enzyme responsible for the production of 2-AG almost completely abolished cell proliferation in the mouse SVZ, while inhibiting FAAH also increased neurogenesis (Goncalves et al., 2008). These findings illustrate the importance of basal endocannabinoid tone in maintaining neurogenesis. Elsewhere, complete knockdown of the α subtype of the DAG lipase α (DAGLα) enzyme reduces brain 2-AG and AEA levels by approximately 80% and 40%, respectively, and furthermore leads to a decrease in cell proliferation rate and a 50% reduction in immature DCX positive neurons in the mouse hippocampus (Gao et al., 2010). The same study shows that a reduction in central 2-AG alone can also interfere with neurogenesis; knockdown of the DAGLβ subtype reduces 2-AG levels in the brain without significantly affecting AEA and results in a decrease in cell proliferation in the hippocampus. Further evidence supporting a role for endocannabinoid signalling in adult hippocampal neurogenesis can be found in studies involving cannabinoid receptor knockout animals; a CB1−/− genotype is accompanied by a 50% decrease in proliferating cells in the dentate gyrus (Jin et al., 2004; Kim et al., 2006). Furthermore, Aguado and colleagues (2007) have demonstrated that kainic acid-induced hippocampal NPC proliferation is attenuated in CB1−/− mice, indicating the role of CB1 in neurogenesis induced by excitotoxicity. Intricate data from the same group indicates that CB1−/− mice have reduced cortical thickness at postnatal day 2, indicating the integral role of CB1 receptors in controlling the specification of upper- and deep-layer cortical neurons (Diaz-Alonso et al., 2012). Finally, CB2−/− animals also exhibit a decreased proliferation rate illustrating the importance of both the CB1 and CB2 receptors (Palazuelos et al., 2006). Taken together, these studies suggest that the endocannabinoid system, acting via multiple complex mechanisms, is a key player in the regulation of adult neurogenesis in vivo.
In vitro effect of cannabinoids on adult neurogenesis
It is known that NPCs (Aguado et al., 2005) express a functional endocannabinoid system and are targeted by cannabinoids to promote neurosphere generation and NPC proliferation (see Table 2000). In addition, endocannabinoids are central in regulating neural differentiation and migration. Indeed, in embryonic murine precursors derived from the cortex, AEA enhances cell differentiation towards a neuronal lineage via a CB1-dependent mechanism (Compagnucci et al., 2013). Furthermore, using freshly dissected RMS tissue from the postnatal brain, Oudin et al. (2011) have shown that endocannabinoid tone is central in controlling neuroblast migration from RMS explants (Oudin et al., 2011). Elsewhere, Butti et al. (2012) demonstrate that SVZ adult mouse NPCs are producers of AEA and that AEA regulates spontaneous EPSCs in medium spiny neurons (Butti et al., 2012). Furthermore, the synthetic cannabinoid WIN-55,212-2, in addition to the selective FAAH inhibitor, URB597, have been shown to promote neurosphere generation, while WIN-55,212-2, URB597 and endocannabinoids (both AEA and 2-AG) increase the number of BrdU+ NPCs from dissociated neurospheres (Aguado et al., 2005). In further experiments from this group using postnatal rat cortical neural progenitors, WIN-55,212-2, URB597, AEA and 2-AG increased the number of GFAP+ cells with a concomitant decrease in β-tubulin III+ cells after differentiation for 2 days, indicating the progliogenic action of synthetic and endogenous cannabinoids during the differentiation process (Aguado et al., 2006). Elsewhere, the CB2 specific agonist AM1241 has been shown to promote the proliferation/differentiation of human NSCs in the presence of the HIV-1 glycoprotein Gp120, and furthermore, AM1241 prevents DNA fragmentation induced by administration of Gp120, which suggests a neuroprotective role of CB2 receptors against impaired neurogenesis, with relevance to the cognitive deficits seen in HIV-1 patients (Avraham et al., 2014). Indeed, CB2 knockout reduces the self-renewal (as determined by neurosphere generation in vitro) of murine embryonic cortical NPCs (Palazuelos et al., 2006), while both HU-308 and JWH-133 increase both primary neurosphere generation and neural progenitor self-renewal in vitro (Palazuelos et al., 2006). Rubio-Araiz et al. demonstrated that both CB1 (ACEA) and CB2 (JWH-056) agonists stimulate the proliferation of primary murine cortical neurospheres (Rubio-Araiz et al., 2008) and recently it has also been demonstrated that hemopressin (a CB1 inverse agonist) promotes oligodendroglial differentiation within SVZ NSC/NPC cultures derived from neonatal mice (Xapelli et al., 2014). In support of this, the CB1 receptor agonist ACEA promotes murine neural precursor differentiation via CB1, with the CB2 receptor agonist JWH-133 being ineffective (Compagnucci et al., 2013).
Table 2.
Literature assessing the in vitro effects of cannabinoids in neurogenesis
| Treatment | Measurement | Observation | Reference |
|---|---|---|---|
| HU-210/AEA | Proliferation of embryonic hippocampal NPCs/NSCs | Enhanced | Jiang et al. (2005) |
| HU-308 | Proliferation of HiB5 NPCs | Enhanced | Palazuelos et al. (2012) |
| HU-308 | Proliferation of cortical progenitors in organotypic cultures | Enhanced | Palazuelos et al. (2012) |
| AEA/ACEA | Differentiation of embryonic murine neural precursors derived from the cortex towards neural lineage | Enhanced | Compagnucci et al. (2013) |
| ACEA/JWH-133 | Migration of Cor-1 NSC line | Enhanced | Oudin et al. (2011) |
| AM251/JTE-907/DAGL inhibitors | RMS neuroblast migration | Reduced | Oudin et al.(2011) |
| ACEA/JWH-133 | RMS neuroblast migration | Enhanced | Oudin et al. (2011) |
| ACEA/JWH-056 | Proliferation of neurospheres | Enhanced | Rubio-Araiz et al. (2008) |
| WIN-55,212-2/URB597 | Neurosphere generation | Enhanced | Aguado et al. (2005) |
| WIN-55,212-2/URB597/ AEA/2-AG | Number of BrdU+ NPCs from dissociated neurospheres | Enhanced | Aguado et al. (2005) |
| WIN-55,212-2/URB597/ AEA/2-AG | Number of GFAP+ cells after differentiation of postnatal NPCs for 2 days | Enhanced | Aguado et al. (2006) |
| WIN-55,212-2/URB597/ AEA/2-AG | Number of β-tubulin III+ cells after differentiation of postnatal NPCs for 2 days | Decreased | Aguado et al. (2006) |
| AM1241 | Proliferation/differentiation of human NSCs in presence of Gp120 | Enhanced | Avraham et al. (2014) |
| CB2−/− | Neurosphere generation of murine embryonic cortical NPCs | Reduced | Palazuelos et al. (2006) |
| HU-308/JWH-133 | Primary neurosphere generation and NPC self-renewal | Increased | Palazuelos et al. (2006) |
| Hemopressin | Oligodendroglial differentiation within SVZ NPC/NSC cultures | Increased | Xapelli et al. (2014) |
Hemopressin is a CB1 inverse agonist.
Mechanisms of cannabinoid-induced regulation of intrinsic/extrinsic signalling in adult neurogenesis
The cellular signalling events orchestrated by cannabinoids in NPCs continue to be elucidated, with particular roles for ERK, PI3K and Akt pathways suggested (see Figure 1). In particular, CB2 couples to the ERK and PI3K/Akt cascades (Palazuelos et al., 2006; 2012; Molina-Holgado et al., 2007) and the CB2 agonist HU-308 promotes the proliferation of NPCs via ERK and PI3K/Akt signalling (Palazuelos et al., 2006). In support of this, HU-308 is a robust activator of the PI3K/Akt pathway in the HiB5 hippocampal progenitor cell line (Palazuelos et al., 2012). Interestingly, mammalian target of rapamycin complex 1 (mTORC1) signalling is a target of the PI3K/Akt pathway and hence is central in neural cell survival/death decision; mTORC signalling also contributes to CB2-regulated NPC proliferation. Indeed, HU-308 induces cell proliferation in both embryonic organotypic cortical slices and in adult hippocampal NPCs via an mTORC1-dependent mechanism (Palazuelos et al., 2012). Elsewhere, both CB1 (ACEA) and CB2 (JWH-056) agonists have been shown to stimulate the proliferation of mouse neural precursor cells via PI3K/Akt pathways (Molina-Holgado et al., 2007) and TNF-α signalling mechanisms (Rubio-Araiz et al., 2008). Both the synthetic cannabinoid HU-210 and AEA promote the proliferation of cultured embryonic hippocampal NPCs in a concentration-dependent manner involving Gi/o proteins and the ERK signalling pathways (Jiang et al., 2005). Further in vitro evidence indicates that ACEA enhances murine neural precursor differentiation to neurons by targeting ERK signalling (Compagnucci et al., 2013). In addition, ACEA reduces ERK phosphorylation in neural precursor cells and this reduction promotes neuronal differentiation. Using neurogenesis and PCR arrays, Compagnucci et al. (2013) recently demonstrated that CB1 activation promotes the expression of genes involved in neuronal maturation and commitment to a neuronal lineage (Compagnucci et al., 2013). In contrast, the endogenous cannabinoid AEA has been shown to inhibit cortical neuron progenitor differentiation to mature neuronal phenotype, decrease the proliferation of primary postnatal murine NPCs (Soltys et al., 2010) and inhibit the differentiation of the human NSC line, HNSC.100 (Rueda et al., 2002). These events are CB1 receptor-dependent and as AEA inhibits NGF-induced ERK activation in PC12 cells via CB1 receptors, this suggests that AEA inhibits NPC differentiation through attenuation of the ERK pathway (Rueda et al., 2002).
Figure 1.
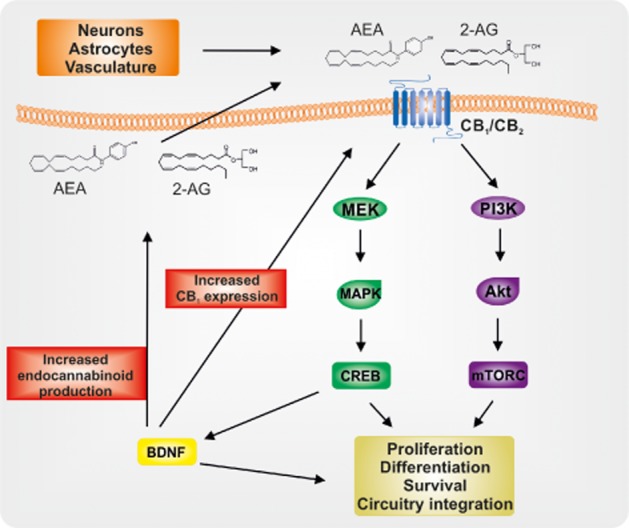
Endocannabinoid signalling regulates NPCs in the adult brain. Endocannabinoids acting in an autocrine and paracrine fashion may activate CB1 and/or CB2 receptors. CB1 and CB2 activity can induce both PI3K/Akt/mTORC and MEK/MAPK/CREB signalling pathways that influence cell proliferation, differentiation and survival, while also promoting integration of immature neurons into existing circuitry. In addition, CREB can induce transcription of BDNF that can directly influence cell fate and may also increase CB1 expression and endocannbinoid production, possibly leading to positive feedback within the signalling system.
Further data elsewhere indicate that signalling involving CREB transcription factor may govern cannabinoid-induced regulation of NPCs. Indeed, exposure of murine NPCs to AEA promotes glial and neuronal differentiation, with a possible role for CREB (Soltys et al., 2010). Much data indicate that CREB is a cannabinoid target, with recent evidence indicating that CB2 agonists target CREB signalling in the rat cortex after subarachnoid haemorrhage (Fujii et al., 2014) and cerebral ischaemia (Choi et al., 2013). In support of this, THC (Casu et al., 2005) and AEA (Isokawa, 2009) administration has been shown to regulate the expression of phosphorylated CREB in the rat cerebellum and hippocampus, respectively, while the CB2 receptor agonist, trans-caryophyllene, promotes the phosphorylation of neural CREB (Choi et al., 2013).
The Sox2 gene family regulate NSC proliferation in the hippocampus and recent evidence indicates that CB1 receptor activation enhances the number of Sox2+ cells via Notch signalling in cultured mouse SVZ cells, suggesting that CB1 receptor activation promotes the self-renewal of SVZ cultures (Xapelli et al., 2013). Cannabinoids also regulate the expression of the T-box transcription factor, Tbr, which may be central in mediating the neurogenic effects of cannabinoids. Indeed, Saez et al. (2014) has recently demonstrated that prenatal exposure of rats to WIN-55,212-2 differentially regulates the number of glutamatergic intermediate progenitors (Tbr2+) and post-mitotic neurons (Tbr1+) during embryonic development in the cortex (Saez et al., 2014). Interestingly, this indicates that prenatal exposure to WIN-55,212-2 impacts the differentiation of glutamatergic neurons in the developing cerebral cortex. In support of this, data from CB1-deficient murine embryos indicate that there is a decrease in Tbr2+ cells in the SVZ (Diaz-Alonso et al., 2014) while Tbr1+ post-mitotic cells accumulate abnormally during embryogenesis in deep bins of the cortical plate of CB1-deficient mice when compared with wild-type littermates (Diaz-Alonso et al., 2012).
Neurotrophic factors are strongly linked to adult neurogenesis and recent evidence suggests that there is functional interplay between BDNF and CB1 receptors in the brain (De Chiara et al., 2010). In support of this, Maison et al. (2009) demonstrated that BDNF increases the expression of CB1 receptors in rat cultured cerebellar granule neurons (Maison et al., 2009), while BDNF can also promote the production of cortical endocannabinoids (Lemtiri-Chlieh and Levine, 2010). In human studies, D'Souza et al. (2009) demonstrated that i.v. administration of THC enhances the expression of peripheral BDNF in serum (D'Souza et al., 2009) and this is supported by evidence that CB2 receptor stimulation promotes BDNF expression in rat neurons (Choi et al., 2013). Recent evidence also suggests that CB1 receptors can cross-talk with NGF signalling in adult mouse dorsal root ganglion neurons (Wang et al., 2014). In addition, intricate new data from Keimpema et al. (2013) indicate that NGF affects endocannabinoid signalling to promote cholinergic differentiation in mice (Keimpema et al., 2013).
A body of literature indicates that signalling involving adenosine, PKC, growth factors and IL-1 receptors may govern cannabinoid-induced regulation of NPCs. Indeed, using adult neural precursor cells prepared from the whole brains of 8-week-old mice, Shinjoy and Di Marzo (2013) recently demonstrated that the major non-THC phytocannabinoid, cannabichromene (CBC), promotes cell survival during differentiation while blunting cell differentiation into astroglia. The authors suggest the involvement of ERK, ATP and adenosine signalling cascades in mediating the effects of CBC on neural cells (Shinjyo and Di Marzo, 2013). Recent evidence also indicates that cannabinoids can target the actin-bundling protein fascin, which plays a role in the migration of neuroblasts and neural development (Sonego et al., 2013). Indeed, the CB1 agonist ACEA controls the interaction between fascin and PKC, which indicates that CB1-dependent signalling may regulate actin-bundling activity, with a subsequent effect on neuroblast migration (Sonego et al., 2013). EGFR signalling is key in controlling NSC survival, and using the Cor-1 NSC line, data from Sutterlin et al. (2013) demonstrate that CB1 and CB2 receptors cooperate with EGFR in the regulation of NSC expansion (Sutterlin et al., 2013). Similarly, the CB1 receptor has been shown to couple activated FGF receptors to axonal growth in rat cerebellar granule neurons (Williams et al., 2003). Finally, Garcia-Ovejero et al. (2013) have demonstrated that both CB1 and CB2 receptors are co-expressed with IL-1 receptor, type I and IL-1 receptor, type II in mouse brain neurospheres and both ACEA and JWH-133 affect IL-1 signalling in primary cultures of mouse brain-derived neurospheres, increasing IL-1β, while decreasing IL-1Rα production by neurospheres. This is significant given that IL-1β negatively regulates neurosphere proliferation (Garcia-Ovejero et al., 2013).
Concluding remarks
While much progress has been made in recent decades in understanding the process of adult neurogenesis, the underlying mechanisms have yet to be fully elucidated. As highlighted in this review, the microenvironment clearly determines the rate of proliferation of NSCs and NPCs, their survival and their differentiation into mature neurons that are integrated into functional networks. Endocannabinoids may play pivotal roles in at least some of these phases of neurogenesis. Of particular interest are the varying temporal effects of synthetic, endogenous and plant-derived cannabinoids on the proliferation and survival phases of neurogenesis, indicating complex physiological regulation of this process that may be modulated by drugs that target the endocannabinoid system. The functional importance of neurogenesis has yet to be clarified; however, the weight of evidence indicates that impaired neurogenesis is associated with depression and cognitive impairment. Pharmacological targeting of the cannabinoid system as a regulator of neurogenesis may prove a fruitful strategy in the prevention or treatment of mood or memory disorders.
Acknowledgments
This work was supported by the College of Medicine and Health (University College Cork), the Department of Anatomy and Neuroscience, University College Cork, and the Department of Physiology, School of Medicine, Trinity College Dublin.
Glossary
- 2-AG
2-arachidonoylglycerol
- AA
arachidonic acid
- ACEA
arachidonyl-2′-chloroethylamide
- AEA
anandamide
- BDNF
brain-derived neurotrophic factor
- BMP
bone morphogenetic protein
- BrdU
5-bromo-2'deoxyuridine
- CB
cannabinoid receptor
- CBC
cannabichromene
- CBD
cannabidiol
- CREB
cAMP response element-binding protein
- DAGL
DAG lipase
- DCX
double cortin
- FAAH
fatty acid amide hydrolase
- GFAP
glial fibrillary acidic protein
- IGF-1
insulin-like growth factor-1
- mTORC1
mammalian target of rapamycin complex 1
- NGF
nerve-growth factor
- NPC
neural progenitor cell
- NSC
neural stem cell
- Ptc1
patched 1
- RMS
rostral migratory stream
- SGZ
subgranular zone
- Shh
Sonic Hedgehog
- Smo
smoothened
- SVZ
subventricular zone
- THC
Δ9-tetrahydrocannabinol
- TRPV1
transient receptor potential cation channel subfamily V member 1
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abboussi O, Tazi A, Paizanis E, El Ganouni S. Chronic exposure to WIN55,212-2 affects more potently spatial learning and memory in adolescents than in adult rats via a negative action on dorsal hippocampal neurogenesis. Pharmacol Biochem Behav. 2014;120:95–102. doi: 10.1016/j.pbb.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, et al. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci. 2010;30:10484–10492. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704–1706. doi: 10.1096/fj.05-3995fje. [DOI] [PubMed] [Google Scholar]
- Aguado T, Palazuelos J, Monory K, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci. 2006;26:1551–1561. doi: 10.1523/JNEUROSCI.3101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado T, Romero E, Monory K, Palazuelos J, Sendtner M, Marsicano G, et al. The CB1 cannabinoid receptor mediates excitotoxicity-induced neural progenitor proliferation and neurogenesis. J Biol Chem. 2007;282:23892–23898. doi: 10.1074/jbc.M700678200. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–327. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: ligand-gated ion channels. Br J Pharmacol. 2013b;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: ion channels. Br J Pharmacol. 2013c;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: catalytic receptors. Br J Pharmacol. 2013d;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: enzymes. Br J Pharmacol. 2013e;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- Avraham HK, Jiang S, Fu Y, Rockenstein E, Makriyannis A, Zvonok A, et al. The cannabinoid CB(2) receptor agonist AM1241 enhances neurogenesis in GFAP/Gp120 transgenic mice displaying deficits in neurogenesis. Br J Pharmacol. 2014;171:468–479. doi: 10.1111/bph.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci. 2007;27:5936–5947. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch AM, Kelly AM. Chronic intracerebroventricular infusion of nerve growth factor improves recognition memory in the rat. Neuropharmacology. 2013;75:255–261. doi: 10.1016/j.neuropharm.2013.07.023. [DOI] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill MS, Snapyan M, Wohlfrom H, Ninkovic J, Jawerka M, Mastick GS, et al. A dlx2- and pax6-dependent transcriptional code for periglomerular neuron specification in the adult olfactory bulb. J Neurosci. 2008;28:6439–6452. doi: 10.1523/JNEUROSCI.0700-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Davis S, Rampon C, Laroche S. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci. 2006;26:5888–5893. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butti E, Bacigaluppi M, Rossi S, Cambiaghi M, Bari M, Cebrian Silla A, et al. Subventricular zone neural progenitors protect striatal neurons from glutamatergic excitotoxicity. Brain. 2012;135(Pt 11):3320–3335. doi: 10.1093/brain/aws194. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, Ortega Z, Palazuelos J, Fogaca MV, Aguiar DC, Diaz-Alonso J, et al. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid system. Int J Neuropsychopharmacol. 2013;16:1407–1419. doi: 10.1017/S1461145712001502. [DOI] [PubMed] [Google Scholar]
- Casu MA, Pisu C, Sanna A, Tambaro S, Spada GP, Mongeau R, et al. Effect of delta9-tetrahydrocannabinol on phosphorylated CREB in rat cerebellum: an immunohistochemical study. Brain Res. 2005;1048:41–47. doi: 10.1016/j.brainres.2005.04.053. [DOI] [PubMed] [Google Scholar]
- Choi IY, Ju C, Anthony Jalin AM, da Lee I, Prather PL, Kim WK. Activation of cannabinoid CB2 receptor-mediated AMPK/CREB pathway reduces cerebral ischemic injury. Am J Pathol. 2013;182:928–939. doi: 10.1016/j.ajpath.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Compagnucci C, Di Siena S, Bustamante MB, Di Giacomo D, Di Tommaso M, Maccarrone M, et al. Type-1 (CB1) cannabinoid receptor promotes neuronal differentiation and maturation of neural stem cells. PLoS ONE. 2013;8:e54271. doi: 10.1371/journal.pone.0054271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Eriksson PS, Faull RL. Progenitor cells and adult neurogenesis in neurodegenerative diseases and injuries of the basal ganglia. Clin Exp Pharmacol Physiol. 2007;34:528–532. doi: 10.1111/j.1440-1681.2007.04609.x. [DOI] [PubMed] [Google Scholar]
- De Chiara V, Angelucci F, Rossi S, Musella A, Cavasinni F, Cantarella C, et al. Brain-derived neurotrophic factor controls cannabinoid CB1 receptor function in the striatum. J Neurosci. 2010;30:8127–8137. doi: 10.1523/JNEUROSCI.1683-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari GV, Inestrosa NC. Wnt signaling function in Alzheimer's disease. Brain Res Brain Res Rev. 2000;33:1–12. doi: 10.1016/s0165-0173(00)00021-7. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Diaz-Alonso J, Aguado T, Wu CS, Palazuelos J, Hofmann C, Garcez P, et al. The CB(1) cannabinoid receptor drives corticospinal motor neuron differentiation through the Ctip2/Satb2 transcriptional regulation axis. J Neurosci. 2012;32:16651–16665. doi: 10.1523/JNEUROSCI.0681-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Alonso J, Aguado T, de Salas-Quiroga A, Ortega Z, Guzman M, Galve-Roperh I. CB1 cannabinoid receptor-dependent activation of mTORC1/Pax6 signaling drives Tbr2 expression and basal progenitor expansion in the developing mouse cortex. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu039. [DOI] [PubMed] [Google Scholar]
- Dimarzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Freund TF, Piomelli D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids. 2002;121:149–158. doi: 10.1016/s0009-3084(02)00150-0. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer EJ. Cannabinoids and innate immunity: taking a toll on neuroinflammation. Scientificworldjournal. 2011;11:855–865. doi: 10.1100/tsw.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza DC, Pittman B, Perry E, Simen A. Preliminary evidence of cannabinoid effects on brain-derived neurotrophic factor (BDNF) levels in humans. Psychopharmacology (Berl) 2009;202:569–578. doi: 10.1007/s00213-008-1333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta. 2013;1830:2435–2448. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Glass M. Cannabinoid receptors and their endogenous agonists. Annu Rev Pharmacol Toxicol. 1998;38:179–200. doi: 10.1146/annurev.pharmtox.38.1.179. [DOI] [PubMed] [Google Scholar]
- Ferri AL, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Frielingsdorf H, Simpson DR, Thal LJ, Pizzo DP. Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiol Dis. 2007;26:47–55. doi: 10.1016/j.nbd.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Fujii M, Sherchan P, Soejima Y, Hasegawa Y, Flores J, Doycheva D, et al. Cannabinoid receptor type 2 agonist attenuates apoptosis by activation of phosphorylated CREB-Bcl-2 pathway after subarachnoid hemorrhage in rats. Exp Neurol. 2014;261:396–403. doi: 10.1016/j.expneurol.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, et al. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Garcia-Ovejero D, Arevalo-Martin A, Navarro-Galve B, Pinteaux E, Molina-Holgado E, Molina-Holgado F. Neuroimmmune interactions of cannabinoids in neurogenesis: focus on interleukin-1β (IL-1β) signalling. Biochem Soc Trans. 2013;41:1577–1582. doi: 10.1042/BST20130198. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Gifford AN, Volkow ND, Lan RX, Makriyannis A. I-123-labeled AM251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors. Eur J Pharmacol. 1996;307:331–338. doi: 10.1016/0014-2999(96)00279-8. [DOI] [PubMed] [Google Scholar]
- Gerard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves MB, Suetterlin P, Yip P, Molina-Holgado F, Walker DJ, Oudin MJ, et al. A diacylglycerol lipase-CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age-dependent manner. Mol Cell Neurosci. 2008;38:526–536. doi: 10.1016/j.mcn.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Hegarty SV, O'Keeffe GW, Sullivan AM. BMP-Smad 1/5/8 signalling in the development of the nervous system. Prog Neurobiol. 2013;109:28–41. doi: 10.1016/j.pneurobio.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Kambo JS, Sun JC, Gorzalka BB, Galea LA. Endocannabinoids modulate stress-induced suppression of hippocampal cell proliferation and activation of defensive behaviours. Eur J Neurosci. 2006;24:1845–1849. doi: 10.1111/j.1460-9568.2006.05061.x. [DOI] [PubMed] [Google Scholar]
- Hill MN, Titterness AK, Morrish AC, Carrier EJ, Lee TT, Gil-Mohapel J, et al. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus. 2010;20:513–523. doi: 10.1002/hipo.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, et al. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J Pharmacol Exp Ther. 1999;289:1427–1433. [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, et al. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge RD, Nelson BR, Kahoud RJ, Yang R, Mussar KE, Reiner SL, et al. Tbr2 is essential for hippocampal lineage progression from neural stem cells to intermediate progenitors and neurons. J Neurosci. 2012;32:6275–6287. doi: 10.1523/JNEUROSCI.0532-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JW, Liddle J, Yu S, Aung MM, Abood ME, Wiley JL, et al. 3-(1′,1′-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorg Med Chem. 1999;7:2905–2914. doi: 10.1016/s0968-0896(99)00219-9. [DOI] [PubMed] [Google Scholar]
- Irvin DK, Nakano I, Paucar A, Kornblum HI. Patterns of Jagged1, Jagged2, Delta-like 1 and Delta-like 3 expression during late embryonic and postnatal brain development suggest multiple functional roles in progenitors and differentiated cells. J Neurosci Res. 2004;75:330–343. doi: 10.1002/jnr.10843. [DOI] [PubMed] [Google Scholar]
- Isokawa M. Time-dependent induction of CREB phosphorylation in the hippocampus by the endogenous cannabinoid. Neurosci Lett. 2009;457:53–57. doi: 10.1016/j.neulet.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, et al. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Toni N, Clemenson GD, Jr, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci. 2008;11:888–893. doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, et al. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Xie L, Kim SH, Parmentier-Batteur S, Sun Y, Mao XO, et al. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol Pharmacol. 2004;66:204–208. doi: 10.1124/mol.66.2.204. [DOI] [PubMed] [Google Scholar]
- Katakowski M, Chen J, Zhang ZG, Santra M, Wang Y, Chopp M. Stroke-induced subventricular zone proliferation is promoted by tumor necrosis factor-alpha-converting enzyme protease activity. J Cereb Blood Flow Metab. 2007;27:669–678. doi: 10.1038/sj.jcbfm.9600390. [DOI] [PubMed] [Google Scholar]
- Keimpema E, Tortoriello G, Alpar A, Capsoni S, Arisi I, Calvigioni D, et al. Nerve growth factor scales endocannabinoid signaling by regulating monoacylglycerol lipase turnover in developing cholinergic neurons. Proc Natl Acad Sci U S A. 2013;110:1935–1940. doi: 10.1073/pnas.1212563110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kim SH, Won SJ, Mao XO, Ledent C, Jin K, Greenberg DA. Role for neuronal nitric-oxide synthase in cannabinoid-induced neurogenesis. J Pharmacol Exp Ther. 2006;319:150–154. doi: 10.1124/jpet.106.107698. [DOI] [PubMed] [Google Scholar]
- Kiyota T, Ingraham KL, Swan RJ, Jacobsen MT, Andrews SJ, Ikezu T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 2012;19:724–733. doi: 10.1038/gt.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochman LJ, dos Santos AA, Fornal CA, Jacobs BL. Despite strong behavioral disruption, Delta9-tetrahydrocannabinol does not affect cell proliferation in the adult mouse dentate gyrus. Brain Res. 2006;1113:86–93. doi: 10.1016/j.brainres.2006.07.080. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Rhodes JS. Neurogenesis, inflammation and behavior. Brain Behav Immun. 2013;27:22–32. doi: 10.1016/j.bbi.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, et al. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, Levine ES. BDNF evokes release of endogenous cannabinoids at layer 2/3 inhibitory synapses in the neocortex. J Neurophysiol. 2010;104:1923–1932. doi: 10.1152/jn.00472.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovestone S, Killick R, Di Forti M, Murray R. Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci. 2007;30:142–149. doi: 10.1016/j.tins.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Mackowiak M, Chocyk A, Markowicz-Kula K, Wedzony K. Acute activation of CB1 cannabinoid receptors transiently decreases PSA-NCAM expression in the dentate gyrus of the rat hippocampus. Brain Res. 2007;1148:43–52. doi: 10.1016/j.brainres.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Maison P, Walker DJ, Walsh FS, Williams G, Doherty P. BDNF regulates neuronal sensitivity to endocannabinoids. Neurosci Lett. 2009;467:90–94. doi: 10.1016/j.neulet.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Marchalant Y, Brothers HM, Wenk GL. Cannabinoid agonist WIN-55,212-2 partially restores neurogenesis in the aged rat brain. Mol Psychiatry. 2009;14:1068–1069. doi: 10.1038/mp.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Hanus LO, Pertwee R, Howlett AC. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev Neurosci. 2014;15:757–764. doi: 10.1038/nrn3811. [DOI] [PubMed] [Google Scholar]
- Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, et al. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell. 2010;7:78–89. doi: 10.1016/j.stem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F, Rubio-Araiz A, Garcia-Ovejero D, Williams RJ, Moore JD, Arevalo-Martin A, et al. CB2 cannabinoid receptors promote mouse neural stem cell proliferation. Eur J Neurosci. 2007;25:629–634. doi: 10.1111/j.1460-9568.2007.05322.x. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Oudin MJ, Gajendra S, Williams G, Hobbs C, Lalli G, Doherty P. Endocannabinoids regulate the migration of subventricular zone-derived neuroblasts in the postnatal brain. J Neurosci. 2011;31:4000–4011. doi: 10.1523/JNEUROSCI.5483-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Egia A, Mechoulam R, Guzman M, Galve-Roperh I. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006;20:2405–2407. doi: 10.1096/fj.06-6164fje. [DOI] [PubMed] [Google Scholar]
- Palazuelos J, Ortega Z, Diaz-Alonso J, Guzman M, Galve-Roperh I. CB2 cannabinoid receptors promote neural progenitor cell proliferation via mTORC1 signaling. J Biol Chem. 2012;287:1198–1209. doi: 10.1074/jbc.M111.291294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotto M, Deprez F. Regulation of adult neurogenesis by GABAergic transmission: signaling beyond GABAA-receptors. Front Cell Neurosci. 2014;8:166. doi: 10.3389/fncel.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Platel JC, Dave KA, Gordon V, Lacar B, Rubio ME, Bordey A. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron. 2010;65:859–872. doi: 10.1016/j.neuron.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan MM, Elsohly MA, Slade D, Ahmed SA, Khan IA, Ross SA. Biologically active cannabinoids from high-potency Cannabis sativa. J Nat Prod. 2009;72:906–911. doi: 10.1021/np900067k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Neuroscience. No more cortical neurons for you. Science. 2006;313:928–929. doi: 10.1126/science.1131713. [DOI] [PubMed] [Google Scholar]
- Remaud S, Gothie JD, Morvan-Dubois G, Demeneix BA. Thyroid hormone signaling and adult neurogenesis in mammals. Front Endocrinol (Lausanne) 2014;5:62. doi: 10.3389/fendo.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JR. On the therapeutical uses and toxic effects of cannabis indica. Lancet. 1890;135:637–638. [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, et al. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- Rivera P, Romero-Zerbo Y, Pavon FJ, Serrano A, Lopez-Avalos MD, Cifuentes M, et al. Obesity-dependent cannabinoid modulation of proliferation in adult neurogenic regions. Eur J Neurosci. 2011;33:1577–1586. doi: 10.1111/j.1460-9568.2011.07650.x. [DOI] [PubMed] [Google Scholar]
- Robson PJ. Therapeutic potential of cannabinoid medicines. Drug Test Anal. 2014;6:24–30. doi: 10.1002/dta.1529. [DOI] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Araiz A, Arevalo-Martin A, Gomez-Torres O, Navarro-Galve B, Garcia-Ovejero D, Suetterlin P, et al. The endocannabinoid system modulates a transient TNF pathway that induces neural stem cell proliferation. Mol Cell Neurosci. 2008;38:374–380. doi: 10.1016/j.mcn.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Rueda D, Navarro B, Martinez-Serrano A, Guzman M, Galve-Roperh I. The endocannabinoid anandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. J Biol Chem. 2002;277:46645–46650. doi: 10.1074/jbc.M206590200. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Palma V, Dahmane N. Hedgehog-Gli signalling and the growth of the brain. Nat Rev Neurosci. 2002;3:24–33. doi: 10.1038/nrn704. [DOI] [PubMed] [Google Scholar]
- Saez TM, Aronne MP, Caltana L, Brusco AH. Prenatal exposure to the CB1 and CB2 cannabinoid receptor agonist WIN 55,212-2 alters migration of early-born glutamatergic neurons and GABAergic interneurons in the rat cerebral cortex. J Neurochem. 2014;129:637–648. doi: 10.1111/jnc.12636. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, et al. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain pathology. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinjyo N, Di Marzo V. The effect of cannabichromene on adult neural stem/progenitor cells. Neurochem Int. 2013;63:432–437. doi: 10.1016/j.neuint.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Soltys J, Yushak M, Mao-Draayer Y. Regulation of neural progenitor cell fate by anandamide. Biochem Biophys Res Commun. 2010;400:21–26. doi: 10.1016/j.bbrc.2010.07.129. [DOI] [PubMed] [Google Scholar]
- Sonego M, Gajendra S, Parsons M, Ma Y, Hobbs C, Zentar MP, et al. Fascin regulates the migration of subventricular zone-derived neuroblasts in the postnatal brain. J Neurosci. 2013;33:12171–12185. doi: 10.1523/JNEUROSCI.0653-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Sukagawa A, Kondo S, Yamashita A, Waku K. Dual synthetic pathways of N-arachidonoylethanolamine (anandamide) – an endogenous cannabinoid receptor ligand in rat-brain. J Neurochem. 1995;65:S27. [Google Scholar]
- Suh H, Consiglio A, Ray J, Sawai T, D'Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell stem cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- Sutterlin P, Williams EJ, Chambers D, Saraf K, von Schack D, Reisenberg M, et al. The molecular basis of the cooperation between EGF, FGF and eCB receptors in the regulation of neural stem cell function. Mol Cell Neurosci. 2013;52:20–30. doi: 10.1016/j.mcn.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2010;15:80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonchev AB, Yamashima T, Guo J, Chaldakov GN, Takakura N. Expression of angiogenic and neurotrophic factors in the progenitor cell niche of adult monkey subventricular zone. Neuroscience. 2007;144:1425–1435. doi: 10.1016/j.neuroscience.2006.10.052. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traiffort E, Charytoniuk DA, Faure H, Ruat M. Regional distribution of Sonic Hedgehog, patched, and smoothened mRNA in the adult rat brain. J Neurochem. 1998;70:1327–1330. doi: 10.1046/j.1471-4159.1998.70031327.x. [DOI] [PubMed] [Google Scholar]
- Van Kampen JM, Hagg T, Robertson HA. Induction of neurogenesis in the adult rat subventricular zone and neostriatum following dopamine D3 receptor stimulation. Eur J Neurosci. 2004;19:2377–2387. doi: 10.1111/j.0953-816X.2004.03342.x. [DOI] [PubMed] [Google Scholar]
- Wang ZY, McDowell T, Wang P, Alvarez R, Gomez T, Bjorling DE. Activation of CB1 inhibits NGF-induced sensitization of TRPV1 in adult mouse afferent neurons. Neuroscience. 2014;277:679–689. doi: 10.1016/j.neuroscience.2014.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EJ, Walsh FS, Doherty P. The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J Cell Biol. 2003;160:481–486. doi: 10.1083/jcb.200210164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Bick-Sander A, Fabel K, Leal-Galicia P, Tauber S, Ramirez-Rodriguez G, et al. Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun Signal. 2010;8:12. doi: 10.1186/1478-811X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JP, Kuo JS, Liu YL, Tzeng SF. Tumor necrosis factor-alpha modulates the proliferation of neural progenitors in the subventricular/ventricular zone of adult rat brain. Neurosci Lett. 2000;292:203–206. doi: 10.1016/s0304-3940(00)01472-5. [DOI] [PubMed] [Google Scholar]
- Xapelli S, Agasse F, Sarda-Arroyo L, Bernardino L, Santos T, Ribeiro FF, et al. Activation of type 1 cannabinoid receptor (CB1R) promotes neurogenesis in murine subventricular zone cell cultures. PLoS ONE. 2013;8:e63529. doi: 10.1371/journal.pone.0063529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xapelli S, Agasse F, Grade S, Bernardino L, Ribeiro FF, Schitine CS, et al. Modulation of subventricular zone oligodendrogenesis: a role for hemopressin? Front Cell Neurosci. 2014;8:59. doi: 10.3389/fncel.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zhao M, Li D, Shimazu K, Zhou YX, Lu B, Deng CX. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol Psychiatry. 2007;62:381–390. doi: 10.1016/j.biopsych.2006.10.019. [DOI] [PubMed] [Google Scholar]


