Abstract
Vascular dysfunction plays a pivotal role in the development of systemic complications associated with arterial hypertension and diabetes. The endothelium, or more specifically, various factors derived from endothelial cells tightly regulate vascular function, including vascular tone. In physiological conditions, there is a balance between endothelium-derived factors, that is, relaxing factors (endothelium-derived relaxing factors; EDRFs) and contracting factors (endothelium-derived contracting factors; EDCFs), which mediate vascular homeostasis. However, in disease states, such as diabetes and arterial hypertension, there is an imbalance between EDRF and EDCF, with a reduction of EDRF signalling and an increase of EDCF signalling. Among EDCFs, COX-derived vasoconstrictor prostanoids play an important role in the development of vascular dysfunction associated with hypertension and diabetes. Moreover, uridine adenosine tetraphosphate (Up4A), identified as an EDCF in 2005, also modulates vascular function. However, the role of Up4A in hypertension- and diabetes-associated vascular dysfunction is unclear. In the present review, we focused on experimental and clinical evidence that implicate these two EDCFs (vasoconstrictor prostanoids and Up4A) in vascular dysfunction associated with hypertension and diabetes.
Table of Links
| TARGETS | |
|---|---|
| Enzymesa | GPCRsc |
| Akt | AT1 receptor |
| AMPK, AMP-activated protein kinase | EP receptor |
| CDK2, cyclin-dependent kinase 2 | DP receptor |
| COX-1 | GPER (GPR30) |
| COX-2 | IP receptor |
| cPLA2, cytosolic PLA2 | P2Y2 receptor |
| ERK1/2 | P2Y4 receptor |
| HO-1, haem oxygenase-1 | P2Y6 receptor |
| L-PGDS, lipocalin-type PGD synthase | TP receptor |
| NOS | Catalytic receptorsd |
| PGIS, prostacyclin synthase (CYP8A1) | PDGFR, PDGF receptor |
| TxS, thromboxane synthase (CYP5A1) | Nuclear hormone receptorse |
| ROCK, Rho kinase | Erα oestrogen receptor (NR3A1) |
| Ligand-gated ion channelsb | Erβ oestrogen receptor (NR3A2) |
| P2X1 receptor |
| LIGANDS | |
|---|---|
| 20-HETE | LY294002 |
| AA, arachidonic acid | NO |
| ADP | Metformin |
| Angiotensin II | PD98059 |
| Diclofenac | PGD2 |
| Clonidine | PGE2 |
| DOCA, deoxycorticosterone acetate | PGF2α |
| E2, 17β-oestradiol | PGI2 |
| EPA, eicosapentaenoic acid | Rapamycin |
| ET-1, endothelin-1 | Suramin |
| L-arginine | Testosterone |
| Ip5I | TxA2, thromboxane A2 |
| L-NAME | U46619 |
| Losartan | VP, vasopressin |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,c,d,eAlexander et al., 2013a,b,c,d,e,,,,).
The endothelium plays a pivotal role in the regulation of vascular tone (Vapaatalo and Mervaala, 2001; Pries and Kuebler, 2006; Flammer and Luscher, 2010; Toda et al., 2010; Flammer et al., 2012; Favero et al., 2014). In response to mechanical forces (e.g. shear stress) and endogenous ligands, endothelial cells (ECs) release a diversity of factors that mediate or directly induce vascular smooth muscle contraction or relaxation (Vapaatalo and Mervaala, 2001; Flammer and Luscher, 2010; Flammer et al., 2012). Accordingly, these factors are largely divided into relaxing and contracting factors [namely, endothelium-derived relaxing factors (EDRFs) and endothelium-derived contracting factors (EDCFs) respectively]. Nitric oxide (NO), endothelium-derived hyperpolarizing factor (EDHF) and prostacyclin (PGI2) are well-established EDRFs (Furchgott and Zawadzki, 1980; Chen et al., 1988; Feletou and Vanhoutte, 2004; Feletou, 2009; Garland et al., 2011; Flammer et al., 2012). On the other hand, vasoconstrictor prostanoids, endothelin-1 (ET-1), angiotensin II and more recently uridine adenosine tetraphosphate (Up4A) have been described as major EDCFs (Yanagisawa et al., 1988; Jankowski et al., 2005; Vanhoutte et al., 2005; 2009,; Vanhoutte and Tang, 2008; Matsumoto et al., 2014a).
Endothelial dysfunction is a hallmark of the pathophysiology of diabetes and arterial hypertension and contributes to macrovascular and microvascular complications associated with these disease states (De Vriese et al., 2000; Kobayashi et al., 2000; Vanhoutte et al., 2005; 2009,; Versari et al., 2009; Ding and Triggle, 2010; Barton et al., 2012; Bazi et al., 2012; Campia et al., 2012). An imbalance between EDRFs and EDCFs plays a key role in the development of vascular dysfunction in hypertension and diabetes (Vanhoutte et al., 2005; 2009,; Michel et al., 2008a; Vanhoutte and Tang, 2008; Ding and Triggle, 2010; Tang and Vanhoutte, 2010; Mukohda et al., 2012). EDRF production, bioavailability and signalling in smooth muscle cells (SMCs) are decreased in vessels under diabetic and hypertensive conditions (Kamata et al., 1989; De Vriese et al., 2000; Vanhoutte et al., 2005; 2009,; Vanhoutte and Tang, 2008; Ding and Triggle, 2010). On the other hand, EDCF production and/or signalling are increased in vessels in the presence of those diseases (Vanhoutte et al., 2005; 2009,; Vanhoutte and Tang, 2008; Feletou et al., 2009; 2010a; 2011,,; Tang and Vanhoutte, 2010; Matsumoto et al., 2014a). Therefore, the fine regulation of this balance may be important in order to prevent the development of diabetes- and hypertension-associated vasculopathies.
EDRFs have attracted great attention and several excellent reviews have been written on EDRF-mediated vascular signalling in metabolic and cardiovascular diseases including diabetes and hypertension (De Vriese et al., 2000; Griffith, 2004; Triggle et al., 2005; Feletou et al., 2008; 2009,; Matsumoto et al., 2008a; Grgic et al., 2009; Barton, 2010; Ding and Triggle, 2010; Edwards et al., 2010; Forstermann and Li, 2011; Campia et al., 2012; Tousoulis et al., 2012; Sena et al., 2013; Toda et al., 2013; Bruder-Nascimento et al., 2014; Manrique et al., 2014; Goulopoulou and Davidge, 2015). The role of EDCF in vascular dysfunction associated with these disease states, however, is not well understood. Constrictor prostanoids are EDCFs with potent and complex effects on vascular smooth muscle function and have been implicated in both diabetes and hypertension (Vanhoutte et al., 2005; Feletou et al., 2009; 2011,; Versari et al., 2009; Barton et al., 2012). Up4A, identified as an EDCF in 2005 (Jankowski et al., 2005), also modulates vascular function (Matsumoto et al., 2011a); however, the pathophysiological role of Up4A is unclear.
In this review, we focus on the contribution of constrictor prostanoids and Up4A to vascular dysfunction in diabetes and arterial hypertension. We address the mechanisms associated with the production and signalling of prostanoids and Up4A in vascular SMCs and the interactions between EDCFs with EDRFs. Further, we discuss experimental evidence that implicates prostanoids and Up4A in diabetes- and hypertension-associated vascular pathology. Potential therapeutic strategies to target the overproduction and actions of EDCFs and to decrease EDCF-mediated responses are also reviewed. The influence of sex and sex steroid hormones on vascular function have been previously described (Tostes et al., 2003; 2008,; Orshal and Khalil, 2004; Khalil, 2005; Arnal et al., 2010; Huxley and Wang, 2010; Hart et al., 2011; Nilsson et al., 2011; Bubb et al., 2012; Miao and Li, 2012; Kittikulsuth et al., 2013) and considerable progress has been made in our understanding of the cellular and molecular mechanisms underlying the effects of sex hormones and their receptors in the vascular system (Tostes et al., 2003; 2008,; Orshal and Khalil, 2004; Khalil, 2005; Arnal et al., 2010; Huxley and Wang, 2010; Hart et al., 2011; Nilsson et al., 2011; Bubb et al., 2012; Miao and Li, 2012; Kittikulsuth et al., 2013). In the present review, therefore, the effect of sex on EDCF vascular signalling in the context of diabetes and arterial hypertension is also discussed.
COX-mediated production of vasoconstrictor prostanoids
The production and signalling of vasoconstrictor prostanoids are increased in arterial SMCs of subjects with hypertension and diabetes (De Vriese et al., 2000; Vanhoutte et al., 2005; 2009,; Vanhoutte and Tang, 2008; Feletou et al., 2009; 2010a,b,; 2011; Versari et al., 2009; Ding and Triggle, 2010; Tang and Vanhoutte, 2010; Wong and Vanhoutte, 2010; Barton et al., 2012; Figure 1). Increased constrictor prostanoid production and activity are attributable to the enhanced expression and/or activity of COXs (COX-1, COX-2) and to increased production of reactive oxygen species (ROS). ROS are considered EDCFs themselves as well as enhancers/modulators of EDCF responses (Tang et al., 2007; Tang and Vanhoutte, 2010) in hypertensive arteries (Vanhoutte et al., 2005; 2009; Vanhoutte and Tang, 2008; Feletou et al., 2009; 2010a; 2011,,; Tang and Vanhoutte, 2010).
Figure 1.
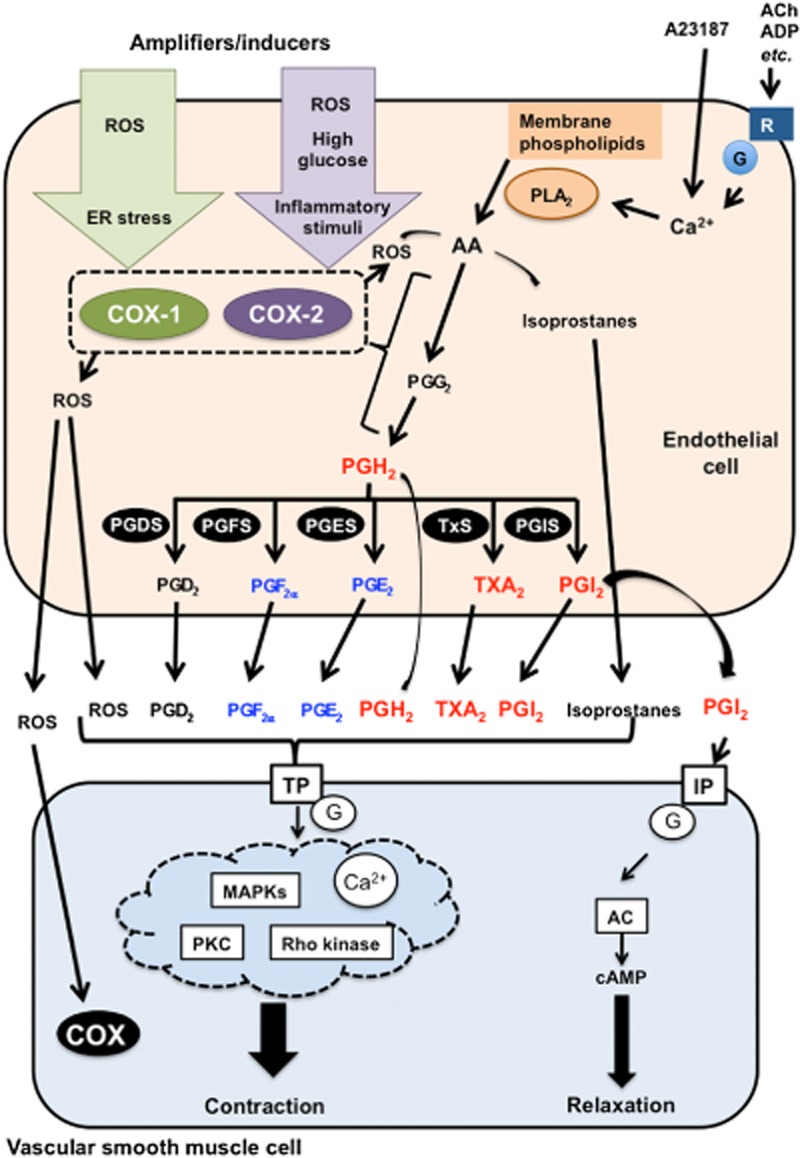
Major EDCF-mediated signalling pathways in hypertension and diabetes. When intracellular Ca2+ levels are increased via the activation of GPCR by, for example, ACh, ADP or Ca2+ ionophore (A23187), AA is produced from membrane phospholipids by PLA2. AA is metabolized to endoperoxides [PGG2 and PGH2] through activation of COXs (COX-1 and COX-2). PGH2 is further metabolized into PGD2, PGE2, PGF2α, PGI2 and TxA2 by specific synthases including PGDS, PGES, PGFS, PGIS and TxS. All these prostanoids and endoperoxides can activate TP receptors in vascular SMC leading to contraction via multiple pathways. Although PGI2 usually leads to vasodilatation through activation of the IP receptor/adenylate cyclase (AC)/cAMP pathway, PGI2 responses are impaired in some conditions, including hypertension and diabetes. Instead of abnormalities in the IP receptor/AC/cAMP pathway, other prostanoids such as PGE2, PGF2α and TxA2, and endoperoxides may be increased and then stimulate TP receptors. In hypertensive arteries, mainly PGI2, TxA2 and PGH2 mediate EDCF responses (in red). In addition to these prostanoids, PGE2 and PGF2α can also behave as EDCF in arteries from diabetic subjects (in blue). PGD2 another EDCF has less significant effects compared with other PGs. COX generates ROS. ROS are considered EDCFs themselves as well as enhancers/modulators of EDCF responses, since ROS (1) further activate endothelial and smooth muscle COX and (2) increase production of isoprostanes via AA, leading to TP receptor activation. COX activity is regulated and further enhanced by several amplifier/inducers in hypertension and diabetes. Details are described in the text.
Stimulation of ECs (e.g. by ACh and ADP) leads to an increase in intracellular concentration of calcium (Ca2+) and subsequent release of arachidonic acid (AA), which is then metabolized by COXs (Vanhoutte et al., 2005; 2009,; Vanhoutte and Tang, 2008; Feletou et al., 2009; 2010a,b,; 2011; Tang and Vanhoutte, 2009; 2010,). PLA2 significantly contributes to this process via Ca2+-dependent and -independent mechanisms (Tang and Vanhoutte, 2009; Feletou et al., 2010a,b,). Elevated cytosolic concentration of Ca2+ is a trigger for endothelium-dependent contractions, probably by activating Ca2+-dependent PLA2 in ECs (Tang and Vanhoutte, 2009; 2010,; Vanhoutte, 2009; Vanhoutte et al., 2009; Feletou et al., 2010a,b,). Indeed, inhibition of Ca2+-dependent PLA2 suppresses EDCF-mediated contractions (Tang et al., 2007; Ishida et al., 2011). Endothelial ligands such as ACh, ET-1 and nucleotides lead to increased intracellular Ca2+ and then release of COX-derived EDCFs (Feletou et al., 2010a,b,; Tang and Vanhoutte, 2010). The calcium ionophore A23187 directly increases intracellular Ca2+ in ECs, and therefore, A23187 may act as an inducer of EDCF production (Shi et al., 2007; Tang et al., 2007). Excessive accumulation of intracellular Ca2+ in ECs is critical and triggers the production of COX-derived EDCFs. This abnormality has been reported in arteries from hypertensive and diabetic subjects (Shi et al., 2007; Tang et al., 2007; Tang and Vanhoutte, 2010). Ca2+-independent PLA2 inhibition also suppresses ACh-induced EDCF production and contraction in aorta of spontaneously hypertensive rats (SHR), a genetic rat model of hypertension (Wong et al., 2010a). Thus, both Ca2+-dependent and -independent mechanisms are involved in the vascular production of EDCF in hypertensive and diabetic conditions.
COXs (COX-1 and COX-2) are rate-limiting enzymes in the AA cascade (Rouzer and Marnett, 2009; Feletou et al., 2011; Nakano, 2015). AA is enzymically cyclized and oxygenated to produce the endoperoxide PGG2. COX, which catalyses this cyclooxygenation reaction, also reduces a hydroperoxyl in PGG2 to a hydroxyl to form PGH2 via a separate peroxidase site on the enzyme (Simmons et al., 2004; Rouzer and Marnett, 2009; Salvemini et al., 2013). COX-1 is constitutively expressed and is usually abundant in ECs, whereas endothelial COX-2 is mainly induced by inflammatory stimuli (Feletou et al., 2009; 2010a,b,; 2011; Tang and Vanhoutte, 2009; 2010,; Vanhoutte, 2009; Grosser et al., 2010). A growing body of evidence suggests that COX-1 is the primary isoform related to endothelium-dependent contractions (Tang et al., 2005b; Tang and Vanhoutte, 2008; Vanhoutte, 2009; Wong et al., 2009; Feletou et al., 2010a,b,; Rovati et al., 2010). For instance, endothelium-dependent contractions are abolished by specific COX-1 inhibitors, but are relatively insensitive to specific COX-2 inhibitors (Yang et al., 2003a; 2004a,). In rat aorta, both COX-1 and COX-2 are detected; however, the amount of COX-2 transcripts in ECs or SMCs is markedly less than that of COX-1 (Tang and Vanhoutte, 2008). Moreover, endothelium-dependent contractions are seen in aortae isolated from wild-type and COX-2 deficient mice, but not from COX-1-deficient mice, suggesting that COX-1 is necessary for endothelium-dependent contractions (Tang et al., 2005b).
Although in healthy conditions the contribution of COX-2 to endothelium-dependent contractions is negligible, COX-2 is implicated in such contractions in hypertension, diabetes and aging (Matsumoto et al., 2007a; Shi et al., 2008). In arteries of SHR or diabetic rats, COX-1 expression is up-regulated, and COX-1 inhibitors block the augmented endothelium-dependent contractions (Yang et al., 2002; Gluais et al., 2006; Shi et al., 2007). COX-1-derived PGI2, thromboxane A2 (TxA2) or endoperoxides all contribute to endothelium-dependent contractions in hypertension (Gluais et al., 2006; 2007,). In hamster aortae, endothelium-dependent, TxA2/endoperoxide (TP) receptor-mediated contraction induced by ACh is mediated by PGF2α. In this study, PGF2α derived from COX-2 and not from COX-1, enhanced the COX-2/PGF2α/TP pathway (Wong et al., 2009). Human renal arteries also exhibit TP receptor-mediated ACh- or PGF2α-induced contractions and COX-2-dependent release of PGF2α (Wong et al., 2009). Endothelial COX-2 rather than COX-1 seems to be responsible for enhanced generation of EDCF in aortae from rats under chronic treatment with the NOS inhibitor L-NAME (Qu et al., 2010).
In diabetic animal models, COX-2-derived prostanoids have been suggested to induce abnormal vasoconstrictor responses or to account for the development of endothelium-derived vasoconstrictor activity (Quilley and Chen, 2003; Bagi et al., 2005; Guo et al., 2005; Nacci et al., 2009; Lopez-Lopez et al., 2011; Ramos-Alves et al., 2012a,b,; Vessieres et al., 2013). Indeed, COX-2 expression and activity are increased in diabetic arteries (Bagi et al., 2006; Sanchez et al., 2010; Kassan et al., 2013; Martinez et al., 2014). Moreover, at the cellular level, high glucose, which is a characteristic of diabetes (De Vriese et al., 2000; Rask-Madsen and King, 2007; 2013,; Forbes and Cooper, 2013), increases COX-2 expression and activity in ECs (Cosentino et al., 2003; Sheu et al., 2005). Others, however, have reported up-regulation of COX-1 and increased production of COX-1-derived prostanoids in arteries of diabetic subjects (Matsumoto et al., 2007a; 2009b,; Shi et al., 2007; Shi and Vanhoutte, 2008; Feletou et al., 2011). Using COX-1-deficient mice fed with a high-fat diet and treated with streptozotocin (STZ; animal model of type 1 diabetes), Zhu et al. (2014) demonstrated that COX-1 remains the major contributor to the synthesis of endothelial PGI2 that leads to vasoconstrictor activity in these conditions (Zhu et al., 2014). Discrepancies about the contribution of COX-1 and COX-2 to endothelium-mediated contractions in hypertension and diabetes may be attributed to different species, vascular beds, disease models and disease duration.
COX is involved in the endothelial generation of ROS (Tang and Vanhoutte, 2009; Feletou et al., 2010a; Sena et al., 2013; Hernanz et al., 2014). Increased COX-derived endothelial production of ROS contributes to the development of endothelial dysfunction in SHR aorta (Tang et al., 2007). ROS affects NO bioavailability (Hattori et al., 1991; Kamata and Kobayashi, 1996; Matsumoto et al., 2007b) and COX activation (Korbecki et al., 2013; Hernanz et al., 2014) and thus, it may also affect the balance between EDCF and EDRF signalling. Indeed, excessive formation of superoxide eliminates the vasodilatory, anti-thrombotic and anti-adhesive effects of NO and PGI2 and stimulates the potent vasoconstrictor, pro-thrombotic and pro-inflammatory actions of PGH2 and TxA2 (Zou, 2007). Upon diffusion into vascular SMCs, ROS activate COX and further stimulates the production of contractile prostanoids (Katusic and Vanhoutte, 1989; Yang et al., 2002; Feletou et al., 2010a,b,; Tang and Vanhoutte, 2010). Moreover, ROS induce the production of isoprostanes such as 8-iso-PGF2α and 8-iso-PGE2, which can activate TP receptors on vascular SMC, leading to contraction (Bauer et al., 2014). Thus, ROS are key players in EDCF-mediated signalling (Tang et al., 2007; Tang and Vanhoutte, 2010) in hypertensive arteries (Vanhoutte et al., 2005; 2009,; Vanhoutte and Tang, 2008; Feletou et al., 2009; 2010a; 2011; Tang and Vanhoutte, 2010).
Prostanoids: endothelium-derived contracting factors
In the prostanoid pathway, both COXs oxidize AA to form unstable PGH2 via the intermediate PGG2. PGH2 is further metabolized into PGD2, PGE2, PGF2α, PGI2 and TxA2 by specific synthases including PGD synthase (PGDS), PGE synthase (PGES), PGF synthase (PGFS), PGI synthase (PGIS) and thromboxane synthase (TxS) respectively (Simmons et al., 2004; Tang and Vanhoutte, 2009; Figure 1). Among these prostanoids, PGI2 and TxA2 are primarily implicated in EDCF-mediated responses; however, PGD2, PGE2 and PGF2α may also contribute to EDCF responses (Vanhoutte et al., 2009; Wong and Vanhoutte, 2010; Figure 1). Although endoperoxides have a very short life, they have contractile actions (Feletou et al., 2009). Stimulus (i.e. ligand), type of vessel and disease state are all determinants of which prostanoids and what amount of each prostanoid (and combination) would contribute to endothelium-dependent contraction. For example, in SHR aorta, ACh-induced endothelium-dependent contractions are largely due to the release of PGI2 rather than other PGs (Gluais et al., 2005). However, TxA2 contributes to EDCF-mediated contraction induced by other ligands such as ADP, A23187 and ET-1 (Gluais et al., 2006; 2007; Vanhoutte et al., 2009; Wong and Vanhoutte, 2010). ACh-induced increased production of TxA2 and PGE2, but not PGF2α or PGI2 has been reported in superior mesenteric arteries from type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats (Matsumoto et al., 2007a; 2008b).
Prostacyclin is the major COX-derived metabolite of AA via PGIS in ECs (Tang and Vanhoutte, 2009; Wong and Vanhoutte, 2010; 2011). Gene expression of PGIS is by far the most abundant and averages more than 90% of the total expression of prostanoid synthases in EC of SHR aorta (Tang and Vanhoutte, 2008). Moreover, the extent of co-localization of PGIS with COX-1 in EC is greater than that with COX-2 (Kawka et al., 2007) and PGIS expression is increased in the aortae of SHR [vs. control Wistar-Kyoto rats (WKY)] (Numaguchi et al., 1999; Tang and Vanhoutte, 2008). Thus, the majority of the endothelial COX-1-derived endoperoxides are transformed into PGI2 in hypertensive arteries (Gluais et al., 2005; 2006,; Tang and Vanhoutte, 2008; 2010,; Feletou et al., 2009). Prostacyclin-induced IP receptor activation usually results in endothelium-dependent vasodilation via the activation of the adenylyl cyclase/cAMP pathway (Alfranca et al., 2006; Feletou et al., 2010b). However, in experimental hypertension, PGI2 binds to TP receptors to evoke vasoconstriction (Gluais et al., 2005; 2006,). This may be due to dysfunction of IP receptors and the AC pathway (Gomez et al., 2008). Indeed, the relaxation induced by PGI2 or its stable analog iloprost is lost in aortae from SHR and aged WKY (Rapoport and Williams, 1996; Gluais et al., 2005). Although high concentrations of PGI2 led to vasoconstriction via the activation of TP receptors, PGI2 may be only weakly effective at TP receptors, as PGI2 is rapidly degraded into its inactive metabolite 6-keto-PGF1α (Gluais et al., 2005). In disease states including hypertension and diabetes, PGI2 abnormalities refer to its interaction with the IP receptor and its downstream signalling as well as its synthesis by PGIS. In hypertension and diabetes, ROS interact with NO to form peroxynitrite (Zou et al., 2004; Szabo, 2009; Forstermann and Li, 2011). Peroxynitrite inhibits PGIS activity via nitration of tyrosine residues (Zou et al., 2002; Nie et al., 2006; Zou, 2007). Thus, the regulation of PGIS activity (e.g. suppression of nitrosative stress) and of IP receptors are important to improve PGI2 signalling in hypertension- and diabetes-associated vasculopathy (Szabo, 2009; Forstermann and Li, 2011; Vanhoutte, 2011).
TxA2, a metabolite of AA through TxS enzymic activity, mediates a number of cellular responses including vasoconstriction and platelet aggregation (Narumiya et al., 1999; Nakahata, 2008). Alterations in TxA2 biosynthesis and actions have been investigated in a series of pathophysiological conditions such as atherosclerosis, myocardial ischaemia, asthma, diabetes, hypertension and pregnancy-induced hypertension (Meagher and FitzGerald, 1993; Hopkins, 2013; Taguchi et al., 2014). The contribution of TxA2 to endothelium-dependent contraction may be ligand, vessel and disease specific. ACh-induced endothelium-dependent contractions are not affected by TxS inhibitors in SHR aortae (Auch-Schwelk et al., 1990; Tang and Vanhoutte, 2009) or canine basilar arteries (Katusic et al., 1988). On the other hand, inhibitors of TxS suppressed ADP-, A23187- and AA-induced endothelium-dependent contraction in the same preparations (Katusic et al., 1988; Gluais et al., 2006; 2007,). Treatment of type 2 diabetic OLETF rats with a TxS inhibitor suppressed ACh-induced TxA2 production, partly improved ACh-induced endothelium-dependent, NO-dependent and EDHF-dependent relaxations, and inhibited ACh-induced endothelium-dependent contractions in superior mesenteric arteries. These data suggest that TxS inhibition normalizes endothelial dysfunction in type 2 diabetes (Matsumoto et al., 2009c).
As mentioned above, PGD2, PGE2 and PGF2α also induce endothelium-dependent contractions; however, the contribution of these prostanoids to EDCF-mediated contractions is considered marginal in most cases, including disease states (Vanhoutte and Tang, 2008; Tang and Vanhoutte, 2009).
Expression of PGDS is increased in response to fluid shear stress (Taba et al., 2000). Studies using lipocalin-type PGDS (L-PGDS)-deficient mice indicate that L-PGDS plays an important role in regulating insulin sensitivity and the development of atherosclerosis in type 2 diabetes (Ragolia et al., 2005). PGD2 may act as an EDCF via activation of TP receptors rather than DP receptors (Gluais et al., 2005; Feletou et al., 2011).
Expression of PGES and PGFS has been detected in aortae from the SHR (Tang and Vanhoutte, 2008; Tang et al., 2008) but their expression was lower than the expression of PGIS. Further, there was no difference in production of either PG between WKY and SHR aortae (Gluais et al., 2005; 2006; 2007,,). Under normal physiological conditions, PGE2 and PGF2α are natural agonists of the EP and FP receptors, respectively (Alfranca et al., 2006; Feletou et al., 2011). In hypertension, however, PGE2 and PGF2α activate the TP receptor (Gluais et al., 2005; Feletou et al., 2011). PGE2 and PGF2α can act as EDCFs when PGIS is inhibited or the metabolism of PGH2 is diverted (Gluais et al., 2005), a phenomenon that may occur when severe oxidative stress leads to tyrosine nitration of PGIS (Zou et al., 2002). Therefore, in the SHR aortae, TxA2, PGH2, PGI2 and, depending on the situation, PGE2 and PGF2α can all act as EDCFs (Feletou et al., 2010a).
Interactions between vasoconstrictor prostanoids and other endothelium-derived factors
In healthy ECs, the production and/or release of EDCFs is tempered by the presence of NO (Tang et al., 2005a; Vanhoutte and Tang, 2008; Tang and Vanhoutte, 2009; Vanhoutte et al., 2009) and EDHFs (Michel et al., 2008a). Endothelium-dependent contractions induced by ACh and nucleotides were relatively weak under basal conditions, but NOS inhibition amplified these contractions (Yang et al., 2003b; 2004a,b,; Matsumoto et al., 2007a; Ishida et al., 2011). Accordingly, in cases of endothelial dysfunction (viz. impaired bioavailability of EDRFs), the production of EDCF predominates. Treatment with L-arginine, a substrate for NOS (Forstermann and Li, 2011), inhibited contractions and normalized relaxation responses to ACh in renal arteries from hypertensive Dahl salt rats, whereas U46619-induced contraction and nitroprusside-induced endothelium-independent relaxation were not affected by L-arginine treatment (Zhou et al., 2001). Endothelium-dependent contractions were suppressed in arteries exposed to endogenous or exogenous NO (Tang et al., 2005a; Feletou et al., 2008). EDCF, on the other hand, leads to reduced NO bioavailability, suggesting a reciprocal relationship between NO and EDCF. ROS derived from stimulation of EC or TP receptor activation (Zhang et al., 2008; Del Turco et al., 2014) may reduce NO bioavailability. Activation of TP receptors in ECs has inhibitory effects on NO production, suppressing endothelium-dependent vasodilation (Liu et al., 2009). EDCF can also suppress EDHF signalling. Michel et al. (2008a) found that EDCF-mediated contraction in rat renal arteries was amplified not only by NOS inhibition, but also EDHF inhibition. In contrast, TxA2 can interact with signalling pathways associated with EDHF (Ellinsworth et al., 2014). TP receptor stimulation is associated with loss of small-conductance Ca2+-activated potassium (SKCa) channel activity and with decreased EDHF-mediated responses in the rat mesenteric artery (Crane and Garland, 2004).
ET-1 is an EDCF with an important role in the pathogenesis of hypertension and diabetes (Maguire and Davenport, 2014; Matsumoto et al., 2014a; Sandoval et al., 2014). Interactions between ET-1 and vasoconstrictor prostanoids have been previously reported. Earlier studies showed that incubation of human aortic ECs with ET-1 led to production of the PGI2 metabolite 6-keto-PGF1α, the TxA2 metabolite TxB2 and PGE2 (Hollenberg et al., 1994). In rat aorta, ET-1 induced the release of PGH2 from ECs and this effect was greater in the hypertensive state (Asano et al., 1994). Basal and ET-1-stimulated release of TxB2 was found in endothelium-intact aortae from SHR, but not WKY rats. ET-1-induced contraction was reduced by denudation of the endothelial layer, inhibition of TxS, or antagonism of TP receptors in aortae from SHR, but not WKY (Taddei and Vanhoutte, 1993). Furthermore, exogenous ET-1-induced vasoconstriction was largely blocked by intrabrachial administration of indomethacin (Taddei et al., 2000) in patients with essential hypertension, but not in normotensive subjects. Thus, ET-1/prostanoid interactions may be essential in the regulation of vascular tone.
Several studies in humans also suggest interactions between EDCF and other endothelium-derived factors. In healthy subjects, infusion of ACh into the brachial artery led to a concentration-dependent increase in flow-mediated dilation, which was diminished by NOS, but not by COX inhibition (Taddei and Vanhoutte, 1993; Taddei et al., 1993; 1998,). In contrast, ACh-induced vasodilatation was blunted in patients with essential hypertension and was resistant to NOS inhibition (Dohi et al., 1990). COX inhibition enhanced ACh-induced vasodilatation in these patients, suggesting that COX-derived vasoconstrictors are largely responsible for the abnormal response to endothelium-dependent vasodilators in essential human hypertension (Vanhoutte et al., 2005; Versari et al., 2009; Virdis et al., 2010).
Vasoconstrictor prostanoid-mediated signalling in vascular SMCs: focus on TP receptors
Prostanoids signal their constrictor effects on vascular SMC primarily via activation of the TP receptor, a member of the seven transmembrane GPCR superfamily (Nakahata, 2008; Woodward et al., 2011; Alexander et al., 2013,,,,). In humans, two distinct TP receptor isoforms have been found, namely TPα and TPβ, but these are not present in other species, such as non-human primates and rodents (Hopkins, 2013). TP receptors are expressed in endothelial and vascular SMC. Those located in vascular SMC are the primary contributors to the hypertensive phenotype (Sparks et al., 2013) and to COX-dependent, endothelium-dependent vasoconstriction (Yang et al., 2003a).
TxA2-induced contractions in isolated bovine aortic SMC and isolated vascular tissue from various species involve both Ca2+-dependent and -independent mechanisms. Various mouse arteries exhibit contractile responses to ACh (carotid > abdominal aorta > femoral) and these responses are abolished by TP receptor antagonism (Zhou et al., 2005), suggesting that endothelium-dependent contractile responses in healthy vascular tissues are evoked through activation of TP receptors. Although TxA2 is the preferred TP receptor ligand, high concentrations of endoperoxides, other PGs, as well as isoprostanes and hydroxyeicosatetraenoic acids (HETEs; Roman, 2002; Miyata and Roman, 2005) also activate the TP receptor (Feletou et al., 2010a; 2011,). 20-HETE has been shown to induce arterial contraction via TP receptor activation. The rate-limiting step of this action is the conversion of 20-HETE to 20-endoperoxides (20-OH-PGH2, 20-OH-PGG2) by COX (Escalante et al., 1989; Schwartzman et al., 1989). 20-HETE-induced contraction is partly dependent on the presence of the endothelium (Schwartzman et al., 1989; Randriamboavonjy et al., 2003) and is abolished by COX inhibition (Escalante et al., 1989; Schwartzman et al., 1989) and by TP receptor antagonists (Schwartzman et al., 1989). In cerebral arteries, flow-mediated constriction involves increased production of ROS and COX activation, and is mediated by the interaction between 20-HETE and TP receptors (Toth et al., 2011). These data suggest that TP receptors may be an integral part of all eicosanoid pathways.
Activation of TP receptors mainly involves stimulation of Gq-dependent phospholipase C (PLC) β, leading to Ca2+-dependent activation of myosin light chain (MLC) kinase and 20 kDa MLC (MLC20) phosphorylation (Fukata et al., 2001; Wilson et al., 2005). Increases in Ca2+ influx due to opening of both receptor-operated and voltage-gated Ca2+channels contribute to TP activation-induced Ca2+-dependent effects (Okon et al., 2002). One of the signalling molecules activated by TP receptors in smooth muscle is Rho kinase (Somlyo and Somlyo, 2003). The activation of Rho kinase leads to inhibition of myosin light chain phosphatase, which reduces MLC20 dephosphorylation (Somlyo and Somlyo, 2000; Nunes et al., 2010). Activation of TP receptors involves receptor co-coupling to G12 and activation of RhoA/Rho kinase (ROCK) signalling, engaging Ca2+-independent increases in overall levels of phosphorylated MLC20, which leads to vascular contraction (Wilson et al., 2005). To study the relationship between ROCK and EDCF signalling, Chan et al. (2009) investigated the expression of ROCK and the effect of Rho kinase inhibitors on endothelium-dependent contraction in aorta isolated from 1-year old SHR and WKY. They found that in the presence of an NOS inhibitor, ROCK inhibitors reduced endothelium-dependent contractions induced by ACh and the Ca2+ ionophore A23187 in aortae from both SHR and WKY. ROCK inhibitors did not affect the production of 6-keto PG F1α (PGI2 metabolite), but suppressed U46619- and PGF2α-induced contraction and ROCK expression in both groups (Chan et al., 2009). These findings suggest that the suppression of EDCF-mediated contraction by ROCK inhibition is mainly due to direct suppression of EDCF-mediated signalling in vascular SMCs and not due to an effect on EDCF release (Chan et al., 2009). Denniss et al. (2010) reported that increased ACh-induced contractions in SHR (vs. WKY) were abolished by endothelial denudation or COX-1 inhibition, and nearly eliminated by TP receptor antagonists or by ROCK inhibition (Denniss et al., 2010). ACh-induced PGI2 production was greater in carotid arteries from SHR (vs. WKY), but ROCK inhibition did not affect PGI2 production. Protein expression of RhoA, but not ROCK-II, as well as Rho activation was increased in carotid arteries of SHR (vs. WKY artery). In addition, RhoA activation was increased by ACh stimulation. Quenching superoxide with tiron or NAD(P)H oxidase inhibition by apocynin reduced ACh-induced contraction in SHR, whereas the superoxide dismutase mimetic tempol amplified the response. Exogenous H2O2-induced contractions in carotid arteries were greater in SHR than in WKY and these responses were abolished by COX-1 inhibition, and greatly reduced by TP receptor antagonism or ROCK inhibition (Denniss et al., 2010). These results suggest that the RhoA/ROCK pathway may be a molecular switch, transducing signals from endothelium-derived PGs and ROS, to turn on vascular SMC contractile pathways (Denniss et al., 2010). This evidence suggests that RhoA/ROCK is a key mediator of abnormal EDCF signalling in hypertension. In rat isolated mesenteric resistance and uterine arteries, inhibition of PKC, ERK1/2 and p38 MAPK (significantly reduced TP receptor-dependent contractions (Goulopoulou et al., 2012). These data indicate that in addition to RhoA/ROCK, PKC and MAPKs are also important downstream effectors of activated TP receptors (Bolla et al., 2002; Nakahata, 2008).
Activation of TP receptors significantly contributes to the pathogenesis of hypertension (Keen et al., 1997; Francois et al., 2004). TP receptor-deficient mice do not develop hypertension in response to chronic angiotensin II infusion and have a reduced BP response to chronic treatment with L-NAME (Keen et al., 1997; Francois et al., 2004; 2008,). Spontaneously hypertensive rats have increased endothelium-dependent contractions in response to ACh and endoperoxides and these responses appear after the development of the hypertensive phenotype (Ge et al., 1995; Yang et al., 2002; 2003a,). TP receptor antagonism reversed ACh-induced vasoconstriction to vasodilatory responses in cerebral arterioles and abolished isometric contractions in aortic rings from hypertensive rats (Okon et al., 2002; Yang et al., 2002). Moreover, contractions induced by the TP receptor agonist U46619 were increased in various arteries from SHR (vs. WKY; Gluais et al., 2005; Chan et al., 2007; Garcia-Redondo et al., 2015). Thus, TP receptors are critical mediators of endothelium-dependent contractions in hypertension.
As described above, ROS, which are enhancers/modulators of EDCF responses, modulate TP receptor function, density and stability. Hydrogen peroxide prevents the translocation and degradation of TP receptors, increasing their density at the cell membrane (Valentin et al., 2004), and TP receptor activation increases the stability of the receptors through a ROS-dependent post-transcriptional mechanism (Wilson et al., 2009). Thus, it is reasonable to speculate that in hypertension, a condition characterized by increased production of ROS, an increase in TP density and stability contributes to augmented endothelium-dependent contractions. Interestingly, there are no differences in the mRNA and protein expression of TP receptors between aortae of WKY and SHR (Tang and Vanhoutte, 2008; Tang et al., 2008), suggesting that an increase in TP receptor density is not the reason for the enhanced endothelium-dependent contractions found in the SHR aorta. Thus, an increase in EDCF production as well as alterations in signalling pathways downstream of TP receptors are more likely to contribute to the hyper-responsiveness to EDCF in hypertensive vessels.
Enhanced activation of TP receptors and augmented endothelium- and TP receptor-dependent vascular contractions are also observed in various metabolic diseases and experimental models of diabetes and hypercholesterolemia (Traupe et al., 2002; Jerez et al., 2008; Michel et al., 2008b). Increased U46619-induced contractions are seen in arteries from obese and diabetic animals (Nobe et al., 2008; Yang et al., 2008; Baretella et al., 2014; Matsumoto et al., 2014b). Obesity and diabetes augment the responses to EDCF, possibly due to an increase in TP receptor gene expression (Traupe et al., 2002). PG receptors other than TP are also involved in diabetes-associated increases in EDCF-induced contractions. In a rat model of type 1 diabetes (STZ-treated rats), PGE2 promotes vascular contractions via EP receptor activation (Shi et al., 2007). RhoA/ROCK signalling is central in diabetes-associated EDCF-induced contractions (Ishida et al., 2012). However, TP receptor-induced augmented vasoconstriction in penile arteries from pre-diabetic obese Zucker rats is coupled to enhanced Ca2+ influx rather than ROCK-mediated augmentation of myofilament Ca2+ sensitization (Villalba et al., 2011).
In summary, in hypertension and diabetes, endothelium-dependent contractions are mainly TP receptor-dependent. The molecular mechanisms downstream of TP receptors involve Ca2+- dependent and -independent pathways, various kinases (ERK1/2, PKC, Rho kinase, p38 MAPK) and their predominance may be vascular bed and disease specific (Figure 1). TP receptors are a promising pharmacological target because their antagonism may suppress EDCF-induced contractions and restore the balance between EDRF and EDCF vasoactive actions (Belhassen et al., 2003; Zuccollo et al., 2005; Gelosa et al., 2010).
Pharmacological targets for the control of EDCF signalling in diabetes and hypertension
Therapies aimed to correct the imbalance in the synthesis/release and signalling of vasoconstrictor and vasodilator prostanoids may be important in the treatment of hypertensive and diabetic subjects. Accordingly, putative targets for the control of EDCF signalling in hypertensive arteries have been suggested.
The regulation of COX activity plays an important role in the control of EDCF signalling. Non-steroidal anti-inflammatory drugs (NSAIDs) inhibit production of PGs by acting on COX-1 and/or COX-2 (Amer et al., 2010). Non-selective NSAIDs, such as naproxen and ibuprofen, inhibit both COX-1 and COX-2 whereas selective NSAIDs act on COX-1 (aspirin) or COX-2 (celecoxib) isoenzymes (Amer et al., 2010). Although COX inhibition seems a possible strategy to prevent COX-associated vascular complications, the incidence of serious adverse cardiovascular effects with COX-2 selective inhibitors (rofecoxib or Vioxx®) has greatly antagonized this concept (Bunimov and Laneuville, 2008; Wong et al., 2010b). There is consensus, however, that low-dose aspirin exerts protective vascular effects. Further investigation of COX inhibitors is required, especially towards their specificity and/or direct inhibition of PGIS activity (Wong et al., 2010b). Meanwhile, antagonism of TP receptors may emerge as a therapeutic alternative to reverse prostanoid-mediated vascular dysregulations (Chamorro, 2009; Jones et al., 2009; Giannarelli et al., 2010; Siller-Matula et al., 2010; Wong et al., 2010b; Davi et al., 2012). Since the actions of prostanoids are complex, the regulation of prostanoid signalling may need to be carefully planned, on a disease-by-disease basis.
Other molecular targets for the suppression of EDCF responses in hypertension and diabetes have been reported (Table 2006). Ford and Rush reported that 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR), an activator of AMP-activated protein kinase (AMPK; Lempiäinen et al., 2012), suppresses EDCF-mediated responses in aortas from SHR and this was reversed in the presence of the AMPK inhibitor Compound C (Ford and Rush, 2011). Spitler et al. recently found that endoplasmic reticulum (ER) stress contributed to increased COX-1 expression and prostanoid release (Spitler et al., 2013). In addition, ER stress increased phosphorylation of ERK1/2 and cytosolic PLA2 (cPLA2), contributing to enhanced EDCF-mediated responses. Treatment with chemical chaperones [ER stress inhibitor, taurourosodeoxycholic acid or 4-phenylbutyric acid] decreased ACh- and AA-induced contraction, COX-1 expression and phosphorylation of cPLA2 and ERK1/2 in SHR aorta. Accordingly, pharmacological inhibition of ER stress suppressed EDCF-mediated responses in aortas and lowered BP in SHR (Spitler et al., 2013). Li et al. (2011) found that treatment with haemin, an inducer of haem oxygenase-1 (HO-1; Ndisang et al., 2001), decreased EDCF-mediated contractions induced by ACh or A23187 via suppression of COX-1, but not COX-2 in aortae from SHR (Li et al., 2011). Moreover, induction of HO-1 with haemin improved endothelium-dependent vascular relaxation through suppression of ROS production and inhibition of COX-2 upregulation induced by diabetes (Wang et al., 2014). Therefore, drugs aimed to increase AMPK or HO-1 activity to inhibit ER stress may represent therapeutic strategies for the suppression of EDCF-induced contractions and the improvement of treatment of vascular function in hypertension.
Table 1.
Potential pharmacological tools to suppress enhanced vascular EDCF-mediated signalling in hypertensive and diabetic models
| Compound name | General action | Main outcome (animal model) | References |
|---|---|---|---|
| PDTC | Low-molecular-weight thiol antioxidant and potent inhibitor of NF-κB (Cau et al., 2011) | Suppresses EDCF- and AA (a source of EDCF)-mediated contraction, as well as the production of prostanoids (OLETF rat) | Matsumoto et al., 2009a |
| Metformin | A biguanide derivative and one of the most commonly used drugs for the treatment of type 2 diabetes (Almabrouk et al., 2014) | Reduces EDCF production and contraction induced by ACh and reduces oxidative stress (OLETF rat) | Matsumoto et al., 2008b |
| AICAR | An activator of AMPK (AMP-activated protein kinase), a putative regulator of metformin signalling (Lempiäinen et al., 2012) | Suppresses ACh-induced EDCF- mediated contraction and the production of vasoconstrictor prostanoids (TxA2 and PGE2; OLETF rat and SHR) | Matsumoto et al., 2008b; Ford and Rush, 2011 |
| Pravastatin | One of the statins which inhibit the enzyme HMG-CoA reductase and the production of cholesterol (Kobayashi et al., 2000) | Pravastatin reduces EDCF-mediated responses by suppressing Rho kinase activity and by stimulating antioxidant activity (OLETF rat) | Ishida et al., 2012 |
| Losartan | Angiotensin AT1 receptor antagonist (Kobayashi et al., 2008) | Reduces ACh-induced production of prostanoids and EDCF(PGE2)-mediated contraction in SMCs and normalizes oxidative stress (OLETF rat) | Matsumoto et al., 2010; Ishida et al., 2011 |
| Suppresses EDCF-mediated responses induced by purinergic receptor stimulation by suppressing COX-2 expression and cPLA2 phosphorylation in arteries (GK rat) | |||
| TUDCA PBA | Chemical chaperones (Kraskiewicz and FitzGerald, 2012) | Suppresses ACh- and AA-induced vascular contractions and decreases expression of COX-1 and activities of cPLA2 and ERK (SHR) | Spitler et al., 2013 |
| Haemin | HO-1 inducer (Ndisang et al., 2001) | Suppresses ACh- and A23187-induced vascular contraction via a decrease in COX-1, but not COX-2 (SHR) | Li et al., 2011 |
| EPA | An omega-3 PUFA that modulates cell membrane fatty acid composition (Calder, 2012). | Suppresses EDCF- and AA-induced contractions and improves EDRFs (NO and EDHF)-mediated relaxation – beneficial effects that are associated with reduced COX-2 expression and activities of ERK and NF-κB (OLETF) | Matsumoto et al., 2009b |
PBA, 4-phenylbutyric acid; PDTC, pyrrolidine dithiocarbamate; PUFA polyunsaturated fatty acid; TUDCA, tauroursodeoxycholic acid.
In OLETF rats, a model of type 2 diabetes associated with the metabolic syndrome, we found increased production and signalling of COX-derived vasoconstrictor prostanoids in mesenteric arteries (Matsumoto et al., 2007a). Although the causal factors of enhanced vasoconstrictor prostanoids in OLETF rats remain unclear, potential pharmacological tools leading to suppression of EDCF-mediated signalling have been reported (Table 2006). AICAR, an AMPK activator (Lempiäinen et al., 2012), inhibited ACh-induced contraction and production of vasoconstrictor prostanoids (Matsumoto et al., 2008b). Likewise, metformin (Almabrouk et al., 2014) decreased ACh-induced contractions, as well as prostanoid superoxide production (Matsumoto et al., 2008b). Treatment with pyrrolidine dithiocarbamate, an inhibitor of NF-κB (Cau et al., 2011), suppressed ACh- and AA-induced contraction and ACh-induced prostanoid release (Matsumoto et al., 2009a). Treatment with pravastatin [an hydroxymethylglutaryl-CoA (HMG-CoA) reductase inhibitor] attenuated endothelium-dependent contractions by inhibiting ROCK activity and up-regulating antioxidant activity (Ishida et al., 2012). The angiotensin II AT1 receptor antagonist, losartan (Kobayashi et al., 2008), suppressed ACh-stimulated prostanoid production, ACh- and AA-induced contraction and reduced superoxide production (Matsumoto et al., 2010). Treatment with losartan suppressed endothelium-dependent contraction by nucleotides and cPLA2 phosphorylation in superior mesenteric arteries from type 2 diabetic Goto-Kakizaki (GK) rats at the chronic stage of the disease (Ishida et al., 2011). The fish oil, eicosapentaenoic acid (EPA), an omega-3 polyunsaturated fatty acid that modulates cell membrane fatty acid composition (Calder, 2012) has several beneficial effects against vascular dysfunction. EPA has anti-inflammatory, anti-platelet, anti-fibrotic, anti-oxidant and anti-atherogenic effects and also increases NO bioavailability (Balakumar and Taneja, 2012; Calder, 2012; Yates et al., 2014). Treatment of OLETF rats with EPA suppressed ACh- and AA-induced contraction, improved ACh-, NO- and EDHF-mediated relaxation, reduced ACh-stimulated prostanoid release, COX-2 expression and activities of ERK1/2 and NF-κB (Matsumoto et al., 2009b). A summary of the actions of these compounds on EDCF signalling is presented in Table 2006. Collectively, these studies indicate that the beneficial effects of these drugs or dietary supplements may be partly attributable to their capacity to normalize EDCF-mediated signalling. In other words, the regulation of EDCF signalling may be a therapeutic target for diabetic and/or hypertensive vasculopathy.
Effects of sex hormones on prostanoid-induced vascular response and signalling
Sex hormones influence EC function and, consequently, EDRFs and EDCF synthesis and their signalling pathways in physiological and pathological conditions (Orshal and Khalil, 2004; Khalil, 2005; Miller and Mulvagh, 2007; Duckles and Miller, 2010). Oestrogen stimulates the release of various endothelium-derived factors (Tostes et al., 2003; Hermenegildo et al., 2006; Miller and Mulvagh, 2007). 17β-oestradiol (E2) stimulates NOS in human and animal ECs – ECs from human umbilical vein (HUVEC), bovine aortae and human aortae (Hayashi et al., 1995; Hishikawa et al., 1995). E2 also induces the production of PGI2 in HUVEC (Mikkola et al., 1995; Sobrino et al., 2010) and ovine fetal pulmonary artery ECs (Sherman et al., 2002). Moreover, oestrogen leads to increased EDHF-mediated signalling (Burger et al., 2009). In cerebral arteries, E2 reduced vascular tone by shifting the primary end product of the endothelial COX-1 pathway from the constrictor PGH2 to dilator PGI2 (Ospina et al., 2003).
Effects of oestrogens are mediated by multiple receptors such as the classic oestrogen receptors ERα and ERβ and the novel G protein-coupled oestrogen receptor (GPER, previously termed GPR30; Revankar et al., 2005; Prossnitz and Barton, 2011) cloned from human ECs (Takada et al., 1997). Natural oestrogens such as E2, a non-specific agonist of ERα, ERβ and GPER (Prossnitz and Barton, 2011), modulate vasoconstrictor prostanoid signalling (Miller and Vanhoutte, 1990; Dantas et al., 1999; Zhang and Kosaka, 2002; Li and Stallone, 2005; Hermenegildo et al., 2006; Li et al., 2008). Moreover, inhibitory effects of E2 on COX-dependent responses to vasoconstrictors have suggested a role for oestrogen receptors (Meyer et al., 1997) on endothelium-dependent contraction. However, the specific receptor(s) involved in this mechanism have not been described.
GPER activation is associated with beneficial and protective effects in the vasculature (Meyer et al., 2011; Han et al., 2013; Ferreira et al., 2015). Meyer et al. (2012) showed that acute GPER antagonism enhanced endothelium-dependent contractions and reduced endothelial NO bioactivity. Chronic GPER deficiency was associated with increased endothelial prostanoid-induced vasoconstriction but had no effect on endothelial NO bioactivity, eNOS and TP receptor gene expression or vascular structure. GPER deletion also increased TP receptor-mediated contraction (Meyer et al., 2012). Therefore, the regulation of GPER activity may have therapeutic potential for vascular complications associated with vasoconstrictor prostanoid signalling in hypertension and diabetes.
Testosterone also influences the release of endothelium-derived factors by both inhibiting EDRF release and increasing EDCF expression (Farhat et al., 1995; Hutchison et al., 1997). Testosterone has been shown to stimulate TxS as well as COX-1 and COX-2 in rat thoracic aorta and mesenteric arteries (Cheuk et al., 2000; Song et al., 2004). Moreover, in vitro treatment with testosterone increased the number of functional TP receptors both in cultured rat aorta (Masuda et al., 1991) and in vascular SMCs of the guinea pig coronary artery (Schror et al., 1994). In addition to its effects on vascular tone under physiological conditions, testosterone also has pro-inflammatory effects. Accordingly, long-term treatment with testosterone, or with its non-aromatizable androgen receptor agonist dihydrotestosterone, exacerbates endotoxin-induced inflammation in the cerebral circulation by mechanisms that involve increased nuclear NF-κB activation and increased levels of COX-2 and inducible NOS (Gonzales et al., 2009). Reinforcing a role for testosterone in vascular inflammation, data from our laboratory demonstrate that testosterone induces leukocyte migration by COX-2-dependent mechanisms (Chignalia et al., 2015).
The modulatory effects of sex hormones on vascular tone may also be due to their influence on the production of vasoconstricor prostanoids. Miller and Vanhoutte (1990) found that AA-induced endothelium-dependent contractions of aortic rings were enhanced by treatment of ovariectomized rabbits with oestrogen for 2 weeks (vs. placebo treatment). In addition, oestrogen treatment augmented contractions induced by PGI2 but not PGE2, PGF2α or U46619, whereas indomethacin suppressed noradrenaline-induced contraction in endothelium-intact aortae from estrogen-treated rabbits. These results suggested that chronic treatment with oestrogens could affect noradrenaline-induced contraction via an endothelium-dependent mechanism that may involve the metabolism of AA by COX, and that altered sensitivity of the SMC to PGI2 may contribute in part to the enhanced contractions to AA upon oestrogen treatment (Miller and Vanhoutte, 1990). Oestrogen also potentiates vascular reactivity to vasopressin (VP), which releases TxA2 and PGI2 from both male and female rat aortae (Li et al., 2008). Whereas ovariectomy attenuated, oestrogen therapy restored VP-stimulated release of TxA2 and PGI2, an effect mediated by upregulation of COX-2 and TxS expression in both ECs and vascular SMCs and up-regulation of TP expression in vascular SMCs (Li et al., 2008). Moreover, sex differences in the endothelial regulation of vasoconstrictor responses due to modulatory effects on vasoconstrictor prostanoids have been described. Whereas the endothelium negatively modulates clonidine (α2-adrenoceptor agonist)-induced contraction entirely via NO in female rats, an endothelial vasoconstrictor prostanoid contributes to clonidine responses in male animals (Tejera et al., 1999).
Important sex differences in endothelial (dys)function have been reported in hypertensive and diabetic subjects (Kauser and Rubanyi, 1995; Hermenegildo et al., 2006; Aloysius et al., 2012). In hypertensive rats, E2 affects the release and/or action of endothelium-derived NO (Huang et al., 1997; Costa et al., 1998) and enhances endothelium-dependent relaxation in aortae of female SHR (Williams et al., 1988). Further, it antagonizes the increased tone in renal arteries of female Dahl salt-sensitive rats by enhancing NO-dependent relaxation and suppressing EDCF-mediated responses via NO-independent mechanisms (Zhang and Kosaka, 2002). Regarding the effects of female sex hormones on the synthesis and the effects of EDCFs in the vasculature, Kahonen et al. demonstrated that diclofenac, a COX inhibitor, abolished sex differences in ACh vascular responses in SHR (Kahonen et al., 1998). In addition, the removal of ovarian steroid hormones increased the generation of COX-derived vasoconstrictors, such as PGH2/PGF2α (Davidge and Zhang, 1998; Dantas et al., 1999). Sex differences in renal prostanoid production have been reported in arterial hypertension, with female SHR exhibiting enhanced urinary excretion of PGE2 and TxA2 metabolites along with enhanced renal microsomal PGES and COX-2 expression, compared with male SHR (Sullivan et al., 2005). In rabbit isolated carotid arteries, testosterone induces a concentration-dependent relaxation, which is increased in diabetic conditions by mechanisms that involve increased release of NO and COX-2-derived PGI2 rather than the absence of COX-1-derived TxA2 (Marrachelli et al., 2010). An imbalance of prostanoid synthesis, with overproduction of vasoconstrictor prostanoids and reduced PGI2 production has been observed in diabetes-associated vascular dysfunction in males (Bolego et al., 2006; Du et al., 2006; Nie et al., 2006; Matsumoto et al., 2007a; 2008b; 2009b; 2010,,,; Ishida et al., 2011). Using a model of diabetes in female rats, Akamine et al. (2006) reported that ACh-induced relaxation, which was decreased in arterioles of diabetic female rats, was ameliorated by diclofenac. In addition, improved production of PGF2α and 6-keto PGF1α, but not TxB2, as well as superoxide generation were observed in ACh-stimulated arterioles from diabetic rats, and diclofenac normalized these alterations. These data suggest that increased release of constrictor prostanoids, most likely PGF2α, is involved in the reduced endothelium-dependent vasodilation in diabetic females and that enhanced COX activity may be a source of superoxide generation in this model (Akamine et al., 2006).
The above-mentioned reports indicate that sex and sex hormones influence EDCF synthesis and EDCF-mediated signalling in physiological conditions and that EDCF-mediated responses are disrupted in diabetic and hypertensive conditions. While this adds further complexity to the regulation of EDCF signalling, it raises the possibility of using different therapeutic treatments to target EDCF-mediated responses in male and female patients.
Uridine adenosine tetraphosphate
Up4A, a dinucleotide with purine and pyrimidine moieties, was identified by Jankowski et al. as a novel potent EDCF (Jankowski et al., 2005). Up4A is released from ECs in response to various stimuli such as mechanical stress, endogenous ligands (ACh, ET-1, ATP and UTP) and Ca2+ ionophore (A23187; Jankowski et al., 2005). Although the molecular mechanisms underlying the production/release of Up4A remain unclear, Jankowski et al. recently demonstrated that the intrinsic enzymic activity of the VEGF receptor 2 leads to the production of Up4A in ECs (Jankowski et al., 2013). Only two papers have been published on circulating Up4A levels in disease states. Jankowski et al. found that circulating levels of Up4A were increased in juvenile hypertensive patients compared with normotensives (Jankowski et al., 2007) and Schuchardt et al. found that patients with chronic kidney disease had a higher plasma Up4A concentration compared with healthy subjects (Schuchardt et al., 2012).
The effects of Up4A on vascular functions have been explored in various animal models (Matsumoto et al., 2011a; Figure 2). Up4A induces vascular calcification (Schuchardt et al., 2012), proliferation and migration of vascular SMC (Gui et al., 2011; Wiedon et al., 2012). Because these events play important roles in the development of vascular dysfunction in diabetes and hypertension (Touyz and Schiffrin, 2004; Chen and Moe, 2012), the regulation of Up4A-induced signalling in vascular SMCs may be a potential therapeutic target. Furthermore, Up4A has been shown to modulate vascular tone in various arteries (Matsumoto et al., 2011a). For example, Up4A induces relaxation of rat aorta (Linder et al., 2008) and swine coronary artery (Zhou et al., 2013) and stimulates contraction in the rat pulmonary artery (Gui et al., 2008), rat aorta (Linder et al., 2008), mouse aorta (Hansen et al., 2010) and rat perfused kidney (Jankowski et al., 2005). Although evidence from vascular functional studies were all derived from non-disease models, we recently found that Up4A-induced contraction is altered in various arteries isolated from deoxycorticosterone-acetate (DOCA)-salt hypertensive rats (Matsumoto et al., 2011b; 2012) and type 2 diabetic GK rats (Matsumoto et al., 2014b). In DOCA-salt hypertensive rats, Up4A-induced contraction is increased in basilar, femoral and renal arteries and is decreased in small mesenteric arteries (vs. control uninephrectomized rats; Matsumoto et al., 2011b; 2012). In renal arteries from hypertensive rats, increased contraction to Up4A may be attributable to increased ERK1/2 activity in SMCs rather than alteration of P2Y receptors, which are putative Up4A receptors (Jankowski et al., 2005; Matsumoto et al., 2011b). Moreover, we recently found that the Up4A-induced contraction is increased in renal arteries from GK rats (vs. control Wistar rats), and this might be due to the enhanced activation of COXs/TP receptor signalling (Matsumoto et al., 2014b). These findings provide the possibility of cross-talk between Up4A and vasoconstrictor prostanoids in the presence of diabetes.
Figure 2.
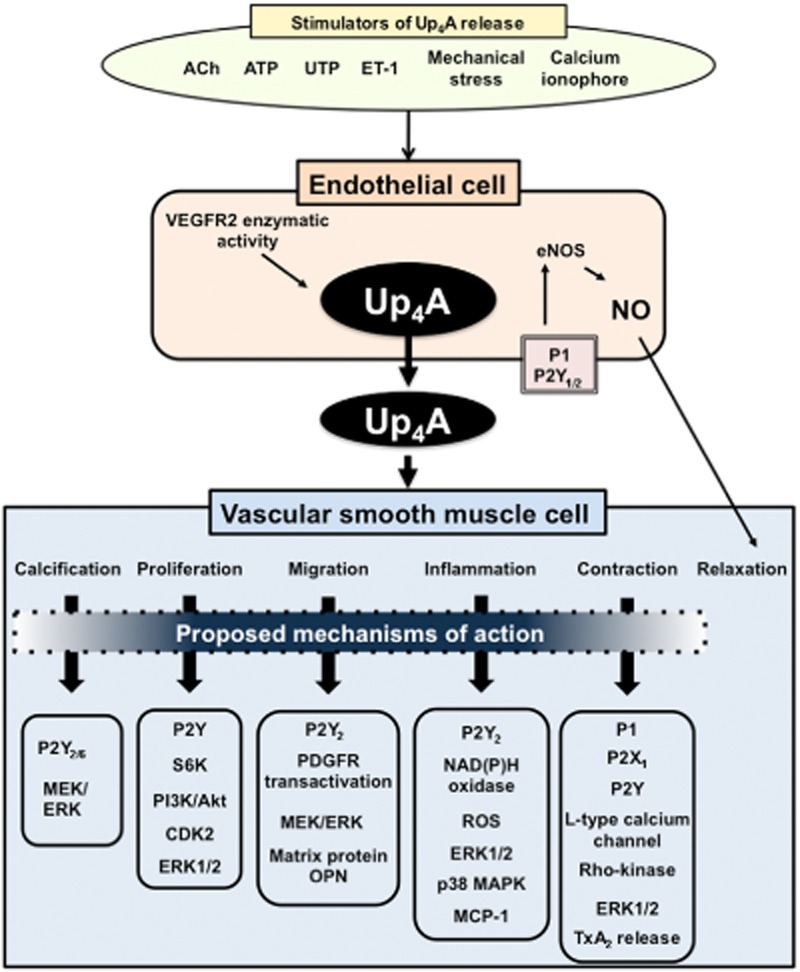
Effects of Up4A on vascular smooth muscle functions. When EC are stimulated with, for example, ACh, ATP, uridine triphosphate (UTP), ET-1, mechanical stress or Ca2+ ionophore (A23187), Up4A is generated. Released Up4A acts in both ECs and vascular SMC. In ECs, Up4A binds to P2Y1/2 or P1 receptor and activates endothelial nitric oxide synthase (eNOS); releasing NO and producing relaxation. In vascular SMCs, Up4A leads to calcification, proliferation, migration, inflammation and contraction. Details are shown in text.
Uridine adenosine tetraphosphate-mediated signalling in vascular SMCs
Since Up4A has purine and pyrimidine moieties, Up4A may also exert its effects via activation of purinergic receptors in vascular SMCs. Purinergic receptors have been classified into two subtypes including P1 (i.e. adenosine receptor) and P2 [P2X (ionotropic) and P2Y (metabotropic)] receptors (Abbracchio et al., 2006; Burnstock, 2007; Erlinge and Burnstock, 2008; Alexander et al., 2013b,c,; Burnstock and Ralevic, 2013). There are reports showing a link between Up4A and purinoceptors in the vasculature. For instance, Jankowski's group demonstrated that Up4A-induced vasoconstriction in perfused kidneys results from the activation of P2X1 receptors and probably also of P2Y2 and P2Y4 receptors (Jankowski et al., 2005). More recently, the same group found that in addition to smooth muscle P2X1 receptor-mediated vasoconstriction in the rat perfused kidney, Up4A also induces concentration-dependent P2Y2 receptor-mediated, long- lasting vasoconstriction (Tolle et al., 2010). Moreover, Up4A-induced vasoconstriction is followed by vasodilation due to P2Y1 and P2Y2 receptor activation on ECs, which leads to NO release (Tolle et al., 2010). Using rat pulmonary arteries, Gui et al. (2008) found that Up4A induces concentration-dependent contraction that is inhibited by suramin, a non-selective P2 receptor antagonist, but not by Ip5I, an antagonist of P2X receptors, or by desensitization of P2X receptors with α,β-methylene-ATP (Gui et al., 2008). We also confirmed that Up4A-induced rat renal arterial contraction is inhibited by suramin but not by Ip5I (Matsumoto et al., 2011b). Linder et al. found that Up4A-induced contraction in rat aorta is blocked by P1 and P2X receptor antagonists (Linder et al., 2008).
The downstream pathways of Up4A-induced responses in vascular SMCs have been investigated (Matsumoto et al., 2011a; Figure 2). Linder et al. found that Up4A-induced rat aortic contraction is suppressed by L-type Ca2+ channel blockade or by ROCK inhibition (Linder et al., 2008). On the other hand, Gui et al. suggested that Up4A-induced contraction in rat pulmonary artery involves extracellular Ca2+ influx and Ca2+ release from intracellular stores, but not Ca2+ sensitization via the ROCK pathway (Gui et al., 2008). In rat renal arteries, we found that Up4A-induced contraction was inhibited by the ERK1/2 pathway inhibitor PD98059, whereas the ERK1/2 activity (determined by phosphorylated ERK1/2 levels) was increased upon Up4A stimulation in renal arteries from DOCA-salt hypertensive rats (vs. control uninephrectomized rat; Matsumoto et al., 2011b). These data suggest that Up4A-induced responses rely on intracellular calcium-dependent and -independent (e.g. kinases) pathways in vascular SMCs.
Up4A-induced vascular SMC migration dramatically depends on secretion of osteopontin (OPN), a multifunctional molecule associated with cell migration, adhesion, survival and tissue remodeling (Scatena et al., 2007). Simultaneous incubation with Up4A and an OPN-blocking antibody suppressed vascular SMC migration (Wiedon et al., 2012). Using specific and non-specific purinoceptor antagonists and inhibitors of MEK/ERK pathway, Wiedon and colleagues demonstrated that Up4A-induced vascular SMC migration was mediated by MEK/ERK pathway upon P2Y2 receptor activation. Furthermore, Up4A-induced migration was reduced by a platelet-derived growth factor receptor (PDGFR) antagonist and by PDGFR-β siRNA treatment, suggesting that transactivation of the PDGFR plays a role in vascular SMC migration mediated by Up4A (Wiedon et al., 2012). Schuchardt et al. found that exogenous application of Up4A increased mineral deposition in mouse and rat aortae and in rat vascular SMCs. In addition, Up4A increased the expression of different genes specific for osteochondrogenic vascular SMCs such as Cbfa1 (Steitz et al., 2001; Naik et al., 2012), while decreasing the expression of SM22α, a specific marker for vascular SMCs (Steitz et al., 2001; Dong et al., 2012). The influence of different P2Y receptor antagonists on Up4A actions indicated that P2Y2/6 receptors might be involved. Mechanisms downstream of P2Y receptor signalling involved activation of the MEK/ERK1/2 pathway. Therefore, Up4A activation of P2Y receptor could affect phenotypic transdifferentiation of vascular SMCs to osteochondrogenic cells, suggesting that purinergic signalling upon Up4A stimulation may be involved in vascular calcification (Schuchardt et al., 2012). Gui et al. (2011) found that Up4A-induced increase in bromodeoxyuridine incorporation was blocked by the mammalian target of rapamycin and the MEK/ERK1/2 inhibitor, PD98059, in human vascular SMCs. In addition, Up4A-induced phosphorylation and activation of S6 kinase (S6K) and ERK1/2 were inhibited by PD98059, whereas S6K but not ERK1/2 activity was inhibited by rapamycin. Up4A also increased Akt phosphorylation, which was inhibited by the PI3K inhibitor, LY294002. Up4A-induced activation of S6K, but not ERK1/2, was also prevented by LY294002, whereas a P2 receptor antagonist, suramin, but not a P2X receptor antagonist, Ip5I, inhibited Up4A-induced phosphorylation and kinase activity of S6K and ERK1/2. Finally, Up4A increased protein expression of cyclin-dependent kinase 2 (CDK2), which was prevented by rapamycin, PD98059 and suramin. These results demonstrate that the signalling mechanisms underlying Up4A-induced proliferation of vascular SMCs are mediated by P2Y receptors and involve the PI3K/Akt and ERK1/2 pathways, leading to the independent activation of S6K and increased in CDK2 expression (Gui et al., 2011). In addition, Up4A promotes the production of the chemokine CCL2 in vascular SMC (Schuchardt et al., 2011), suggesting a role for this EDCF in vascular inflammation. Up4A induced ROS generation via NAD(P)H oxidase activation, which further stimulated CCL2 formation through ERK1/2 and p38 MAPK activation in vascular SMCs. This process by Up4A requires the activation of P2Y2 receptors (Schuchardt et al., 2011).
Intracellular signalling of Up4A in the vasculature of hypertensive and diabetic subjects has not been fully characterized. The effects of sex and sex steroid hormones of Up4A production and activity is also an unexplored area of study.
Conclusions and perspectives
In conclusion, vasoconstrictor prostanoids and Up4A play an important role in vascular dysfunction associated with diabetes and arterial hypertension. The signalling pathways activated by these EDCFs may differ based on vessel type, stages of disease (e.g. early or chronic stage) and sex. The complexity of the intracellular signalling and the interactions between vasoconstrictor prostanoids and Up4A provide an exciting area of investigation for the pursuit of new pharmacological targets for the management of vascular dysfunction in hypertension and diabetes. Sex and sex steroid hormones are essential mediators of these pathways and should be considered in the design of experimental studies and the development of therapeutic compounds.
Acknowledgments
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology, Japan, by the Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private Schools of Japan, and by JSPS KAKENHI Grant Number 26460107 and by the American Heart Association (13SDG17050056, S. G.) and by Fundaçao de Amparo a Pesquisa do Estado de Sao Paulo and Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, Brazil.
Glossary
- AA
arachidonic acid
- AICAR
5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside
- AMPK
AMP-activated protein kinase
- CDK2
cyclin-dependent kinase 2
- cPLA2
cytosolic PLA2
- DOCA
deoxycorticosterone-acetate
- E2
17β-oestradiol
- ECs
endothelial cells
- EDCF
endothelium-derived contracting factor
- EDHF
endothelium-derived hyperpolarizing factor
- EDRF
endothelium-derived relaxing factor
- EPA
eicosapentaenoic acid
- ER
endoplasmic reticulum
- ET-1
endothelin-1
- GK
Goto-Kakizaki
- GPER
G protein-coupled oestrogen receptor
- HETEs
hydroxyeicosatetraenoic acid
- HO-1
haem oxygenase-1
- HUVEC
human umbilical vein endothelial cells
- L-NAME
NG-nitro-l-arginine methyl ester
- L-PGDS
lipocalin-type PGD synthase
- MLC20
myosin light chain 20
- NO
nitric oxide
- NSAIDs
non-steroidal anti-inflammatory drugs
- OLETF
Otsuka Long-Evans Tokushima Fatty
- OPN
osteopontin
- PDGFR
platelet-derived growth factor receptor
- PGI2
prostacyclin
- PGIS
prostacyclin synthase
- ROCK
Rho kinase
- ROS
reactive oxygen species
- S6K
S6 kinase
- SHR
spontaneously hypertensive rats
- SMCs
smooth muscle cells
- STZ
streptozotocin
- TP
TxA2/endoperoxide receptor
- TxA2
thromboxane A2
- TxS
thromboxane synthase
- Up4A
uridine adenosine tetraphosphate
- VP
vasopressin
- WKY
Wistar-Kyoto rats
Conflict of interest
There are no potential conflicts of interest among the authors regarding the publication of this manuscript.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International union of pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamine EH, Urakawa TA, de Oliveira MA, Nigro D, de Carvalho MH, de Cassia A, et al. Decreased endothelium-dependent vasodilation in diabetic female rats: role of prostanoids. J Vasc Res. 2006;43:401–410. doi: 10.1159/000094790. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013a;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-gated ion channels. Br J Pharmacol. 2013b;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013c;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic Receptors. Br J Pharmacol. 2013d;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Nuclear Hormone Receptors. Br J Pharmacol. 2013e;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfranca A, Iniguez MA, Fresno M, Redondo JM. Prostanoid signal transduction and gene expression in the endothelium: role in cardiovascular disease. Cardiovasc Res. 2006;70:446–456. doi: 10.1016/j.cardiores.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Almabrouk TA, Ewart MA, Salt IP, Kennedy S. Perivascular fat, AMP-activated protein kinase and vascular diseases. Br J Pharmacol. 2014;171:595–617. doi: 10.1111/bph.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloysius UI, Achike FI, Mustafa MR. Mechanisms underlining gender differences in phenylephrine contraction of normoglycaemic and short-term streptozotocin-induced diabetic WKY rat aorta. Vascul Pharmacol. 2012;57:81–90. doi: 10.1016/j.vph.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Amer M, Bead VR, Bathon J, Blumenthal RS, Edwards DN. Use of nonsteroidal anti-inflammatory drugs in patients with cardiovascular disease: a cautionary tale. Cardiol Rev. 2010;18:204–212. doi: 10.1097/CRD.0b013e3181ce1521. [DOI] [PubMed] [Google Scholar]
- Arnal JF, Fontaine C, Billon-Gales A, Favre J, Laurell H, Lenfant F, et al. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol. 2010;30:1506–1512. doi: 10.1161/ATVBAHA.109.191221. [DOI] [PubMed] [Google Scholar]
- Asano H, Shimizu K, Muramatsu M, Iwama Y, Toki Y, Miyazaki Y, et al. Prostaglandin H2 as an endothelium-dependent contracting factor modulates endothelin-1-induced contraction. J Hypertens. 1994;12:383–390. [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Thromboxane A2 receptor antagonists inhibit endothelium-dependent contractions. Hypertension. 1990;15:699–703. doi: 10.1161/01.hyp.15.6.699. [DOI] [PubMed] [Google Scholar]
- Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, et al. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostanoids. Arterioscler Thromb Vasc Biol. 2005;25:1610–1616. doi: 10.1161/01.ATV.0000172688.26838.9f. [DOI] [PubMed] [Google Scholar]
- Bagi Z, Erdei N, Papp Z, Edes I, Koller A. Up-regulation of vascular cyclooxygenase-2 in diabetes mellitus. Pharmacol Rep. 2006;58(Suppl):52–56. [PubMed] [Google Scholar]
- Balakumar P, Taneja G. Fish oil and vascular endothelial protection: bench to bedside. Free Radic Biol Med. 2012;53:271–279. doi: 10.1016/j.freeradbiomed.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Baretella O, Chung SK, Barton M, Xu A, Vanhoutte PM. Obesity and heterozygous endothelial overexpression of prepro-endothelin-1 modulate responsiveness of mouse main and segmental renal arteries to vasoconstrictor agents. Life Sci. 2014;118:206–212. doi: 10.1016/j.lfs.2013.12.214. [DOI] [PubMed] [Google Scholar]
- Barton M. Obesity and aging: determinants of endothelial cell dysfunction and atherosclerosis. Pflugers Arch. 2010;460:825–837. doi: 10.1007/s00424-010-0860-y. [DOI] [PubMed] [Google Scholar]
- Barton M, Beretella O, Meyer MR. Obesity and risk of vascular disease: importance of endothelium-dependent vasoconstriction. Br J Pharmacol. 2012;165:591–602. doi: 10.1111/j.1476-5381.2011.01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Ripperger A, Frantz S, Ergun S, Schwedhelm E, Benndorf RA. Pathophysiology of isoprostanes in the cardiovascular system: implications of isoprostane-mediated thromboxane A2 receptor activation. Br J Pharmacol. 2014;171:3115–3131. doi: 10.1111/bph.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazi Z, Feher A, Cassuto J. Microvascular responsiveness in obesity: implications for therapeutic intervention. Br J Pharmacol. 2012;165:544–560. doi: 10.1111/j.1476-5381.2011.01606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhassen L, Pelle G, Dubois-Rande JL, Adnot S. Improved endothelial function by the thromboxane A2 receptor antagonist S18886 in patients with coronary artery disease treated with aspirin. J Am Coll Cardiol. 2003;41:1198–1204. doi: 10.1016/s0735-1097(03)00048-2. [DOI] [PubMed] [Google Scholar]
- Bolego C, Buccellanti C, Radaelli T, Cetin I, Puglisi L, Folco G, et al. eNOS, COX-2, and prostacyclin production are impaired in endothelial cells from diabetics. Biochem Biophys Res Commun. 2006;339:188–190. doi: 10.1016/j.bbrc.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Bolla M, Matrougui K, Loufrani L, Maclouf J, Levy B, Levy-Toledano S, et al. p38 mitogen-activated protein kinase activation is required for thromboxane- induced contraction in perfused and pressurized rat mesenteric resistance arteries. J Vasc Res. 2002;39:353–360. doi: 10.1159/000065547. [DOI] [PubMed] [Google Scholar]
- Bruder-Nascimento T, da Silva MA, Tostes RC. The involvement of aldosterone on vascular insulin resistance: implications in obesity and type 2 diabetes. Diabetol Metab Syndr. 2014;6:90. doi: 10.1186/1758-5996-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb KJ, Khambata RS, Ahluwalia A. Sexual dimorphism in rodent models of hypertension and atherosclerosis. Br J Pharmacol. 2012;167:298–312. doi: 10.1111/j.1476-5381.2012.02036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunimov N, Laneuville O. Cyclooxygense inhibitors: instrumental drugs to understand cardiovascular homeostasis and arterial thrombosis. Cardiovasc Hematol Disord Drug Targets. 2008;8:268–277. doi: 10.2174/187152908786786250. [DOI] [PubMed] [Google Scholar]
- Burger NZ, Kuzina OY, Osol G, Gokina NI. Estrogen replacement enhances EDHF-mediated vasodilation of mesenteric and uterine resistance arteries: role of endothelial cell Ca2+ Am J Physiol Endocrinol Metab. 2009;296:E503–E512. doi: 10.1152/ajpendo.90517.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev. 2013;66:102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142:592S–599S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- Campia U, Tesauro M, Cardillo C. Human obesity and endothelium-dependent responsiveness. Br J Pharmacol. 2012;165:561–573. doi: 10.1111/j.1476-5381.2011.01661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau SB, Guimaraes DA, Rizzi E, Ceron CS, Souza LL, Tirapelli CR, et al. Pyrrolidine dithiocarbamate down-regulates vascular matrix metalloproteinases and ameliorates vascular dysfunction and remodeling in renovascular hypertension. Br J Pharmacol. 2011;164:372–381. doi: 10.1111/j.1476-5381.2011.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro A. TP receptor antagonism: a new concept in atherothrombosis and stroke prevention. Cerebrovasc Dis. 2009;27(Suppl. 3):20–27. doi: 10.1159/000209262. [DOI] [PubMed] [Google Scholar]
- Chan CK, Mak JC, Man RY, Vanhoutte PM. Rho kinase inhibitors prevent endothelium-dependent contractions in the aorta. J Pharmacol Exp Ther. 2009;329:820–826. doi: 10.1124/jpet.108.148247. [DOI] [PubMed] [Google Scholar]
- Chan YC, Leung FP, Yao X, Lau CW, Vanhoutte PM, Huang Y. Raloxifene modulates pulmonary vascular reactivity in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2007;49:355–361. doi: 10.1097/FJC.0b013e318046f329. [DOI] [PubMed] [Google Scholar]
- Chen G, Suzuki H, Weston AH. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol. 1988;95:1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NX, Moe SM. Vascular calcification: pathophysiology and risk factors. Curr Hypertens Rep. 2012;14:228–237. doi: 10.1007/s11906-012-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheuk BL, Leung PS, Lo AC, Wong PY. Androgen control of cyclooxygenase expression in the rat epididymis. Biol Reprod. 2000;63:775–780. doi: 10.1093/biolreprod/63.3.775. [DOI] [PubMed] [Google Scholar]
- Chignalia AZ, Oliveira MA, Debbas V, Dull RO, Laurindo FR, Touyz RM, et al. Testosterone induces leukocyte migration by NADPH oxidase-driven ROS- and COX2-dependent mechanisms. Clin Sci. 2015;129:39–48. doi: 10.1042/CS20140548. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, et al. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation. 2003;107:1017–1023. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- Costa SG, Anversa P, Scavone C, Sucupira M, Carvalho MHC. Nitric oxide synthase activity in microvessels of SHR and normotensive rats: effects of estrogen. J Hypertens. 1998;25:S80. [Google Scholar]
- Crane GJ, Garland CJ. Thromboxane receptor stimulation associated with loss of SKCa activity and reduced EDHF responses in the rat isolated mesenteric artery. Br J Pharmacol. 2004;142:43–50. doi: 10.1038/sj.bjp.0705756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas AP, Scivoletto R, Fortes ZB, Nigro D, Carvalho MH. Influence of female sex hormones on endothelium-derived vasoconstrictor prostanoid generation in microvessels of spontaneously hypertensive rats. Hypertension. 1999;34:914–919. doi: 10.1161/01.hyp.34.4.914. [DOI] [PubMed] [Google Scholar]
- Davi G, Santilli F, Vazzana N. Thromboxane receptors antagonists and/or synthase inhibitors. Handb Exp Pharmacol. 2012;210:261–286. doi: 10.1007/978-3-642-29423-5_11. [DOI] [PubMed] [Google Scholar]
- Davidge ST, Zhang Y. Estrogen replacement suppresses a prostaglandin H synthase-dependent vasoconstrictor in rat mesenteric arteries. Circ Res. 1998;83:388–395. doi: 10.1161/01.res.83.4.388. [DOI] [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Turco S, Basta G, Lazzerini G, Chancharme L, Lerond L, De Caterina R. Involvement of the TP receptor in TNF-α-induced endothelial tissue factor expression. Vascul Pharmacol. 2014;62:49–56. doi: 10.1016/j.vph.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Denniss SG, Jeffery AJ, Rush JW. RhoA-Rho kinase signaling mediates endothelium- and endoperoxidase-dependent contractile activities characteristic of hypertensive vascular dysfunction. Am J Physiol Heart Circ Physiol. 2010;298:H1391–H1405. doi: 10.1152/ajpheart.01233.2009. [DOI] [PubMed] [Google Scholar]
- Ding H, Triggle CR. Endothelial dysfunction in diabetes: multiple targets for treatment. Pflugers Arch. 2010;459:977–994. doi: 10.1007/s00424-010-0807-3. [DOI] [PubMed] [Google Scholar]
- Dohi Y, Thiel MA, Buhler FR, Luscher TF. Activation of endothelial L-arginine pathway in resistance arteries. Effect of age and hypertension. Hypertension. 1990;16:170–179. doi: 10.1161/01.hyp.16.2.170. [DOI] [PubMed] [Google Scholar]
- Dong LH, Lv P, Han M. Roles of SM22a in cellular plasticity and vascular diseases. Cardiovasc Hematol Disord Drug Targets. 2012;12:119–125. doi: 10.2174/1871529x11202020119. [DOI] [PubMed] [Google Scholar]
- Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006;116:1071–1080. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckles SP, Miller VM. Hormonal modulation of endothelial NO production. Pflugers Arch. 2010;459:841–851. doi: 10.1007/s00424-010-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarizing factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- Ellinsworth DC, Shukla N, Fleming I, Jeremy JY. Interactions between thromboxane A2, thronboxane/prostaglandin (TP) receptors, and endothelium-derived hyperpolarization. Cardiovasc Res. 2014;102:9–16. doi: 10.1093/cvr/cvu015. [DOI] [PubMed] [Google Scholar]
- Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante B, Sessa WB, Flack JR, Yadagiri P, Schwartzman ML. Vasoactivity of 20-hydroxyeicosatetraenoic acid is dependent on metabolism by cyclooxygenase. J Pharmacol Exp Ther. 1989;248:229–232. [PubMed] [Google Scholar]
- Farhat MY, Wolfe R, Vargas R, Foegh ML, Ramwell PW. Effect of testosterone treatment on vasoconstrictor response of left anterior descending coronary artery in male and female pigs. J Cardiovasc Pharmacol. 1995;25:495–500. doi: 10.1097/00005344-199503000-00023. [DOI] [PubMed] [Google Scholar]
- Favero G, Paganelli C, Buffoli B, Rodella LF, Rezzani R. Endothelium and its alterations in cardiovascular diseases: life style intervention. Biomed Res Int. 2014;2014:801896. doi: 10.1155/2014/801896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br J Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. EDHF: new therapeutic targets? Pharmacol Res. 2004;49:565–580. doi: 10.1016/j.phrs.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Feletou M, Tang EH, Vanhoutte PM. Nitric oxide the gatekeeper of endothelial vasomotor control. Front Biosci. 2008;13:4198–4217. doi: 10.2741/3000. [DOI] [PubMed] [Google Scholar]
- Feletou M, Verbeuren TJ, Vanhoutte PM. Endothelium-dependent contractions in SHR: a tale of prostanoid TP and IP receptors. Br J Pharmacol. 2009;156:563–574. doi: 10.1111/j.1476-5381.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, Huang Y, Vanhoutte PM. Vasoconstrictor prostanoids. Pflugers Arch. 2010a;459:941–950. doi: 10.1007/s00424-010-0812-6. [DOI] [PubMed] [Google Scholar]
- Feletou M, Kohler R, Vanhoutte PM. Endothelium-derived vasoactive factors and hypertension: possible roles in pathogenesis and as treatment targets. Curr Hypertens Rep. 2010b;12:267–275. doi: 10.1007/s11906-010-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, Huang Y, Vanhoutte PM. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br J Pharmacol. 2011;164:894–912. doi: 10.1111/j.1476-5381.2011.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira NS, Cau SB, Silva MA, Manzato CP, Mestriner FL, Matsumoto T, et al. Diabetes impairs the vascular effects of aldosterone mediated by G protein-coupled estrogen receptor activation. Front Pharmacol. 2015;6:34. doi: 10.3389/fphar.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flammer AJ, Luscher TF. Three decades of endothelium research: from the detection of nitric oxide to the everyday implementation of endothelial function measurements in cardiovascular diseases. Swiss Med Wkly. 2010;140:w13122. doi: 10.4414/smw.2010.13122. [DOI] [PubMed] [Google Scholar]
- Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- Ford RJ, Rush JW. Endothelium-dependent vasorelaxation to the AMPK activator AICAR is enhanced in aorta from hypertensive rats and is NO and EDCF dependent. Am J Physiol Heart Circ Physiol. 2011;300:H64–H75. doi: 10.1152/ajpheart.00597.2010. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol. 2011;164:213–223. doi: 10.1111/j.1476-5381.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois H, Athirakul K, Mao L, Rockman H, Coffman TM. Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension. 2004;43:364–369. doi: 10.1161/01.HYP.0000112225.27560.24. [DOI] [PubMed] [Google Scholar]
- Francois H, Makhanova N, Ruiz P, Ellison J, Mao L, Rockman HA, et al. A role for the thromboxane receptor in L-NAME hypertension. Am J Physiol Renal Physiol. 2008;295:F1096–F1102. doi: 10.1152/ajprenal.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the regulation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Redondo AB, Briones AM, Martinez-Revelles S, Palao T, Vila L, Alonso MJ, et al. c-Src, ERK1/2 and Rho kinase mediate hydrogen peroxide-induced vascular contraction in hypertension: role of TXA2, NAD(P)H oxidase and mitochondria. J Hypertens. 2015;33:77–87. doi: 10.1097/HJH.0000000000000383. [DOI] [PubMed] [Google Scholar]
- Garland CJ, Hiley CR, Dora KA. EDHF: spreading the influence of the endothelium. Br J Pharmacol. 2011;164:839–852. doi: 10.1111/j.1476-5381.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge T, Hughes H, Junquero DC, Wu KK, Vanhoutte PM, Boulanger CM. Endothelium-dependent contractions are associated with both augmented expression of prostaglandin H synthase-1 and hypersensitivity to prostaglandin H2 in the SHR aorta. Circ Res. 1995;76:1003–1010. doi: 10.1161/01.res.76.6.1003. [DOI] [PubMed] [Google Scholar]
- Gelosa P, Ballerio R, Banfi C, Nobili E, Glanella A, Pignieri A, et al. Terutroban, a thromboxane/prostaglandin endoperoxide receptor antagonist, increases survival in stroke-prone rats by preventing systemic inflammation and endothelial dysfunction: comparison with aspirin and rosuvastatin. J Pharmacol Exp Ther. 2010;334:199–205. doi: 10.1124/jpet.110.165787. [DOI] [PubMed] [Google Scholar]
- Giannarelli C, Zafar MU, Badimon JJ. Prostanoid and TP-receptors in atherothrombosis: is there a role for their antagonism? Thromb Haemost. 2010;104:949–954. doi: 10.1160/TH10-03-0195. [DOI] [PubMed] [Google Scholar]
- Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Feletou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol. 2005;146:834–845. doi: 10.1038/sj.bjp.0706390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluais P, Paysant J, Badier-Commander C, Verbeuren T, Vanhoutte PM, Feletou M. In SHR aorta, calcium ionophore A-23187 releases prostacyclin and thromboxane A2 as endothelium-derived contracting factors. Am J Physiol Heart Circ Physiol. 2006;291:H2255–H2264. doi: 10.1152/ajpheart.01115.2005. [DOI] [PubMed] [Google Scholar]
- Gluais P, Vanhoutte PM, Feletou M. Mechanisms underlying ATP-induced endothelium-dependent contractions in the SHR aorta. Eur J Pharmacol. 2007;556:107–114. doi: 10.1016/j.ejphar.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Gomez E, Schwendemann C, Roger S, Simonet S, Paysant J, Courchay C, et al. Aging and prostacyclin responses in aorta and platelets from WKY and SHR rats. Am J Physiol Heart Circ Physiol. 2008;295:H2198–H2211. doi: 10.1152/ajpheart.00507.2008. [DOI] [PubMed] [Google Scholar]
- Gonzales RJ, Duckles SP, Krause DN. Dihydrotestosterone stimulates cerebrovascular inflammation through NFkappaB, modulating contractile function. J Cereb Blood Flow Metab. 2009;29:244–253. doi: 10.1038/jcbfm.2008.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulopoulou S, Davidge ST. Molecular mechanisms of maternal vascular dysfunction in preeclampsia. Trends Mol Med. 2015;21:88–97. doi: 10.1016/j.molmed.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Goulopoulou S, Hannan JL, Matsumoto T, Webb RC. Pregnancy reduces RhoA/Rho kinase and protein kinase C signaling pathways downstream of thromboxane receptor activation in the rat uterine artery. Am J Physiol Heart Circ Physiol. 2012;302:H2477–H2488. doi: 10.1152/ajpheart.00900.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgic I, Kaistha BP, Hoyer J, Kohler R. Endothelial Ca2+-activated K+ channels in normal and impaired EDHF-dilator response – relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol. 2009;157:509–526. doi: 10.1111/j.1476-5381.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol. 2004;141:881–903. doi: 10.1038/sj.bjp.0705698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser T, Yu Y, Fitzgerald GA. Emotion recollected in tranquility: lessons learned from the COX-2 saga. Annu Rev Med. 2010;61:17–33. doi: 10.1146/annurev-med-011209-153129. [DOI] [PubMed] [Google Scholar]
- Gui Y, Walsh MP, Jankowski V, Jankowski J, Zheng XL. Up4A stimulates endothelium-independent contraction of isolated rat pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2008;294:L733–L738. doi: 10.1152/ajplung.00403.2007. [DOI] [PubMed] [Google Scholar]
- Gui Y, He G, Walsh MP, Zheng XL. Signaling mechanisms mediating uridine adenosine tetraphosphate-induced proliferation of human vascular smooth muscle cells. J Cardiovasc Pharmacol. 2011;58:654–662. doi: 10.1097/FJC.0b013e318231e929. [DOI] [PubMed] [Google Scholar]
- Guo Z, Su W, Allen S, Pang H, Daugherty A, Smart E, et al. COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res. 2005;67:723–735. doi: 10.1016/j.cardiores.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Han G, Li F, Yu X, White RE. GPER: a novel target for non-genomic estrogen action in the cardiovascular system. Pharmacol Res. 2013;71:53–60. doi: 10.1016/j.phrs.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Hansen PB, Hristovska A, Wolff H, Vanhoutte P, Jensen BL, Bie P. Uridine adenosine tetraphosphate affects contractility of mouse aorta and decreases blood pressure in conscious rats and mice. Acta Physiol (Oxf) 2010;200:171–179. doi: 10.1111/j.1748-1716.2010.02135.x. [DOI] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Miller VM. Sex, hormones and neuroeffector mechanisms. Acta Physiol (Oxf) 2011;203:155–165. doi: 10.1111/j.1748-1716.2010.02192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Kawasaki H, Abe K, Kanno M. Superoxide dismutase recovers altered endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol. 1991;261:H1086–H1094. doi: 10.1152/ajpheart.1991.261.4.H1086. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Yamada K, Esaki M, Kuzuya M, Satake S, Ishikawa T, et al. Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochem Biophys Res Commun. 1995;214:847–855. doi: 10.1006/bbrc.1995.2364. [DOI] [PubMed] [Google Scholar]
- Hermenegildo C, Oviedo PJ, Cano A. Cyclooxygenases regulation by estradiol on endothelium. Curr Pharm Des. 2006;12:205–215. doi: 10.2174/138161206775193136. [DOI] [PubMed] [Google Scholar]
- Hernanz R, Briones AM, Salaices M, Alonso MJ. New roles for old pathways? A circuitous relationship between reactive oxygen species and cyclo-oxygenase in hypertension. Clin Sci. 2014;126:111–121. doi: 10.1042/CS20120651. [DOI] [PubMed] [Google Scholar]
- Hishikawa K, Nakaki T, Marumo T, Suzuki H, Kato R, Saruta T. Up-regulation of nitric oxide synthase by estradiol in human aortic endothelial cells. FEBS Lett. 1995;360:291–293. doi: 10.1016/0014-5793(95)00124-r. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Tong W, Shelhamer JH, Lawrence M, Cunnion RE. Eicosanoid production by human aortic endothelial cells in response to endothelin. Am J Physiol. 1994;267:H2290–H2296. doi: 10.1152/ajpheart.1994.267.6.H2290. [DOI] [PubMed] [Google Scholar]
- Hopkins PN. Molecular biology of atherosclerosis. Physiol Rev. 2013;93:1317–1542. doi: 10.1152/physrev.00004.2012. [DOI] [PubMed] [Google Scholar]
- Huang A, Sun D, Kaley G, Koller A. Estrogen maintains nitric oxide synthesis in arterioles of female hypertensive rats. Hypertension. 1997;29:1351–1356. doi: 10.1161/01.hyp.29.6.1351. [DOI] [PubMed] [Google Scholar]
- Hutchison SJ, Sudhir K, Chou TM, Sievers RE, Zhu BQ, Sun YP, et al. Testosterone worsens endothelial dysfunction associated with hypercholesterolemia and environmental tobacco smoke exposure in male rabbit aorta. J Am Coll Cardiol. 1997;29:800–807. doi: 10.1016/s0735-1097(96)00570-0. [DOI] [PubMed] [Google Scholar]
- Huxley VH, Wang J. Cardiovascular sex differences influencing microvascular exchange. Cardiovasc Res. 2010;87:230–242. doi: 10.1093/cvr/cvq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida K, Matsumoto T, Taguchi K, Kamata K, Kobayashi T. Mechanisms underlying altered extracellular nucleotide-induced contractions in mesenteric arteries from rats in later-stage type 2 diabetes: effect of ANG II type 1 receptor antagonism. Am J Physiol Heart Circ Physiol. 2011;301:H1850–H1861. doi: 10.1152/ajpheart.00502.2011. [DOI] [PubMed] [Google Scholar]
- Ishida K, Matsumoto T, Taguchi K, Kamata K, Kobayashi T. Pravastatin normalizes endothelium-derived contracting factor-mediated response via suppression of Rho-kinase signalling in mesenteric artery from aged type 2 diabetic rat. Acta Physiol (Oxf) 2012;205:255–265. doi: 10.1111/j.1748-1716.2011.02403.x. [DOI] [PubMed] [Google Scholar]
- Jankowski V, Tolle M, Vanholder R, Schonfelder G, van der Giet M, Henning L, et al. Uridine adenosine tetraphosphate: a novel endothelium-derived vasoconstrictive factor. Nat Med. 2005;11:223–227. doi: 10.1038/nm1188. [DOI] [PubMed] [Google Scholar]
- Jankowski V, Meyer AA, Schlattmann P, Gui Y, Zhang XL, Stamcou I, et al. Increased uridine adenosine tetraphosphate concentrations in plasma of juvenile hypertensives. Arterioscler Thromb Vasc Biol. 2007;27:1776–1781. doi: 10.1161/ATVBAHA.107.143958. [DOI] [PubMed] [Google Scholar]
- Jankowski V, Schulz A, Kretschmer A, Mischak H, Boehringer F, van der Giet M, et al. The enzymatic activity of the VEGFR2 receptor for the biosynthesis of dinucleotide polyphosphates. J Mol Med. 2013;91:1095–1107. doi: 10.1007/s00109-013-1036-y. [DOI] [PubMed] [Google Scholar]
- Jerez S, Sierra L, Coviello A, de Bruno MP. Endothelial dysfunction and improvement of the angiotensin II-reactivity in hypercholesterolemic rabbits: role of cyclooxygenase metabolites. Eur J Pharmacol. 2008;580:182–189. doi: 10.1016/j.ejphar.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Jones RL, Giembycz MA, Woodward DF. Prostanoid receptor antagonists: development strategies and therapeutic applications. Br J Pharmacol. 2009;158:104–145. doi: 10.1111/j.1476-5381.2009.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahonen M, Tolvanen JP, Sallinen K, Wu X, Porsti I. Influence of gender on control of arterial tone in experimental hypertension. Am J Physiol. 1998;44:H15–H22. doi: 10.1152/ajpheart.1998.275.1.H15. [DOI] [PubMed] [Google Scholar]
- Kamata K, Kobayashi T. Changes in superoxide dismutase mRNA expression by streptozotocin-induced diabetes. Br J Pharmacol. 1996;119:583–589. doi: 10.1111/j.1476-5381.1996.tb15712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata K, Miyata N, Kasuya Y. Impairment of endothelium-dependent relaxation and changes in levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br J Pharmacol. 1989;97:614–618. doi: 10.1111/j.1476-5381.1989.tb11993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassan M, Choi SK, Galan M, Biship A, Umezawa K, Trebak M, et al. Enhanced NF-kB activity impairs vascular function through PARP-1-, SP-1-, and COX-2-dependent mechanisms in type 2 diabetes. Diabetes. 2013;62:2078–2087. doi: 10.2337/db12-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katusic ZS, Vanhoutte PM. Superoxide anion is an endothelium-derived contracting factor. Am J Physiol. 1989;257:H33–H37. doi: 10.1152/ajpheart.1989.257.1.H33. [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Shepherd JT, Vanhoutte PM. Endothelium-dependent contractions to calcium ionophore A23187, arachidonic acid and acetylcholine in canine basilar arteries. Stroke. 1988;19:476–479. doi: 10.1161/01.str.19.4.476. [DOI] [PubMed] [Google Scholar]
- Kauser K, Rubanyi GM. Gender differences in endothelial dysfunction in the aorta of spontaneously hypertensive rats. Hypertension. 1995;25:517–523. doi: 10.1161/01.hyp.25.4.517. [DOI] [PubMed] [Google Scholar]
- Kawka DW, Ouellet M, Hetu PO, Singer II, Riendeau D. Double-label expression studies of prostacyclin synthase, thromboxane synthase and COX isoforms in normal aortic endothelium. Biochim Biophys Acta. 2007;1771:45–54. doi: 10.1016/j.bbalip.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Keen HL, Brands MW, Smith MJ, Jr, Shek EW, Hall JE. Thromboxane is required for full expression of angiotensin hypertension in rats. Hypertension. 1997;29:310–314. doi: 10.1161/01.hyp.29.1.310. [DOI] [PubMed] [Google Scholar]
- Khalil RA. Sex hormones as potential modulators of vascular function in hypertension. Hypertension. 2005;46:249–254. doi: 10.1161/01.HYP.0000172945.06681.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittikulsuth W, Sullivan JC, Pollock DM. ET-1 actions in the kidney: evidence for sex differences. Br J Pharmacol. 2013;168:318–326. doi: 10.1111/j.1476-5381.2012.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Matsumoto T, Kamata K. Mechanisms underlying the chronic pravastatin treatment-induced improvement in the impaired endothelium-dependent aortic relaxation seen in streptozotocin-induced diabetic rats. Br J Pharmacol. 2000;131:231–238. doi: 10.1038/sj.bjp.0703572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Nogami T, Taguchi K, Matsumoto T, Kamata K. Diabetic state, high plasma insulin and angiotensin II combine to augment endothelin-1-induced vasoconstriction via ETA receptors and ERK. Br J Pharmacol. 2008;155:974–983. doi: 10.1038/bjp.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J Physiol Pharmacol. 2013;64:409–421. [PubMed] [Google Scholar]
- Kraskiewicz H, FitzGerald U. Interfering with endoplasmic reticulum stress. Trends Pharmacol Sci. 2012;33:53–63. doi: 10.1016/j.tips.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Lempiäinen J, Finckenberg P, Levijoki J, Mervaala E. AMPK activator AICAR ameliorates ischaemia reperfusion injury in the rat kidney. Br J Pharmacol. 2012;166:1905–1915. doi: 10.1111/j.1476-5381.2012.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Stallone JN. Estrogen potentiates vsopressin-induced contraction of female rat aorta by enhancing cyclooxygenase-2 and thromboxane function. Am J Physiol Heart Circ Physiol. 2005;289:H1542–H1550. doi: 10.1152/ajpheart.01024.2004. [DOI] [PubMed] [Google Scholar]
- Li M, Kuo L, Stallone JN. Estrogen potentiates constrictor prostanoid function in female rat aorta by upregulation of cyclooxygenase-2 and thromboxane pathway expression. Am J Physiol Heart Circ Physiol. 2008;294:H2444–H2455. doi: 10.1152/ajpheart.01121.2007. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang Y, Vanhoutte PM. Upregulation of heme oxygenase 1 by hemin impairs endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Hypertension. 2011;58:926–934. doi: 10.1161/HYPERTENSIONAHA.111.173807. [DOI] [PubMed] [Google Scholar]
- Linder AE, Tumbri M, Linder FF, Webb RC, Leite R. Uridine adenosine tetraphosphate induces contraction and relaxation in rat aorta. Vascul Pharmacol. 2008;48:202–207. doi: 10.1016/j.vph.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CQ, Leung FP, Wong SL, Wong WT, Lau CW, Lu L, et al. Thromboxane prostanoid receptor activation impairs endothelial nitric oxide-dependent vasorelaxations: the role of Rho kinase. Biochem Pharmacol. 2009;78:374–381. doi: 10.1016/j.bcp.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez JG, Moral-Sanz J, Frazziano G, Gomez-Villalobos MJ, Moreno L, Menendez C, et al. Type 1 diabetes-induced hyper-responsiveness to 5-hydroxytryptamine in rat pulmonary arteries via oxidative stress and induction of cyclooxygenase-2. J Pharmacol Exp Ther. 2011;338:400–407. doi: 10.1124/jpet.111.179515. [DOI] [PubMed] [Google Scholar]
- Maguire JJ, Davenport AP. Endothelin@25 – new agonists, antagonists, inhibitors and emerging research frontiers: IUPHAR Review 12. Br J Pharmacol. 2014;171:5555–5572. doi: 10.1111/bph.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique C, Lastra G, Sowers JR. New insights into insulin action and resistance in the vasculature. Ann N Y Acad Sci. 2014;1311:135–150. doi: 10.1111/nyas.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrachelli VG, Miranda FJ, Centeno JM, Salom JB, Torregrosa G, Jover-Mengual T, et al. Role of NO-synthases and cyclooxygenases in the hyperreactivity of male rabbit carotid artery to testosterone under experimental diabetes. Pharmacol Res. 2010;61:62–70. doi: 10.1016/j.phrs.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Martinez AC, Hernandez M, Novella S, Martinez MP, Pagan RM, Hermenegildo C, et al. Diminished neurogenic femoral artery vasoconstrictor response in a Zucker obese rat model: differential regulation of NOS and COX derivatives. PLoS ONE. 2014;9:e106372. doi: 10.1371/journal.pone.0106372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda A, Mathur R, Halushka PV. Testosterone increases thromboxane A2 receptors in cultured rat aortic smooth muscle cells. Circ Res. 1991;69:638–643. doi: 10.1161/01.res.69.3.638. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kakami M, Noguchi E, Kobayashi T, Kamata K. Imbalance between endothelium-derived relaxing and contracting factors in mesenteric arteries from aged OLETF rats, a model of Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007a;293:H1480–H1490. doi: 10.1152/ajpheart.00229.2007. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Noguchi E, Kobayashi T, Kamata K. Mechanisms underlying the chronic pioglitazone treatment-induced improvement in the impaired endothelium-dependent relaxation seen in aortas from diabetic rats. Free Radic Biol Med. 2007b;42:993–1007. doi: 10.1016/j.freeradbiomed.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kobayashi T, Kamata K. Relationships among ET-1, PPARgamma, oxidative stress and endothelial dysfunction in diabetic animals. J Smooth Muscle Res. 2008a;44:41–55. doi: 10.1540/jsmr.44.41. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Noguchi E, Ishida K, Kobayashi T, Yamada N, Kamata K. Metformin normalizes endothelial function by suppressing vasoconstrictor prostanoids in mesenteric arteries from OLETF rats, a model of type 2 diabetes. Am J Physiol Heart Circ Physiol. 2008b;295:H1165–H1176. doi: 10.1152/ajpheart.00486.2008. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Ishida K, Kobayashi T, Kamata K. Pyrrolidine dithiocarbamate reduces vascular prostanoid-induced responses in aged type 2 diabetic rat model. J Pharmacol Sci. 2009a;110:326–333. doi: 10.1254/jphs.09116fp. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Nakayama N, Ishida K, Kobayashi T, Kamata K. Eicosapentaenoic acid improves imbalance between vasodilator and vasoconstrictor actions of endothelium-derived factors in mesenteric arteries from rats at chronic stage of type 2 diabetes. J Pharmacol Exp Ther. 2009b;329:324–334. doi: 10.1124/jpet.108.148718. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Takaoka E, Ishida K, Nakayama N, Noguchi E, Kobayashi T, et al. Abnormalities of endothelium-dependent responses in mesenteric arteries from Otsuka Long-Evans Tokushima Fatty (OLETF) rats are improved by chronic treatment with thromboxane A2 synthase inhibitor. Atherosclerosis. 2009c;205:87–95. doi: 10.1016/j.atherosclerosis.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Ishida K, Nakayama N, Taguchi K, Kobayashi T, Kamata K. Mechanisms underlying the losartan treatment-induced improvement in the endothelial dysfunction seen in mesenteric arteries from type 2 diabetic rats. Pharmacol Res. 2010;62:271–281. doi: 10.1016/j.phrs.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Tostes RC, Webb RC. The role of uridine adenosine tetraphosphate in the vascular system. Adv Pharmacol Sci. 2011a;2011:435132. doi: 10.1155/2011/435132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Tostes RC, Webb RC. Uridine adenosine tetraphosphate-induced contraction is increased in renal but not pulmonary arteries from DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol. 2011b;301:H409–H417. doi: 10.1152/ajpheart.00084.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Tostes RC, Webb RC. Alterations in vasoconstrictor responses to the endothelium-derived contracting factor uridine adenosine tetraphosphate are region specific in DOCA-salt hypertensive rats. Pharmacol Res. 2012;65:81–90. doi: 10.1016/j.phrs.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Lopes RA, Taguchi K, Kobayashi T, Tostes RC. Linking the beneficial effects of current therapeutic approaches in diabetes to the vascular endothelin system. Life Sci. 2014a;118:129–135. doi: 10.1016/j.lfs.2013.12.216. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Watanabe S, Kawamura R, Taguchi K, Kobayashi T. Enhanced uridine adenosine tetraphosphate-induced contraction in renal artery from type 2 diabetic Goto-Kakizaki rats due to activated cyclooxygenase/thromboxane receptor axis. Pflugers Arch. 2014b;466:331–342. doi: 10.1007/s00424-013-1330-0. [DOI] [PubMed] [Google Scholar]
- Meagher EF, FitzGerald GA. Disordered eicosanoid formation in pregnancy-induced hypertension. Circulation. 1993;88:1324–1333. doi: 10.1161/01.cir.88.3.1324. [DOI] [PubMed] [Google Scholar]
- Meyer MC, Cummings K, Osol G. Estrogen replacement attenuates resistance artery adrenergic sensitivity via endothelial vasodilators. Am J Physiol. 1997;272:H2264–H2270. doi: 10.1152/ajpheart.1997.272.5.H2264. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Prossnitz ER, Barton M. The G protein-coupled estrogen receptor GPER/GPR30 as a regulator of cardiovascular function. Vascul Pharmacol. 2011;55:17–25. doi: 10.1016/j.vph.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Amann K, Field AS, Hu C, Hathaway HJ, Kanagy NL, et al. Deletion of G protein-coupled estrogen receptor increases endothelial vasoconstriction. Hypertension. 2012;59:507–512. doi: 10.1161/HYPERTENSIONAHA.111.184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao CY, Li ZY. The role of perivascular adipose tissue in vascular smooth muscle cell growth. Br J Pharmacol. 2012;165:643–658. doi: 10.1111/j.1476-5381.2011.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F, Simonet S, Vayssettes-Courchay C, Bertin F, Sansilvestri-Morel P, Bernhardt F, et al. Altered TP receptor function in isolated, perfused kidneys of nondiabetic and diabetic ApoE-deficient mice. Am J Physiol Renal Physiol. 2008b;294:F120–F129. doi: 10.1152/ajprenal.00111.2007. [DOI] [PubMed] [Google Scholar]
- Michel FS, Man GS, Man RY, Vanhoutte PM. Hypertension and the absence of EDHF-mediated responses favour endothelium-dependent contractions in renal arteries of the rat. Br J Pharmacol. 2008a;155:217–226. doi: 10.1038/bjp.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola T, Turunen P, Avela K, Orpana A, Viinikka L, Ylikorkala O. 17 beta-estradiol stimulates prostacyclin, but not endothelin-1, production in human vascular endothelial cells. J Clin Endocrinol Metab. 1995;80:1832–1836. doi: 10.1210/jcem.80.6.7775630. [DOI] [PubMed] [Google Scholar]
- Miller VM, Mulvagh SL. Sex steroids and endothelial function: translating basic science to clinical practice. Trends Pharmacol Sci. 2007;28:263–270. doi: 10.1016/j.tips.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Miller VM, Vanhoutte PM. 17 Beta-estradiol augments endothelium-dependent contractions to arachidonic acid in rabbit aorta. Am J Physiol. 1990;258:R1502–R1507. doi: 10.1152/ajpregu.1990.258.6.R1502. [DOI] [PubMed] [Google Scholar]
- Miyata N, Roman RJ. Role of 20-hydroxyeicosapentaenoic acid (20-HETE) in vascular system. J Smooth Muscle Res. 2005;41:175–193. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- Mukohda M, Okada M, Hara Y, Yamawaki H. Methylglyoxal accumulation in arterial walls causes vascular contractile dysfunction in spontaneously hypertensive rats. J Pharmacol Sci. 2012;120:26–35. doi: 10.1254/jphs.12088fp. [DOI] [PubMed] [Google Scholar]
- Nacci C, Tarquinio M, De Benedictis L, Mauro A, Zigrino A, Carratu MR, et al. Endothelial dysfunction in mice with streptozotocin-induced type 1 diabetes is opposed by compensatory overexpression of cyclooxygenase-2 in the vasculature. Endocrinology. 2009;150:849–861. doi: 10.1210/en.2008-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik V, Leaf EM, Hu JH, Yang HY, Nguyen NB, Giacheli CM, et al. Sources of cells that contribute to atherosclerotic intimal calcification: an in vivo genetic fate mapping study. Cardiovasc Res. 2012;94:545–554. doi: 10.1093/cvr/cvs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata N. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol Ther. 2008;118:18–35. doi: 10.1016/j.pharmthera.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Nakano T. Roles of lipid-modulating enzymes diacylglycerol kinase and cyclooxygenase under pathophysiological conditions. Anat Sci Int. 2015;90:22–32. doi: 10.1007/s12565-014-0265-7. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Ndisang JF, Wang R, Vannacci A, Marzocca C, Fantappie O, Mazzanti R, et al. Haeme oxygenase-1 and cardiac anaphylaxis. Br J Pharmacol. 2001;134:1689–1696. doi: 10.1038/sj.bjp.0704427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Wu JL, Zhang M, Xu J, Zou MH. Endothelial nitric oxide synthase-dependent tyrosine nitration of prostacyclin synthase in diabetes in vivo. Diabetes. 2006;55:3133–3141. doi: 10.2337/db06-0505. [DOI] [PubMed] [Google Scholar]
- Nilsson BO, Olde B, Leeb-Lundberg LM. G protein-coupled oestrogen receptor 1 (GPER1)/GPR30: a new player in cardiovascular and metabolic oestrogenic signalling. Br J Pharmacol. 2011;163:1131–1139. doi: 10.1111/j.1476-5381.2011.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobe K, Nezu Y, Tsumita N, Hashimoto T, Honda K. Intra- and extrarenal arteries exhibit different profiles of contractile responses in high glucose conditions. Br J Pharmacol. 2008;155:1204–1213. doi: 10.1038/bjp.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numaguchi Y, Harada M, Osanai H, Hayashi K, Toki Y, Okumura K, et al. Altered gene expression of prostacyclin synthase and prostacyclin receptor in the thoracic aorta of spontaneously hypertensive rats. Cardiovasc Res. 1999;41:682–688. doi: 10.1016/s0008-6363(98)00239-9. [DOI] [PubMed] [Google Scholar]
- Nunes KP, Rigsby CS, Webb RC. RhoA/Rho-kinase and vascular diseases: what is the link? Cell Mol Life Sci. 2010;67:3823–3836. doi: 10.1007/s00018-010-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon EB, Golbabaie A, van Breemen C. In the presence of L-NAME SERCA blockade induces endothelium-dependent contraction of mouse aorta through activation of smooth muscle prostaglandin H2/thromboxane A2 receptors. Br J Pharmacol. 2002;137:545–553. doi: 10.1038/sj.bjp.0704884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- Ospina JA, Duckles SP, Krause DN. 17Beta-estradiol decreases vascular tone in cerebral arteries by shifting COX-dependent vasoconstriction to vasodilation. Am J Physiol Heart Circ Physiol. 2003;285:H241–H250. doi: 10.1152/ajpheart.00018.2003. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries AR, Kuebler WM. Normal endothelium. Handb Exp Pharmacol. 2006;176:1–40. doi: 10.1007/3-540-32967-6_1. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C, Leung SW, Vahnoutte PM, Man RY. Chronic inhibition of nitric-oxide synthase potentiates endothelium-dependent contractions in the rat aorta by augmenting the expression of cyclooxygenase-2. J Pharmacol Exp Ther. 2010;334:373–380. doi: 10.1124/jpet.110.167098. [DOI] [PubMed] [Google Scholar]
- Quilley J, Chen YJ. Role of COX-2 in the enhanced vasoconstrictor effect of arachidonic acid in the diabetic rat kidney. Hypertension. 2003;42:837–843. doi: 10.1161/01.HYP.0000085650.29823.F2. [DOI] [PubMed] [Google Scholar]
- Ragolia L, Palaia T, Hall CE, Maesaka JK, Eguchi N, Urade Y. Accelerated glucose intolerance, nephropathy, and atherosclerosis in prostaglandin D2 synthase knock-out mice. J Biol Chem. 2005;280:29946–29955. doi: 10.1074/jbc.M502927200. [DOI] [PubMed] [Google Scholar]
- Ramos-Alves FE, de Queiroz DB, Santos-Rocha J, Duarte GP, Xavier FE. Effect of age and COX-2-derived prostanoids on the progression of adult vascular dysfunction in the offspring of diabeic rats. Br J Pharmacol. 2012a;166:2198–2208. doi: 10.1111/j.1476-5381.2012.01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Alves FE, de Queiroz DB, Santos-Rocha J, Duarte GP, Xavier FE. Increased cyclooxygenase-2-derived prostanoids contributes to the hyperreactivity to noradrenaline in mesenteric resistance arteries from offspring of diabetic rats. PLoS ONE. 2012b;7:e50593. doi: 10.1371/journal.pone.0050593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randriamboavonjy V, Busse R, Fleming I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension. 2003;41:801–806. doi: 10.1161/01.HYP.0000047240.33861.6B. [DOI] [PubMed] [Google Scholar]
- Rapoport RM, Williams SP. Role of prostaglandins in acetylcholine-induced contraction of aorta from spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 1996;28:64–75. doi: 10.1161/01.hyp.28.1.64. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 2013;17:20–33. doi: 10.1016/j.cmet.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res. 2009;50:S29–S34. doi: 10.1194/jlr.R800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovati GE, Sala A, Capra V, Dahlen SE, Folco G. Dual COXIB/TP antagonists: a possible new twist in NSAID pharmacology and cardiovascular risk. Trends Pharmacol Sci. 2010;31:102–107. doi: 10.1016/j.tips.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Kim SF, Mollace V. Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: relevance and clinical implications. Am J Physiol Regul Integr Comp Physiol. 2013;304:R473–R487. doi: 10.1152/ajpregu.00355.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Contreras C, Villalba N, Martinez P, Martinez AC, Briones A, et al. Altered arachidonic acid metabolism via COX-1 and COX-2 contributes to the endothelial dysfunction of penile arteries from obese Zucker rats. Br J Pharmacol. 2010;159:604–616. doi: 10.1111/j.1476-5381.2009.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval YH, Atef ME, Levesque LO, Li Y, Anand-Srivastava MB. Endothelin-1 signaling in vascular physiology and pathophysiology. Curr Vasc Pharmacol. 2014;12:202–214. doi: 10.2174/1570161112666140226122054. [DOI] [PubMed] [Google Scholar]
- Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- Schror K, Morinelli TA, Masuda A, Matsuda K, Mathur RS, Halushka PV. Testosterone treatment enhances thromboxane A2 mimetic induced coronary artery vasoconstriction in guinea pigs. Eur J Clin Invest. 1994;24:50–52. doi: 10.1111/j.1365-2362.1994.tb02428.x. [DOI] [PubMed] [Google Scholar]
- Schuchardt M, Prufer J, Prufer N, Wiedon A, Huang T, Chebli M, et al. The endothelium-derived contracting factor uridine adenosine tetraphosphate induces P2Y(2)-mediated pro-inflammatory signaling by monocyte chemoattractant protein-1 formation. J Mol Med. 2011;89:799–810. doi: 10.1007/s00109-011-0750-6. [DOI] [PubMed] [Google Scholar]
- Schuchardt M, Tolle M, Prufer J, Prufer N, Huang T, Jankowski V, et al. Uridine adenosine tetraphosphate activation of the purinergic receptor P2Y enhances in vitro vascular calcification. Kidney Int. 2012;81:256–265. doi: 10.1038/ki.2011.326. [DOI] [PubMed] [Google Scholar]
- Schwartzman ML, Falck JR, Yadagiri P, Escalante B. Metabolism of 20-hydroxyeicosatetraenoic acid by cyclooxygenase. Formation and identification of novel endothelium-dependent vasoconstrictor metabolites. J Biol Chem. 1989;264:11658–11662. [PubMed] [Google Scholar]
- Sena CM, Pereira AM, Seica R. Endothelial dysfunction – a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832:2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Sherman TS, Chambliss KL, Gibson LL, Pace MC, Mendelsohn ME, Pfister SL, et al. Estrogen acutely activates prostacyclin synthesis in ovine fetal pulmonary artery endothelium. Am J Respir Cell Mol Biol. 2002;26:610–616. doi: 10.1165/ajrcmb.26.5.4528. [DOI] [PubMed] [Google Scholar]
- Sheu ML, Ho FM, Yang RS, Chao KF, Lin WW, Lin-Shiau SY, et al. High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler Thromb Vasc Biol. 2005;25:539–545. doi: 10.1161/01.ATV.0000155462.24263.e4. [DOI] [PubMed] [Google Scholar]
- Shi Y, Vanhoutte PM. Oxidative stress and COX cause hyper-responsiveness in vascular smooth muscle of the femoral artery from diabetic rats. Br J Pharmacol. 2008;154:639–651. doi: 10.1038/bjp.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Feletou M, Ku DD, Man RY, Vanhoutte PM. The calcium ionophore A23187 induces endothelium-dependent contractions in femoral arteries from rats with streptozotocin-induced diabetes. Br J Pharmacol. 2007;150:624–632. doi: 10.1038/sj.bjp.0706999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Man RY, Vanhoutte PM. Two isoforms of cyclooxygenase contribute to augmented endothelium-dependent contractions in femoral arteries of 1-year old rats. Acta Pharmacol Sin. 2008;29:185–192. doi: 10.1111/j.1745-7254.2008.00749.x. [DOI] [PubMed] [Google Scholar]
- Siller-Matula JM, Krumphuber J, Jilma B. Pharmacokinetic, pharmacodynamic and clinical profile of novel antiplatelet drugs targeting vascular diseases. Br J Pharmacol. 2010;59:502–517. doi: 10.1111/j.1476-5381.2009.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- Sobrino A, Oviedo PJ, Novella S, Laguna-Fernandez A, Bueno C, Garcia-Perez MA, et al. Estradiol selectively stimulates endothelial prostacyclin production through estrogen receptor-{alpha} J Mol Endocrinol. 2010;44:237–246. doi: 10.1677/JME-09-0112. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction by G-proteins, Rho kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Song D, Arikawa E, Galipeau D, Battell M, McNeill JH. Androgens are necessary for the development of fructose-induced hypertension. Hypertension. 2004;43:667–672. doi: 10.1161/01.HYP.0000118018.77344.4e. [DOI] [PubMed] [Google Scholar]
- Sparks MA, Makhanova NA, Griffiths RC, Snouwaert JN, Koller BH, Coffman TM. Thromboxane receptors in smooth muscle promote hypertension, vascular remodeling, and sudden death. Hypertension. 2013;61:166–173. doi: 10.1161/HYPERTENSIONAHA.112.193250. [DOI] [PubMed] [Google Scholar]
- Spitler KM, Matsumoto T, Webb RC. Suppression of endoplasmic reticulum stress improves endothelium-dependent contractile responses in aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2013;305:H344–H353. doi: 10.1152/ajpheart.00952.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- Sullivan JC, Sasser JM, Pollock DM, Pollock JS. Sexual dimorphism in renal production of prostanoids in spontaneously hypertensive rats. Hypertension. 2005;45:406–411. doi: 10.1161/01.HYP.0000156879.83448.93. [DOI] [PubMed] [Google Scholar]
- Szabo C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br J Pharmacol. 2009;156:713–727. doi: 10.1111/j.1476-5381.2008.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taba Y, Sasaguri T, Miyagi M, Abumiya T, Miwa Y, Ikeda T, et al. Fluid shear stress induces lipocalin-type prostaglandin D2 synthase in vascular endothelial cells. Circ Res. 2000;86:967–973. doi: 10.1161/01.res.86.9.967. [DOI] [PubMed] [Google Scholar]
- Taddei S, Vanhoutte PM. Role of endothelium in endothelin-evoked contractions in the rat aorta. Hypertension. 1993;21:9–15. doi: 10.1161/01.hyp.21.1.9. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Salvetti A. Vasodilation to acetylcholine in primary and secondary forms of human hypertension. Hypertension. 1993;21:929–933. doi: 10.1161/01.hyp.21.6.929. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222–2229. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti A. Vascular effects of endothelin-1 in essential hypertension: relationship with cyclooxygenase-derived endothelium-dependent contracting factors and nitric oxide. J Cardiovasc Pharmacol. 2000;35:S37–S40. doi: 10.1097/00005344-200000002-00009. [DOI] [PubMed] [Google Scholar]
- Taguchi K, Hida M, Matsumoto T, Ikeuchi-Takahashi Y, Onishi H, Kobayashi T. Effect of short-term polyphenol treatment on endothelial dysfunction and thromboxane A2 levels in streptozotocin-induced diabetic mice. Biol Pharm Bull. 2014;37:1056–1061. doi: 10.1248/bpb.b14-00157. [DOI] [PubMed] [Google Scholar]
- Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun. 1997;240:737–741. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics. 2008;32:409–418. doi: 10.1152/physiolgenomics.00136.2007. [DOI] [PubMed] [Google Scholar]
- Tang EH, Vanhoutte PM. Prostanoids and reactive oxygen species: team players in endothelium-dependent contractions. Pharmacol Ther. 2009;122:140–149. doi: 10.1016/j.pharmthera.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Tang EH, Vanhoutte PM. Endothelial dysfunction: a strategic target in the treatment of hypertension? Pflugers Arch. 2010;459:995–1004. doi: 10.1007/s00424-010-0786-4. [DOI] [PubMed] [Google Scholar]
- Tang EH, Feletou M, Huang Y, Man RY, Vanhoutte PM. Acetylcholine and sodium nitroprusside cause long-term inhibition of EDCF-mediated contractions. Am J Physiol Heart Circ Physiol. 2005a;289:H2434–H2440. doi: 10.1152/ajpheart.00568.2005. [DOI] [PubMed] [Google Scholar]
- Tang EH, Ku DD, Tipoe GL, Feletou M, Man RY, Vanhoutte PM. Endothelium-dependent contractions occur in the aorta of wild-type and COX2-/- knockout but not COX1-/- knockout mice. J Cardiovasc Pharmacol. 2005b;46:761–765. doi: 10.1097/01.fjc.0000187174.67661.67. [DOI] [PubMed] [Google Scholar]
- Tang EH, Leung FP, Huang Y, Feletou M, So KF, Man RY, et al. Calcium and reactive oxygen species increase in endothelial cells in response to releasers of endothelium-derived contracting factor. Br J Pharmacol. 2007;151:15–23. doi: 10.1038/sj.bjp.0707190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang EH, Jensen BL, Skott O, Leung GP, Feletou M, Man RY, et al. The role of prostaglandin E and thromboxane-prostanoid receptors in the response to prostaglandin E2 in the aorta of Wistar Kyoto rats and spontaneously hypertensive rats. Cardiovasc Res. 2008;78:130–138. doi: 10.1093/cvr/cvm112. [DOI] [PubMed] [Google Scholar]
- Tejera N, Balfagon G, Marin J, Ferrer M. Gender differences in the endothelial regulation of alpha2-adrenoceptor-mediated contraction in the rat aorta. Clin Sci. 1999;97:19–25. [PubMed] [Google Scholar]
- Toda N, Imamura T, Okamura T. Alteration of nitric oxide-mediated blood flow regulation in diabetes mellitus. Pharmacol Ther. 2010;127:189–209. doi: 10.1016/j.pharmthera.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Toda N, Nakanishi S, Tanabe S. Aldosterone affects blood flow and vascular tone regulated by endothelium-derived NO: therapeutic implications. Br J Pharmacol. 2013;168:519–533. doi: 10.1111/j.1476-5381.2012.02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolle M, Schuchardt M, Wiedon A, Huang T, Klockel L, Jankowski J, et al. Differential effects of uridine adenosine tetraphosphate on purinoceptors in the rat isolated perfused kidney. Br J Pharmacol. 2010;161:530–540. doi: 10.1111/j.1476-5381.2010.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tostes RC, Nigro D, Fortes ZB, Carvalho MH. Effects of estrogen on the vascular system. Braz J Med Biol Res. 2003;36:1143–1158. doi: 10.1590/s0100-879x2003000900002. [DOI] [PubMed] [Google Scholar]
- Tostes RC, Fortes ZB, Callera GE, Montezano AC, Touyz RM, Webb RC, et al. Endothelin, sex and hypertension. Clin Sci. 2008;114:85–97. doi: 10.1042/CS20070169. [DOI] [PubMed] [Google Scholar]
- Toth P, Rozsa B, Springo Z, Doczi T, Koller A. Isolated human and rat cerebral arteries constrict to increases in flow: role of 20-HETE and TP receptors. J Cereb Blood Flow Metab. 2011;31:2096–2105. doi: 10.1038/jcbfm.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10:4–18. doi: 10.2174/157016112798829760. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol. 2004;122:339–352. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- Traupe T, Lang M, Goettsch W, Munter K, Morawietz H, Vetter W, et al. Obesity increases prostanoid-mediated vasoconstriction and vascular thromboxane receptor gene expression. J Hypertens. 2002;20:2239–2245. doi: 10.1097/00004872-200211000-00024. [DOI] [PubMed] [Google Scholar]
- Triggle CR, Howarth A, Cheng ZJ, Ding H. Twenty-five years since the discovery of endothelium-derived relaxing factor (EDRF): does a dysfunctional endothelium contribute to the development of type 2 diabetes? Can J Physiol Pharmacol. 2005;83:681–700. doi: 10.1139/y05-069. [DOI] [PubMed] [Google Scholar]
- Valentin F, Field MC, Tippins JR. The mechanism of oxidative stress stabilization of the thromboxane receptor in COS-7 cells. J Biol Chem. 2004;279:8316–8324. doi: 10.1074/jbc.M306761200. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. COX-1 and vascular disease. Clin Pharmacol Ther. 2009;86:212–215. doi: 10.1038/clpt.2009.108. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. Endothelium-dependent contractions in hypertension: when prostacyclin becomes ugly. Hypertension. 2011;57:526–531. doi: 10.1161/HYPERTENSIONAHA.110.165100. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Tang EH. Endothelium-dependent contractions: when a good guy turns bad! J Physiol. 2008;586:5295–5304. doi: 10.1113/jphysiol.2008.161430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte PM, Feletou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Vapaatalo H, Mervaala E. Clinically important factors influencing endothelial function. Med Sci Monit. 2001;7:1075–1085. [PubMed] [Google Scholar]
- Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol. 2009;157:527–536. doi: 10.1111/j.1476-5381.2009.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessieres E, Guihot AL, Toutain B, Maquigneau M, Fassot C, Loufrani L, et al. COX-2-derived prostanoids and oxidative stress additionally reduce endothelium-mediated relaxation in old type 2 diabetic rats. PLoS ONE. 2013;8:e68217. doi: 10.1371/journal.pone.0068217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba N, Contreras C, Hernandez M, Garcia-Sacristan A, Prieto D. Impaired Ca2+ handling in penile arteries from prediabetic Zucker rats: involvement of Rho kinase. Am J Physiol Heart Circ Physiol. 2011;300:H2044–H2053. doi: 10.1152/ajpheart.01204.2010. [DOI] [PubMed] [Google Scholar]
- Virdis A, Ghiadoni L, Taddei S. Human endothelial dysfunction: EDCFs. Pflugers Arch. 2010;459:1015–1023. doi: 10.1007/s00424-009-0783-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ying L, Chen YY, Shen YL, Guo R, Jin KK, et al. Induction of heme oxygenase-1 ameliorates vascular dysfunction in streptozotocin-induced type 2 diabetic rats. Vascul Pharmacol. 2014;61:16–24. doi: 10.1016/j.vph.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Wiedon A, Tolle M, Bastine J, Schuchardt M, Huang T, Jankowski V, et al. Uridine adenosine tetraphosphate (Up4A) is a strong inductor of smooth muscle cell migration via activation of the P2Y2 receptor and cross-communication to the PDGF receptor. Biochem Biophys Res Commun. 2012;417:1035–1040. doi: 10.1016/j.bbrc.2011.12.088. [DOI] [PubMed] [Google Scholar]
- Williams SP, Shackelford DP, Iams SG, Mustafa SJ. Endothelium-dependent relaxation in estrogen-treated spontaneously hypertensive rats. Eur J Pharmacol. 1988;145:205–207. doi: 10.1016/0014-2999(88)90231-2. [DOI] [PubMed] [Google Scholar]
- Wilson DP, Susnjar M, Kiss E, Sutherland C, Walsh MP. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochem J. 2005;389:763–774. doi: 10.1042/BJ20050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Cavanagh CC, Lesher AM, Frey AJ, Russell SE, Smyth EM. Activation-dependent stabilization of the human thromboxane receptor: role of reactive oxygen species. J Lipid Res. 2009;50:1047–1056. doi: 10.1194/jlr.M800447-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MS, Vanhoutte PM. COX-mediated endothelium-dependent contractions: from the past to recent discoveries. Acta Pharmacol Sin. 2010;31:1095–1102. doi: 10.1038/aps.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MS, Man RY, Vanhoutte PM. Calcium-independent phospholipase A(2) plays a key role in the endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2010a;298:H1260–H1266. doi: 10.1152/ajpheart.01068.2009. [DOI] [PubMed] [Google Scholar]
- Wong SL, Leung FP, Lau CW, Au CL, Yung LM, Yao X, et al. Cyclooxygenase-2-derived prostaglandin F2alpha mediates endothelium-dependent contraction in the aortae of hamsters with increased impact during aging. Circ Res. 2009;104:228–235. doi: 10.1161/CIRCRESAHA.108.179770. [DOI] [PubMed] [Google Scholar]
- Wong SL, Wong WT, Tian XY, Lau CW, Huang Y. Prostaglandins in action indispensable roles of cyclooxygenase-1 and -2 in endothelium-dependent contractions. Adv Pharmacol. 2010b;60:61–83. doi: 10.1016/B978-0-12-385061-4.00003-9. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Jones RL, Narumiya S. International Union of Basic and Clinical Pharmacology. LXXXIII: classification of prostanoid receptors, updating 15 years of progress. Pharmacol Rev. 2011;63:471–538. doi: 10.1124/pr.110.003517. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yang D, Feletou M, Boulanger CM, Wu HF, Levens N, Zhang JN, et al. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol. 2002;136:104–110. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Feletou M, Levens N, Zhang JN, Vanhoutte PM. A diffusible substance(s) mediates endothelium-dependent contractions in the aorta of SHR. Hypertension. 2003a;41:143–148. doi: 10.1161/01.hyp.0000047651.45322.16. [DOI] [PubMed] [Google Scholar]
- Yang D, Levens N, Zhang JN, Vanhotte PM, Feletou M. Specific potentiation of endothelium-dependent contractions in SHR by tetrahydrobiopterin. Hypertension. 2003b;41:136–142. doi: 10.1161/01.hyp.0000047669.93078.a7. [DOI] [PubMed] [Google Scholar]
- Yang D, Gluais P, Zhang JN, Vanhoutte PM, Feletou M. Endothelium-dependent contractions to acetylcholine, ATP and the calcium ionophore A23187 in aortas from spontaneously hypertensive and normotensive rats. Fundam Clin Pharmacol. 2004a;18:321–326. doi: 10.1111/j.1472-8206.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- Yang D, Gluais P, Zhang JN, Vanhoutte PM, Feletou M. Nitric oxide and inactivation of the endothelium-dependent contracting factor released by acetylcholine in spontaneously hypertensive rat. J Cardiovasc Pharmacol. 2004b;43:815–820. doi: 10.1097/00005344-200406000-00011. [DOI] [PubMed] [Google Scholar]
- Yang XQ, Wang YY, Chen AF. Increased superoxide contributes to enhancement of vascular contraction in Ins2(Akita) diabetic mice, an autosomal dominant mutant model. Clin Exp Pharmacol Physiol. 2008;35:1097–1103. doi: 10.1111/j.1440-1681.2007.04756.x. [DOI] [PubMed] [Google Scholar]
- Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther. 2014;141:272–282. doi: 10.1016/j.pharmthera.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kosaka H. Sex-specific acute effect of estrogen on endothelium-derived contracting factor in the renal artery of hypertensive Dahl rats. J Hypertens. 2002;20:237–246. doi: 10.1097/00004872-200202000-00013. [DOI] [PubMed] [Google Scholar]
- Zhang M, Dong Y, Xu J, Xie Z, Wu Y, Song Y, et al. Thromboxane receptor activates the AMP-activated protein kinase in vascular smooth muscle cells via hydrogen peroxide. Circ Res. 2008;102:328–337. doi: 10.1161/CIRCRESAHA.107.163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou MS, Kosaka H, Tian RX, Abe Y, Chen QH, Yoneyama H, et al. L-arginine improves endothelial function in renal artery of hypertensive Dahl rats. J Hypertens. 2001;19:421–429. doi: 10.1097/00004872-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Varadharaj S, Zhao X, Parinandi N, Flavahan NA, Zweier JL. Acetylcholine causes endothelium-dependent contraction of mouse arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1027–H1032. doi: 10.1152/ajpheart.00226.2005. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Merkus D, Cheng C, Duckers HJ, Jan Danser AH, Duncker DJ. Uridine adenosine tetraphosphate is a novel vasodilator in the coronary microcirculation which acts through purinergic P1 but not P2 receptors. Pharmacol Res. 2013;67:10–17. doi: 10.1016/j.phrs.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Zhu N, Liu B, Luo W, Zhang Y, Li H, Li S, et al. Vasoconstrictor role of cyclooxygenase-1-mediated prostacyclin synthesis in non-insulin-dependent diabetic mice induced by high-fat diet and streptozotocin. Am J Physiol Heart Circ Physiol. 2014;307:H319–H327. doi: 10.1152/ajpheart.00022.2014. [DOI] [PubMed] [Google Scholar]
- Zou MH. Peroxynitrite and protein tyrosine nitration of prostacyclin. Prostaglandins Other Lipid Mediat. 2007;82:119–127. doi: 10.1016/j.prostaglandins.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Zou MH, Shi C, Cohen RA. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes. 2002;51:198–203. doi: 10.2337/diabetes.51.1.198. [DOI] [PubMed] [Google Scholar]
- Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11:89–97. doi: 10.1080/10623320490482619. [DOI] [PubMed] [Google Scholar]
- Zuccollo A, Shi C, Mastroianni R, Maitland-Toolan KA, Weisbrod RM, Zang M, et al. The thromboxane A2 receptor antagonist S18886 prevents enhanced atherogenesis caused by diabetes mellitus. Circulation. 2005;112:3001–3008. doi: 10.1161/CIRCULATIONAHA.105.581892. [DOI] [PubMed] [Google Scholar]


