Abstract
Background and Purpose
The discovery that flavonoids are capable of inhibiting platelet function has led to their investigation as potential antithrombotic agents. However, despite the range of studies on the antiplatelet properties of flavonoids, little is known about the mechanisms by which flavonoids inhibit platelet function. In this study, we aimed to explore the pharmacological effects of a polymethoxy flavonoid, nobiletin, in the modulation of platelet function.
Experimental Approach
The ability of nobiletin to modulate platelet function was explored by using a range of in vitro and in vivo experimental approaches. Aggregation, dense granule secretion and spreading assays were performed using washed platelets. Fibrinogen binding, α-granule secretion and calcium mobilization assays were performed using platelet-rich plasma and whole blood was used in impedance aggregometry and thrombus formation experiments. The effect of nobiletin in vivo was assessed by measuring tail bleeding time using C57BL/6 mice.
Key Results
Nobiletin was shown to suppress a range of well-established activatory mechanisms, including platelet aggregation, granule secretion, integrin modulation, calcium mobilization and thrombus formation. Nobiletin extended bleeding time in mice and reduced the phosphorylation of PKB (Akt) and PLCγ2 within the collagen receptor (glycoprotein VI)-stimulated pathway, in addition to increasing the levels of cGMP and phosphorylation of vasodilator-stimulated phosphoprotein, a protein whose activity is associated with inhibitory cyclic nucleotide signalling.
Conclusions and Implications
This study provides insight into the underlying molecular mechanisms through which nobiletin modulates haemostasis and thrombus formation. Therefore, nobiletin may represent a potential antithrombotic agent of dietary origins.
Tables of Link
| LIGANDS | |
|---|---|
| ADP | Luteolin |
| Apigenin | LY294002 |
| Aspirin | Nitric oxide (NO) |
| ATP | ODQ |
| cGMP | Quercetin |
| Clopidogrel | Thrombin |
| Collagen | TXA2 |
| Fibrinogen | von Willebrand factor |
| Indomethacin |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b).
Introduction
Platelet activation is initiated upon binding of various ligand molecules to their cognate cell surface receptors, which trigger intracellular signalling cascades that lead to shape change, release of granule contents, thromboxane A2 (TXA2) synthesis and release and thrombus formation (Gibbins, 2004). A number of currently available anti-thrombotic drugs (e.g. aspirin and clopidogrel) are hampered by associated side effects including severe bleeding (Barrett et al., 2008; Mackman, 2008; Michelson, 2008), and therefore, the development of safer, more efficacious and target-specific drugs to prevent thrombosis is a pressing priority. The association between diet and cardiovascular disease is well established (Wright et al., 2012). Epidemiological studies (Middleton et al., 2000; Joshipura et al., 2001; Hubbard et al., 2004; Benavente-Garcia and Castillo, 2008; Hooper et al., 2012) have suggested that regular intake of foods rich in flavonoids can reduce cardiovascular disease risks. Indeed, dietary flavonoids have been shown to inhibit platelet function, reduce lipid levels and maintain vascular integrity, which may underpin a reduced risk of cardiovascular diseases. The complex nature of the mechanism of action of plant flavonoids on the cardiovascular system including platelets has initiated research to better understand the basis of the relationship between these dietary components and cardiovascular health in order to develop more effective strategies to prevent or treat thrombotic diseases (Wright et al., 2012).
Since extracts from citrus fruits, particularly lemons (Citrus limon) are widely used in traditional medications in India and China, we sought to explore the importance of flavonoids which are abundant in citrus fruits on platelet function. Nobiletin is a highly abundant flavonoid present in these and is structurally similar to tangeretin, another abundant flavonoid in such sources (Benavente-Garcia and Castillo, 2008). Nobiletin has been shown to have several beneficial effects on different cell types including anti-proliferative and anti-apoptotic properties on cancer cells (Akao et al., 2008; Chen et al., 2014), and inhibitory effects on inflammation (Wu et al., 2006). Nobiletin has been shown to reduce the adhesive properties of platelets (Sempinska et al., 1977; Robbins, 1988), although the mechanisms through which this occurs and its potential to reduce thrombus formation have not been explored previously. In a previous study, we reported that tangeretin inhibits platelet function and thrombus formation via elevation of cGMP and inhibition of PI3K signalling (Vaiyapuri et al., 2013). In this study, we demonstrated that nobiletin is a potent inhibitor of platelet function that acts through similar effects on platelet function, and established these mechanisms as common modes of action which flavonoids may share in order to modulate the function of these cells.
Methods
Detailed methods for immunoblotting analysis, cyclic nucleotide, flow cytometry, clot retraction, platelet spreading, in vitro thrombus formation and tail bleeding assays are provided in Supporting Information Appendix S1.
The animal welfare and ethical statement
All animals (C57BL/6 mice) were used following appropriate approval from the University of Reading Local Ethics Review Panel and a license from the British Home Office accordance with the Animal (Scientific Procedures) Act 1986. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). Nobiletin or vehicle control was administered blindly, and the number of mice required was calculated based on previous studies within the authors' laboratory (Vaiyapuri et al., 2013). Every animal used in the experiments was included in the analysis (no exclusions were made). There were no adverse effects observed in mice infused with nobiletin.
Platelet preparation, aggregation and dense granule secretion
Human platelet preparation and aggregation assays were performed using standard protocols. After informed consent had been obtained, blood was collected from healthy, aspirin-free human volunteers into a syringe containing 4% citrate (1:10 ratio). The citrated whole blood was mixed with an equal volume of physiological saline (0.9% NaCl) and was used for impedance aggregometry. The blood was centrifuged for 20 min at 102× g at room temperature to obtain platelet-rich plasma (PRP) for aggregation, flow cytometry, clot retraction and cyclic nucleotide assays. Further centrifugation of PRP [with 125 ng·mL–1 prostaglandin I2 (PGI2)] at 1413× g for 10 min resulted in a platelet pellet that was re-suspended in modified Tyrodes-HEPES buffer (134 mM NaCl, 2.9 mM KCl, 0.34 mM Na2HPO4·12H2O, 12 mM NaHCO3, 20 mM HEPES and 1 mM MgCl2, pH 7.3) and washed by centrifuging again at the same speed for 10 min. The resulting platelet pellet was re-suspended in modified Tyrodes-HEPES buffer to a final density of 4 × 108 cells·mL−1 for aggregation and ATP secretion assays.
Platelet aggregation assays were performed by optical aggregometry using cross-linked collagen-related peptide [CRP-XL, a selective agonist for the platelet collagen receptor glycoprotein (GP) VI, from Prof R Farndale (University of Cambridge)], collagen (Horm collagen, Nycomed, Linz, Austria) and thrombin (Sigma Aldrich, Poole, UK) in the presence or absence of various concentrations of nobiletin (Sigma Aldrich; dissolved in DMSO to prepare stock solutions). The final concentration of DMSO used as required was 0.01% (v/v), which did not affect platelet function and was used as vehicle control. Similarly, dense granule secretion was measured by monitoring the level of ATP released from washed platelets upon activation with CRP-XL using a luciferin-luciferase luminescence substrate (Chrono-log, Havertown, PA, USA) by luminescence aggregometry. All the aggregation assays (optical, impedance and luminescence) were performed using Chrono-Log Model 700 Whole Blood/Optical-Lumi Aggregometer.
Statistical analysis
The data obtained from aggregation, fibrinogen binding, α-granule secretion and in vitro thrombus formation assays were analysed using non-parametric repeated measures anova (Friedman test). Median fluorescence intensity values obtained with fibrinogen binding and α-granule secretion assays were converted into percentages for comparison of controls with nobiletin-inhibited samples. Data obtained from dense granule secretion, clot retraction, spreading and cyclic nucleotide assays were analysed using parametric repeated measures anova. Data from calcium mobilization experiments were analysed by ‘R’ statistical software and the percentage of maximum level of calcium release was analysed using non-parametric repeated measures anova (Friedman test). Tail bleeding assay data were analysed using t-test. All the statistical analyses were performed using GraphPad Prism (version 5.04) from GraphPad Software Inc. (La Jolla, CA, USA).
Results
Nobiletin inhibits human platelet activation
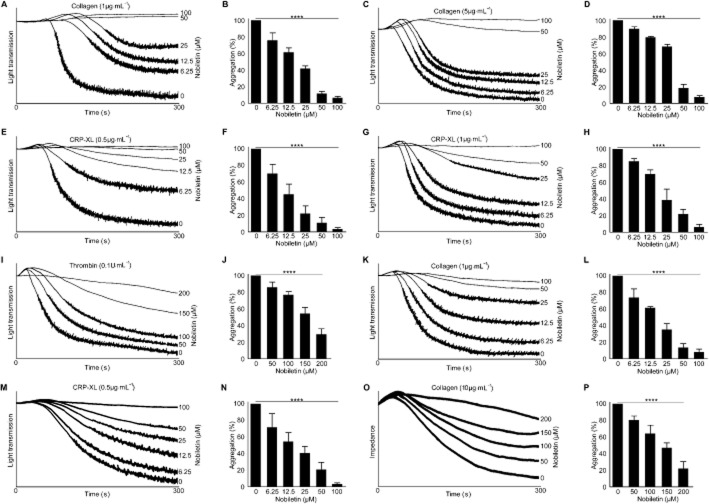
Optical aggregometry was used to analyse the effects of nobiletin on platelet function upon activation with collagen, CRP-XL and thrombin. Washed human platelets were incubated with vehicle (0.01% DMSO) or nobiletin (6.25, 12.5, 25, 50 and 100 μM) for 5 min before stimulation with collagen (1 μg·mL−1) for up to 5 min (Figure 1A) in an optical aggregometer. Nobiletin reduced platelet aggregation in a concentration-dependent manner at 5 min (Figure 1A and B) with 95% inhibition achieved at 100 μM. The inhibitory effects of nobiletin were reduced modestly when a higher concentration of collagen (5 μg·mL−1) was used (Figure 1C and D). Since collagen activates platelets by binding both glycoprotein VI (GPVI) and integrin α2β1, a GPVI-selective agonist, CRP-XL, was used in aggregation assays to determine whether the inhibition of platelet aggregation occurred through the blockade of GPVI signalling. Platelet aggregation stimulated with CRP-XL (0.5 μg·mL−1) was also inhibited by nobiletin in a concentration-dependent manner (Figure 1E and F). Similar to collagen, a higher concentration of CRP-XL (1 μg·mL−1) reduced the level of inhibition induced by nobiletin (Figure 1G and H). In order to determine whether nobiletin is able to affect the signalling cascades activated by G-protein coupled receptors, aggregation assays were performed using thrombin as an agonist. A concentration of 0.1 U·mL−1 of thrombin was chosen to obtain similar levels of aggregation as obtained with other agonists such as collagen (1 μg·mL−1) and CRP-XL (0.5 μg·mL−1). Thrombin (0.1 U·mL−1)-stimulated platelet activation was inhibited by nobiletin only at higher concentrations (50, 100, 150 and 200 μM; Figure 1I and J). Lower concentrations of nobiletin did not inhibit thrombin-induced platelet aggregation (data not shown).
Figure 1.
Nobiletin inhibits agonist-induced platelet activation. Human-washed platelet aggregation performed in the presence or absence of various concentrations of nobiletin was monitored for 5 min by optical aggregometry following stimulation with 1 μg·mL−1 (A and B) and 5 μg·mL−1 collagen (C and D), 0.5 μg·mL−1 (E and F) and 1 μg·mL−1 CRP-XL (G and H) and 0.1 U·mL−1 thrombin (I and J). Similarly, the effects of nobiletin on PRP were analysed following stimulation with 1 μg·mL−1 collagen (K and L). Indomethacin (10 μM)-treated platelets were stimulated with 0.5 μg·mL−1 CRP-XL in the presence and absence of nobiletin (M and N). Whole blood aggregation was performed using 10 μg·mL−1 collagen in the presence and absence of nobiletin by impedance aggregometry (O and P). Cumulative data represent mean values ± SD (n = 3), the level of aggregation obtained at 5 min with vehicle-treated samples (0 μM) was taken as 100%. The P-values shown in the figure are calculated by non-parametric repeated measures anova (Friedman test; ****P < 0.0001).
Some dietary flavonoids such as quercetin (Wright et al., 2012) and tangeretin (Vaiyapuri et al., 2013) have been shown to bind plasma proteins, and therefore, the effect of nobiletin on platelet activation in PRP was examined to compare its effects with washed platelets. Collagen (1 μg·mL−1)-induced platelet aggregation in PRP was inhibited by nobiletin in a concentration-dependent manner to a similar extent to that observed with washed platelets (Figure 1K and L). This suggests that the effects of nobiletin at this concentration are not adversely affected by the presence of plasma proteins. To understand if the effects of nobiletin in collagen-induced platelet aggregation are dependent on secondary activation mediated through molecules that are synthesized and released from platelets, further aggregation assays were performed using indomethacin (an inhibitor of COX, which prevents TXA2 synthesis)-treated washed platelets. CRP-XL (0.5 μg·mL−1)-induced aggregation was reduced significantly by nobiletin in indomethacin (10 μM)-treated platelets (Figure 1M and N) indicating that the effects of nobiletin are not fully dependent on the inhibition of the effects of secondary mediators such as TXA2. To further understand the effects of nobiletin in whole blood in the presence of other cells such as red blood cells and leukocytes, impedance aggregometry was performed. Collagen at a concentration of 10 μg·mL−1 was used to obtain a similar level of aggregation to 1 μg·mL−1 in washed platelets. Despite the higher concentration of collagen, nobiletin (50 μM and above) reduced aggregation significantly (Figure 1O and P). These analyses together indicate that nobiletin predominantly inhibits GPVI-stimulated platelet activation, although it is able to modulate platelet activation induced by other agonists such as thrombin, at higher concentrations.
Integrin αIIbβ3-mediated inside-out signalling is modulated by nobiletin
Platelet inside-out signalling to integrin αIIbβ3 plays an essential role in the modulation of its conformation to increase affinity for plasma fibrinogen and von Willebrand factor (vWF) binding and consequent platelet aggregation (Calderwood, 2004). In order to explore the effects of nobiletin in inside-out signalling that leads to the modulation of integrin αIIbβ3 affinity, fibrinogen binding was measured by flow cytometry in the presence or absence of nobiletin. Consistent with platelet aggregation, CRP-XL (1 μg·mL−1)-induced fibrinogen binding in PRP was inhibited significantly at all concentrations of nobiletin used (6.25, 12.5, 25, 50, 100 and 200 μM; Figure 2A and Supporting Information Fig. S1A).
Figure 2.
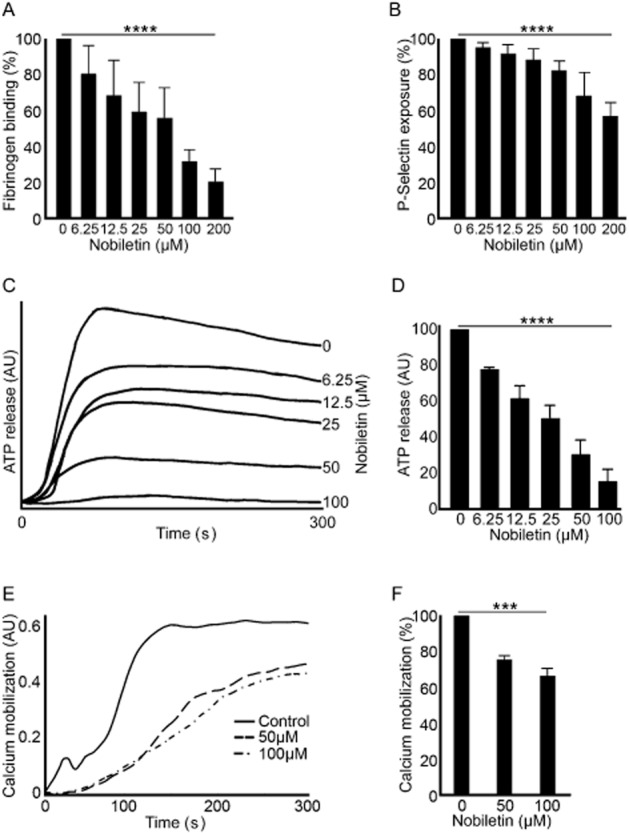
Nobiletin modulates platelet inside-out signalling, granule secretion and calcium mobilization. The effect of nobiletin on CRP-XL (1 μg·mL−1)-induced fibrinogen binding (A) and P-selectin exposure (B) in PRP was measured by flow cytometry. The median fluorescence intensity values were converted into percentages for comparison. The level of fibrinogen binding or P-selectin exposure with vehicle controls was taken as 100%. Data represent mean ± SD (n = 5) and the P-values shown were calculated using non-parametric repeated measures anova (Friedman test). Similarly, the effect of nobiletin on dense granule secretion upon stimulation with CRP-XL (1 μg·mL−1) was measured in washed platelets (C and D). The level of ATP obtained with vehicle controls was taken as 100%. Data represent mean ± SD (n = 4) and the P-values shown were calculated using parametric repeated measures anova. Fluo4-NW dye labelled human PRP was stimulated with 1 μg·mL−1 CRP-XL in the presence of 50 or 100 μM nobiletin or vehicle control and the intracellular calcium levels were measured by flow cytometry (E). Traces shown are representative of four separate experiments. The inhibition of intracellular calcium levels was calculated by comparing the levels obtained at 5 min (F) in the presence or absence (taken as 100%) of nobiletin. Data represent mean ± SD (n = 4). The P-values shown in the figure were as calculated by using non-parametric repeated measures anova (Friedman test; ***P < 0.001 and ****P < 0.0001). AU represents arbitrary units.
Nobiletin inhibits platelet granule secretion
Platelet degranulation is an important step towards thrombus formation as the released granule contents enhance rapid activation of platelets through paracrine/autocrine actions (Gibbins, 2004). Platelet α-granules are rich in proteins such as fibrinogen, vWF and P-selectin and dense granules are rich in non-proteinaceous substances such as ADP, ATP and calcium. To assess the ability of nobiletin to inhibit platelet α-granule secretion, P-selectin exposure on the surface of platelets upon stimulation with CRP-XL was measured by flow cytometry. Different concentrations of nobiletin were pre-incubated with human PRP prior to activation with CRP-XL (1 μg·mL−1) and analysis of P-selectin exposure. Nobiletin inhibited significantly P-selectin exposure at all concentrations used in PRP (Figure 2B and Supporting Information Fig. S1B). Maximal inhibition of approximately 50% was obtained with 200 μM nobiletin. Similarly, the level of ATP secreted from washed platelets assayed in parallel with aggregation was inhibited by nobiletin upon stimulation with CRP-XL (1 μg·mL−1) in a concentration-dependent manner (Figure 2C and 2D). The level of inhibition observed in dense granule secretion was larger than that of α-granule secretion, which is likely due to different conditions (washed platelets, stirring and 37°C for dense granule secretion; PRP, non-stirring and room temperature for α-granule secretion) used in these two assays. Together these data suggest that nobiletin is able to reduce platelet granule secretion and thereby control platelet activation.
Intracellular calcium mobilization is modulated by nobiletin
An elevation of intracellular calcium levels in platelets is important for the regulation of thrombus formation, reorganization of the actin cytoskeleton necessary for shape change (Hathaway and Adelstein, 1979), degranulation and integrin αIIbβ3-mediated signalling (Shattil and Brass, 1987). To analyse the effects of nobiletin on intracellular calcium mobilization, this was measured in human platelets (PRP) by flow cytometry. CRP-XL (1 μg·mL−1)-activated cytoplasmic calcium levels were inhibited significantly by nobiletin (50 and 100 μM). Maximum inhibition of approximately 40% was achieved with 100 μM nobiletin (Figure 2E and 2F). The initial acceleration rate of calcium elevation was inhibited substantially by nobiletin (Figure 2E). These data suggest that nobiletin affects the elevation of intracellular calcium levels to modulate platelet function.
Nobiletin affects integrin αIIbβ3-mediated outside-in signalling
Following binding to fibrinogen, integrin αIIbβ3 clustering transduces signals (outside-in signalling) into platelets to allow spreading and in the latter phase of its formation, clot retraction (Calderwood, 2004). The modulatory effects of nobiletin on integrin αIIbβ3-mediated outside-in signalling were analysed by measuring clot retraction and platelet spreading under static conditions. Clot formation was initiated by adding thrombin to PRP in the absence or presence of nobiletin (12.5, 25, 50 and 100 μM), and the rate of clot retraction was monitored over 2 h by measuring the remaining clot weight. Although the initial clot formation was normal in vehicle and nobiletin-treated samples, clot retraction was reduced significantly in the presence of nobiletin (100 μM) at 2 h compared with the control samples (Figure 3A and 3B). Similarly, nobiletin-treated (100 μM) platelets were unable spread on fibrinogen to a similar extent to control platelets at 45 min. Most nobiletin-treated platelets failed to progress beyond filopodia formation with only a few cells progressing to lamellapodia formation and full spreading (Figure 3C and 3D). These data suggest that outside-in signalling through integrin αIIbβ3, which controls the coordinated process of clot retraction and platelet spreading on fibrinogen, is affected by nobiletin.
Figure 3.
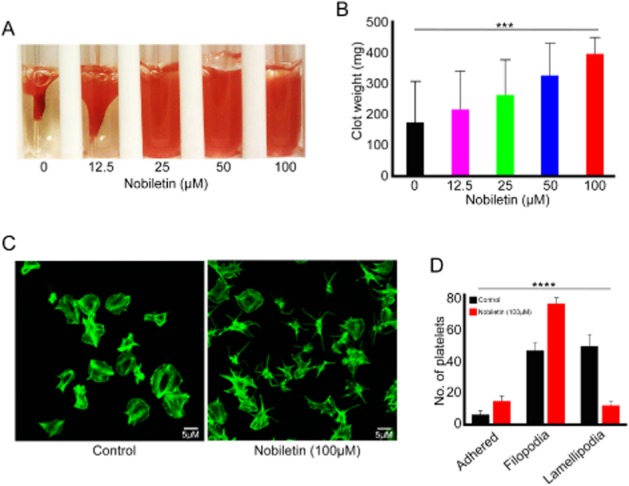
Integrin αIIbβ3-mediated outside-in signalling is inhibited by nobiletin. The effect of various concentrations of nobiletin (12.5, 25, 50 and 100 μM) on clot retraction was analysed in vitro using human PRP (A and B). (A) Representative image of clot retraction at 2 h in the presence or absence of different concentrations of nobiletin. Data in (B) represent mean ± SD (n = 4) of clot weights measured at 2 h. Washed human platelets were allowed to spread for 45 min in the presence and absence of 100 μM nobiletin on 100 μg·mL−1 fibrinogen-coated cover glasses and stained with Alexa fluor 488 labelled phalloidin prior to analysis using a Nikon A1-R-confocal microscope. The images shown in (C) are representative of multiple images acquired from four separate experiments. The images were analysed by ImageJ and the number of platelets found at different states of platelet spreading were calculated (D). The P-values were calculated using parametric repeated measures anova (***P = <0.001 and ****P = <0.0001).
Nobiletin limits thrombus formation in vitro
Given the dramatic effects of nobiletin on platelet function, its effects on thrombus formation were assessed in vitro in whole blood under arterial flow conditions. Fluorescently labelled (DiOC6) human blood was pre-incubated with vehicle control or different concentrations of nobiletin (12.5, 25, 50 and 100 μM) and perfused over collagen-coated Vena8 biochips for 10 min at a shear rate of 20 dynes·cm−2. Normal progression of thrombus formation was observed in vehicle-treated samples, whereas a clear reduction in thrombus volume and fluorescence intensity (Figure 4A and 4B) was observed at all concentrations of nobiletin used. These data suggest that nobiletin is able to reduce both initial platelet adhesion and subsequent thrombus formation.
Figure 4.
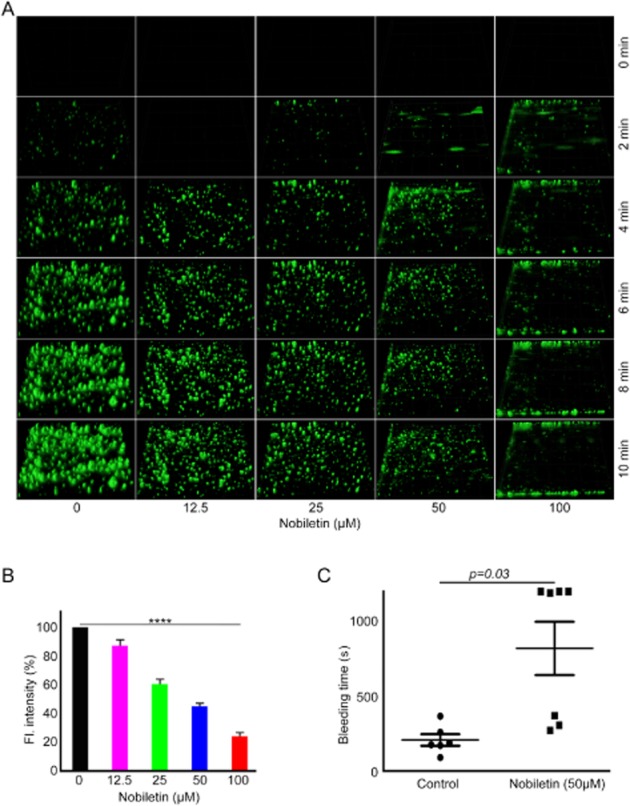
Nobiletin limits thrombus formation in vitro and extends bleeding time in mice. DiOC6 labelled human blood was pre-incubated with vehicle or different concentrations of nobiletin and perfused over collagen-coated Vena8 BioChips. Images were obtained every 30 s for up to 10 min and representative images (at 0, 2, 4, 6, 8 and 10 min) are shown in (A). The fluorescence intensity of thrombi obtained in the absence of nobiletin was taken as 100% and compared with the levels obtained with different concentrations of nobiletin (B). Data represent mean ± SD (n = 4). The P-values shown in the figure are as calculated by non-parametric repeated measures anova (Friedman test; ****P < 0.0001). The effect of nobiletin (estimated 50 μM) on haemostasis in mice was analysed by measuring the bleeding time after removal of 1 mm of tail tip. The bleeding time obtained with vehicle-treated group was compared with nobiletin-treated mice (C). Data represent mean ± SD (n = 7 mice for vehicle control or nobiletin-treated group). The significance between control and treated groups, and the P-values were calculated by Student's t-test.
Nobiletin extends bleeding time in mice
To analyse the effects of nobiletin on haemostasis, tail bleeding assays were performed on mice in the presence of vehicle [DMSO (0.01%)] or nobiletin (at estimated 50 μM concentration based on the mouse weight and respective volume of blood). Vehicle-treated mice bled between 98 and 372 s, whereas administration of nobiletin extended the bleeding to between 278 and 1200 s (Figure 4C). Four of the seven nobiletin-treated mice bled for 1200 s (when the assay was terminated). These data suggest that nobiletin is able to modulate haemostasis in mice, consistent with the effects observed on thrombus formation.
Nobiletin blocks PKB (Akt) activation
Since collagen and CRP-XL-stimulated platelet activation was affected by nobiletin, the phosphorylation levels of different signalling proteins involved in the GPVI pathway were analysed. Washed human platelets were stimulated at 37°C in an aggregometer under conditions (in presence of 1 mM EGTA, 10 μM indomethacin and 2 U·mL−1 apyrase) that disfavour platelet aggregation with CRP-XL (1 μg·mL−1) in the presence of various concentrations of nobiletin (12.5, 25, 50, 100, 150 and 200 μM) or vehicle control, and lysates were prepared to analyse the phosphorylation of different signalling proteins. Total tyrosine phosphorylation (Figure 5A) and the phosphorylation of individual proteins such as Syk (pY323) and LAT (pY200; Figure 5B) were not affected by nobiletin. However, tyrosine phosphorylation of PLCγ2 (pY759) was diminished at all concentrations of nobiletin used (Figure 5B). Similarly, the serine phosphorylation of PKB (or Akt) (pS473) was also affected in a concentration-dependent manner by nobiletin (Figure 5B). Although the phosphorylation levels were quantified under non-aggregation conditions, the level of inhibition of phosphorylation of PLCγ2 and Akt were similar to the inhibitory effects observed on platelet aggregation at the same concentration of agonist (Figure 1G and 1H). For example, 12.5 μM of nobiletin inhibited around 20–30% of CRP-XL-induced platelet aggregation and phosphorylation of PLCγ2 and Akt. These data suggest that nobiletin may not influence the immediate effectors within the GPVI pathway (such as Syk and LAT) although it can modulate the later signalling events controlled by PLCγ2 and Akt, which are shared with activation mechanisms stimulated by other agonists. The inhibitory effects of nobiletin on PLCγ2 and Akt phosphorylation indicate its potential effect on PI3K-mediated platelet signalling.
Figure 5.
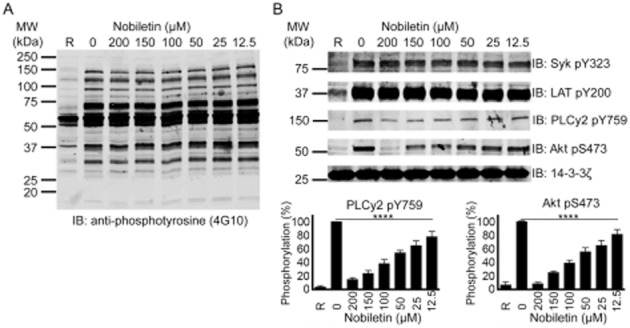
Nobiletin inhibits phosphorylation of PLCγ2 and PKB (Akt). Washed human platelets were stimulated with CRP-XL (1 μg·mL−1) in the absence or presence of various concentrations of nobiletin before the analysis by immunoblotting using anti-phosphotyrosine antibody (A) and phospho-specific antibodies for proteins involved in the GPVI pathway such as Syk pY323, LAT pY200, PLCγ2 pY759 and Akt pS473 (B). Total level of 14-3-3ζ was measured on each sample as a loading control. The blots shown in the figure are representative of four separate experiments. R represents resting platelets. Data presented in (B) represent mean ± SD (n = 4) and P-values were calculated using non-parametric repeated measures anova (Friedman test; ****P < 0.0001).
Nobiletin elevates platelet cGMP levels
Under physiological conditions, endothelium-derived nitric oxide (NO) and PGI2 inhibit platelet function by elevating platelet cGMP and cAMP levels respectively. Since plant flavonoids such as tangeretin have been shown to act on cyclic nucleotide signalling (Ok et al., 2012), the effects of nobiletin on the levels of cGMP and cAMP in platelets were analysed in the presence of vehicle control or different concentrations (50, 100, 150 and 200 μM) of nobiletin. The levels of cGMP (Figure 6A) and cAMP (Figure 6B) were unaltered upon stimulation with CRP-XL (1 μg·mL−1), but the addition of nobiletin elevated cGMP levels significantly. Lower concentrations (less than 50 μM) of nobiletin did not increase the cGMP levels significantly (data not shown). cAMP levels, however, remained unaltered in the presence of all the concentrations of nobiletin used. To explore whether the elevation of cGMP levels by nobiletin is dependent on activation by CRP-XL, the level of cGMP was measured in resting platelets incubated with nobiletin (200 μM) for 5 min. Platelet cGMP levels were increased in platelets treated with nobiletin alone (Figure 6A) indicating that cGMP elevation is not dependent on agonist stimulation or platelet activation. These data suggest that nobiletin may inhibit platelet function through the elevation of cGMP and not through cAMP.
Figure 6.
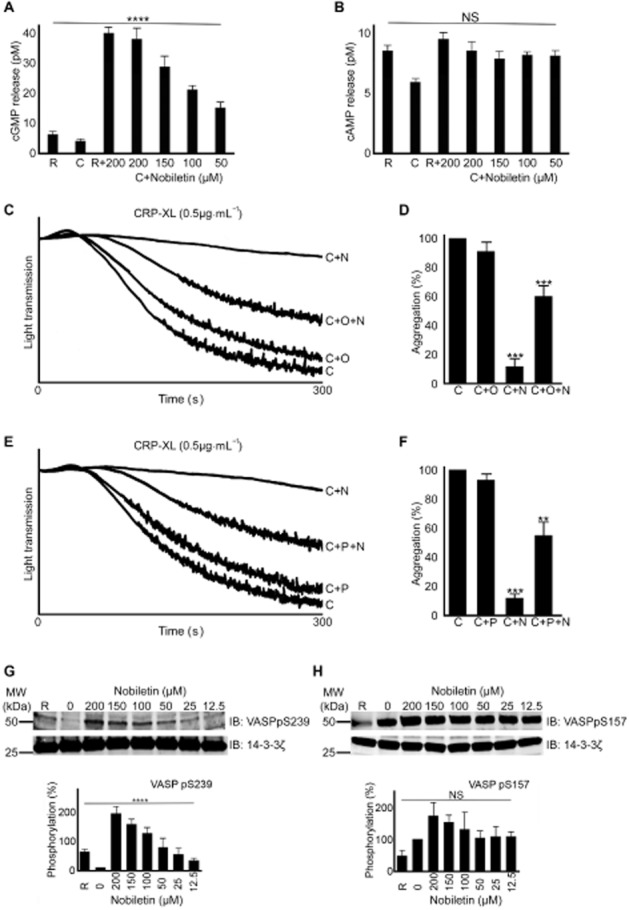
Nobiletin elevates cGMP levels and increases VASP (S239) phosphorylation. The levels of cGMP (A) and cAMP (B) were measured in platelets (PRP) upon stimulation with CRP-XL (1 μg·mL−1) using elisa kits in the presence or absence of various concentrations of nobiletin. The concentration of cGMP and cAMP was calculated based on standard curves. R, resting; C, CRP-XL-treated platelets. Data represent mean ± SD (n = 4) and P-values were calculated using parametric repeated measures anova (****P < 0.0001). Platelet aggregation was measured using CRP-XL (0.5 μg·mL−1) together in the presence and absence of 20 μM ODQ, a soluble guanylate cyclase inhibitor (C and D) or 30 μM PKG inhibitor (Rp-8-Br-PET-cGMPS) (E and F) and 50 μM nobiletin for 5 min. C, O, P and N represent CRP-XL, ODQ, PKG inhibitor and nobiletin respectively. The level of aggregation obtained with CRP-XL at 5 min was taken as 100% to calculate the percentage of inhibition. The data from C + N were compared with C and C + O/P + N were compared with C + N. Data represent mean ± SD (n = 3) and P-values were calculated using Student's t-test as shown (**P < 0.01 and ***P < 0.001). The effect of various concentrations of nobiletin on phosphorylation of VASP [at positions pS239 (G) and pS157 (H)], a substrate for cyclic nucleotide-dependent PKs, was analysed in washed platelets upon stimulation with CRP-XL (1 μg·mL−1) by immunoblotting. The blots are representative of four separate experiments. Data represent mean ± SD (n = 4) and P-values were calculated using non-parametric repeated measures anova (Friedman test; ****P < 0.0001).
To further understand the effects of nobiletin in platelets through the elevation of cGMP levels, aggregation assays were performed in the presence and absence of a soluble guanylate cyclase inhibitor ODQ and a cGMP-dependent PKG inhibitor (Rp-8-bromo-β-phenyl-1,N2-ethenoguanosine 3′,5′-cyclic monophosphorothioate sodium salt [Rp-8-Br-PET-cGMPS]). Pre-incubation of platelets either with ODQ (20 μM) or the PKG inhibitor (30 μM) did not cause a reduction in CRP-XL (0.5 μg·mL−1)-induced platelet aggregation. However, both of these inhibitors reversed the inhibitory actions of nobiletin (50 μM) upon activation with CRP-XL (Figure 6C–F). These data further confirm that the actions of nobiletin in platelets are at least in part explained by its ability to modulate cGMP signalling.
Vasodilator-stimulated phosphoprotein (VASP) is a substrate for cAMP- and cGMP-dependent PKs that mediate cAMP- and cGMP-dependent inhibitory signalling in platelets respectively (Butt et al., 1994; Li et al., 2003b). Upon elevation of cAMP, the cAMP-dependent PKA phosphorylates VASP at position S157, whereas increased levels of cGMP result in phosphorylation of S239 in VASP by cGMP-dependent PKG (Li et al., 2003a,b,). To confirm the ability of nobiletin to modulate cyclic nucleotide signalling, the phosphorylation of VASP was assessed using phospho-specific antibodies upon stimulation with CRP-XL (1 μg·mL−1). Immunoblot analysis showed that nobiletin increased the phosphorylation of VASP at S239 (Figure 6G), but only small changes were observed in phosphorylation of position S157 (Figure 6H). These data suggest that nobiletin may modulate the inhibitory signalling mediated through cGMP and PKG in platelets.
Discussion
A better understanding of the relationship between plant flavonoids and platelet function may aid in the development of improved strategies to treat cardiovascular diseases. In this study, we characterized nobiletin, which is a polymethoxylated flavonoid structurally similar to tangeretin present in the peel of citrus fruits, and analysed its anti-platelet effects. Nobiletin was shown to have anti-adhesive properties in rat platelets (Sempinska et al., 1977; Robbins, 1988), and reduced death rates in rats by ADP induced thrombosis at 8 mM concentration (Robbins, 1973). However, these previous studies did not explore the mechanism of action through which nobiletin exerts its effects in platelets particularly in humans. In our previous study, we demonstrated the effects of a citrus flavonoid, tangeretin on the modulation of platelet function (Vaiyapuri et al., 2013). Tangeretin affected platelet function by elevating cGMP levels and inhibiting PI3K-mediated signalling cascades.
In this study, we report that nobiletin inhibited platelet activation upon stimulation with collagen and CRP-XL in a concentration-dependent manner with notable levels of reduction achieved at as low as 6.25 μM. Increasing concentrations of agonists were able to partially overcome the inhibitory effects of nobiletin, confirming that it does not show any toxic effects to the platelets. Platelet activation stimulated with a GPCR agonist, thrombin, was, however, only inhibited at higher concentrations of nobiletin (as high as 50 μM required to obtain a significant reduction) indicating that it has increased potency for the inhibition of GPVI dependent pathways. Similar inhibitory effects on GPVI and thrombin induced platelet activation were observed with tangeretin although at lower concentrations (Vaiyapuri et al., 2013). These results indicate that nobiletin may be less potent than tangeretin, although it maintained similar levels of inhibition in PRP in the presence of plasma proteins. A number of other plant-derived flavonoids such as quercetin, apigenin and luteolin have been shown to inhibit agonist-induced platelet aggregation at various concentrations (Hubbard et al., 2003; 2004,; Lill et al., 2003; Wright et al., 2010). Consistent with the inhibition of aggregation, nobiletin was able to inhibit the affinity modulation of integrin αIIbβ3 to favour platelet aggregation. In addition, nobiletin inhibited platelet granule secretion similar to tangeretin and other flavonoids.
A number of flavonoids have been shown to inhibit several enzymes, which regulate normal cellular functions such as tyrosine kinases, phospholipase As (PLAs), lipoxygenases, COXs and phosphodiesterases (Benavente-Garcı'a et al., 1997; Benavente-Garcia and Castillo, 2008; Wright et al., 2012). Quercetin and catechin were able to inhibit the phosphorylation of serine/threonine kinases, extracellular signal–related kinase 1/2, c-Jun N-terminal kinase, MAPK and Akt in vascular smooth muscle and endothelial cells (Wright et al., 2012). Similarly, the flavonoids present in purple grape juice were shown to partially inhibit the phosphorylation of PKC in platelets (Freedman et al., 2001). Since nobiletin inhibited collagen-induced platelet activation, further analyses of the signalling proteins involved in the GPVI pathway was performed and revealed that it did not affect early signalling effector molecules such as Syk and LAT. Nobiletin did however diminish the activation of later signalling modulators such as AKT and PLCγ2, both associated with PI3K signalling to regulate platelet function, particularly calcium mobilization. Consistent with diminished PI3K signalling, reduced calcium levels and subsequently retarded integrin αIIbβ3-mediated outside-in signalling (clot retraction and platelet spreading) were observed upon treatment with nobiletin. The inhibition of PLCγ2 and AKT by nobiletin was also observed in other cell types such as liver cancer cells (Shi et al., 2013). Together, these inhibitory effects of nobiletin resulted in reduced thrombus formation in vitro under arterial flow conditions.
Experiments to dissect the direct and indirect actions of these citrus flavonoids on the various components of PI3K signalling may reveal specific targets in the modulation of platelet function. The inhibition of PI3K signalling was also observed with other flavonoids such as quercetin (Matter et al., 1992), which led to the development of LY294002, a commonly used PI3K inhibitor (Vlahos et al., 1994). Due to the broad spectrum nature of flavonoids on different aspects of cell regulation (Wright et al., 2012), it is entirely possible for nobiletin to have other target mechanisms to inhibit platelet function. Flavonoids such as apigenin and luteolin have shown to antagonize TXA2 receptors in order to inhibit platelet function (Lill et al., 2003), and therefore, nobiletin may also have additional targets that may influence on the secondary activation of platelets.
The ability of flavonoids to inhibit phosphodiesterases (PDE; Wright et al., 2012), which are involved in the hydrolysis of cyclic nucleotides such as cAMP and cGMP (Francis et al., 2011), suggests that flavonoids may also modulate platelet function through other inhibitory mechanisms. Nobiletin increased the cGMP levels and VASP phosphorylation at S239, which is phosphorylated by PKG, while it neither increased the cAMP levels nor phosphorylation of VASP at S157, which is regulated by PKA. The increased levels of VASP phosphorylation may directly inhibit platelet function mediated through the phosphorylation of myosin light chain kinase resulting in impaired calmodulin binding and therefore reduce cytoskeletal reorganization essential for platelet shape change (Onselaer et al., 2014). In addition, VASP phosphorylation is able to inhibit the activation of integrin αIIbβ3 and suppress the release of intracellular calcium through inhibiting IP3 receptor activation (Geiger, 2001). We have previously shown that tangeretin was able to increase the levels of cGMP although it did not alter the cAMP levels or inhibit PDE activity (Vaiyapuri et al., 2013), indicating that tangeretin elevates cGMP levels through increased production rather than its reduced hydrolysis. Purple grape juice and flavonoid derivatives have been shown to increase platelet derived NO production in order to inhibit platelet function (Freedman et al., 2001). Elevated levels of NO in platelets are capable of causing an increase in cGMP levels, which in turn activate PKG to phosphorylate its substrate VASP at position S239 (Butt et al., 1994). It is presently unclear whether the effects that we observed are mediated by direct actions of nobiletin on NO production, or cGMP levels. It is presently unclear whether the effects of nobiletin on PI3K and cGMP signalling are mechanistically linked or whether they represent separate modes of action.
Better understanding of the structure–function relationships of flavonoids will reveal their selectivity for target molecules (Wright et al., 2012), and will also enable the understanding of differences between the specificity and efficiency of nobiletin and tangeretin. While structurally similar, a difference at an O-methyl group on the B ring results in considerable changes in their inhibitory effects observed in PRP, suggesting the importance of this methyl group in determination of its ability to bind plasma proteins.
The polymethylated structure of nobiletin has been suggested to maintain its stability and enable resistance against the metabolic changes such as alterations by the cytochrome P450 system, UDP-glucurosyltransferases and sulphotransferases (Walle, 2007). The most common metabolic changes observed in nobiletin are the demethylation on the C4 ring in its structure (Yasuda et al., 2003). The extended half-life of nobiletin compared with tangeretin in the circulation (Walle, 2007; Manthey et al., 2011) suggests this may be potentially administered in order to avail longer bioavailability.
In this study, we demonstrate that 12.5 μM nobiletin is able to significantly inhibit platelet function in vitro in washed platelets, PRP and whole blood. A concentration of 50 μM was shown to moderately affect haemostasis in mice. Tail bleeding assays were performed at 37°C by maintaining the animal on a heated mat. The use of anaesthetics may have effects on platelet function and therefore in haemostasis; however, the parallel comparison of vehicle control and nobiletin-treated mice alleviates this issue. A pharmacokinetic study previously reported (Manthey et al., 2011) that concentrations of tangeretin of around 6.2 μM (including known metabolites) and nobiletin 22.4 μM in rodents were achievable upon oral administration of 50 mg·kg−1 of body weight. This indicates that the ingestion of around 100 mg·kg−1 may reach approximately 50 μM of nobiletin in plasma and affect haemostasis to a similar extent to that observed in this study. As reported in the previous study, the bioavailability of nobiletin even after 12 h of oral administration was maintained above a concentration of approximately 5 μM. The half-life of nobiletin, however, upon the administration through the i.v. route has yet to be explored. Although one would imagine that it is unlikely for these levels of nobiletin and tangeretin to be achieved through regular dietary intake, many additional flavonoids are present in most diets. It may also be possible to achieve the required levels of these flavonoids by regular oral supplementation as suggested previously in rodents. Indeed, a pilot human study reported quercetin to reach 10 μM through oral dietary supplementation (Hubbard et al., 2006) and this suggests that the regular intake of nobiletin through supplementation might show similar effects, which we observed in mice upon i.v. infusion of nobiletin. Therefore, nobiletin and tangeretin, together with other bioactive flavonoids, may collectively modulate platelet function in order to reduce potential thrombosis risk. The effects of tangeretin and nobiletin, however, below the threshold of detection in vivo, may still be relevant. Chronic ingestion of nobiletin and tangeretin from diets may also result in a cumulative effect on the reduction of platelet function over a period of time. Diets rich in bioactive flavonoids also result in intake of a range of complex mixtures of various flavonoids such as quercetin, apigenin and luteolin that may also be beneficial in reducing cardiovascular disease incidence. We conclude that further investigation to explore the therapeutic potentials of nobiletin and tangeretin may enable the formulation of better therapeutic strategies to reduce cardiovascular disease risk.
Acknowledgments
This work was supported by the British Heart Foundation (grants: PG/11/125/29320 and RG/09/011/28094) and Medical Research Council (grant: MR/J002666/1).
Glossary
- GPVI
glycoprotein VI
- sGC
soluble guanylate cyclase
- VASP
vasodilator-stimulated phosphoprotein
Author contributions
S. V. designed the study, performed research, analysed the data and wrote the paper. H. R., M. S. A., A. J. U., A. R. S., G. D. F., M. C., C. I. J. and L. A. M. performed research. J. M. G. designed the study, analysed the data and wrote the paper.
Conflict of interest
The authors declare that there are no conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 Representative histograms show the level of fibrinogen binding (A) or P-selectin exposure (B) on the platelet surface in the absence and presence of various concentrations of nobiletin upon stimulation with 1 μg·mL−1 CRP-XL as measured by flow cytometry.
Appendix S1 Methods.
References
- Akao Y, Itoh T, Ohguchi K, Iinuma M, Nozawa Y. Interactive effects of polymethoxy flavones from Citrus on cell growth inhibition in human neuroblastoma SH-SY5Y cells. Bioorg Med Chem. 2008;16:2803–2810. doi: 10.1016/j.bmc.2008.01.058. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: catalytic receptors. Br J Pharmacol. 2013a;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett NE, Holbrook L, Jones S, Kaiser WJ, Moraes LA, Rana R, et al. Future innovations in anti-platelet therapies. Br J Pharmacol. 2008;154:918–939. doi: 10.1038/bjp.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavente-Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008;56:6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- Benavente-Garcı'a OCJ, Marin FR, Ortun A, Del Rı'o JA. Uses and properties of citrus flavonoids. J Agric Food Chem. 1997;45:4505–4515. [Google Scholar]
- Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, et al. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994;269:14509–14517. [PubMed] [Google Scholar]
- Calderwood DA. Integrin activation. J Cell Sci. 2004;117:657–666. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- Chen C, Ono M, Takeshima M, Nakano S. Antiproliferative and apoptosis-inducing activity of nobiletin against three subtypes of human breast cancer cell lines. Anticancer Res. 2014;34:1785–1792. [PubMed] [Google Scholar]
- Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev. 2011;91:651–690. doi: 10.1152/physrev.00030.2010. [DOI] [PubMed] [Google Scholar]
- Freedman JE, Parker C, 3rd, Li L, Perlman JA, Frei B, Ivanov V, et al. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation. 2001;103:2792–2798. doi: 10.1161/01.cir.103.23.2792. [DOI] [PubMed] [Google Scholar]
- Geiger J. Inhibitors of platelet signal transduction as anti-aggregatory drugs. Expert Opin Investig Drugs. 2001;10:865–890. doi: 10.1517/13543784.10.5.865. [DOI] [PubMed] [Google Scholar]
- Gibbins JM. Platelet adhesion signalling and the regulation of thrombus formation. J Cell Sci. 2004;117:3415–3425. doi: 10.1242/jcs.01325. [DOI] [PubMed] [Google Scholar]
- Hathaway DR, Adelstein RS. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci U S A. 1979;76:1653–1657. doi: 10.1073/pnas.76.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, et al. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95:740–751. doi: 10.3945/ajcn.111.023457. [DOI] [PubMed] [Google Scholar]
- Hubbard GP, Stevens JM, Cicmil M, Sage T, Jordan PA, Williams CM, et al. Quercetin inhibits collagen-stimulated platelet activation through inhibition of multiple components of the glycoprotein VI signaling pathway. J Thromb Haemost. 2003;1:1079–1088. doi: 10.1046/j.1538-7836.2003.00212.x. [DOI] [PubMed] [Google Scholar]
- Hubbard GP, Wolffram S, Lovegrove JA, Gibbins JM. Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. J Thromb Haemost. 2004;2:2138–2145. doi: 10.1111/j.1538-7836.2004.01067.x. [DOI] [PubMed] [Google Scholar]
- Hubbard GP, Wolffram S, de Vos R, Bovy A, Gibbins JM, Lovegrove JA. Ingestion of onion soup high in quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in man: a pilot study. Br J Nutr. 2006;96:482–488. [PubMed] [Google Scholar]
- Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG Group NCRRGW. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ajdic J, Eigenthaler M, Du X. A predominant role for cAMP-dependent protein kinase in the cGMP-induced phosphorylation of vasodilator-stimulated phosphoprotein and platelet inhibition in humans. Blood. 2003a;101:4423–4429. doi: 10.1182/blood-2002-10-3210. [DOI] [PubMed] [Google Scholar]
- Li Z, Xi X, Gu M, Feil R, Ye RD, Eigenthaler M, et al. A stimulatory role for cGMP-dependent protein kinase in platelet activation. Cell. 2003b;112:77–86. doi: 10.1016/s0092-8674(02)01254-0. [DOI] [PubMed] [Google Scholar]
- Lill G, Voit S, Schror K, Weber AA. Complex effects of different green tea catechins on human platelets. FEBS Lett. 2003;546:265–270. doi: 10.1016/s0014-5793(03)00599-4. [DOI] [PubMed] [Google Scholar]
- Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey JA, Cesar TB, Jackson E, Mertens-Talcott S. Pharmacokinetic study of nobiletin and tangeretin in rat serum by high-performance liquid chromatography-electrospray ionization-mass spectrometry. J Agric Food Chem. 2011;59:145–151. doi: 10.1021/jf1033224. [DOI] [PubMed] [Google Scholar]
- Matter WF, Brown RF, Vlahos CJ. The inhibition of phosphatidylinositol 3-kinase by quercetin and analogs. Biochem Biophys Res Commun. 1992;186:624–631. doi: 10.1016/0006-291x(92)90792-j. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson AD. P2Y12 antagonism: promises and challenges. Arterioscler Thromb Vasc Biol. 2008;28:s33–s38. doi: 10.1161/ATVBAHA.107.160689. [DOI] [PubMed] [Google Scholar]
- Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Ok WJ, Cho HJ, Kim HH, Lee DH, Kang HY, Kwon HW, et al. Epigallocatechin-3-gallate has an anti-platelet effect in a cyclic AMP-dependent manner. J Atheroscler Thromb. 2012;19:337–348. doi: 10.5551/jat.10363. [DOI] [PubMed] [Google Scholar]
- Onselaer MB, Oury C, Hunter RW, Eeckhoudt S, Barile N, Lecut C, et al. The Ca(2+) /calmodulin-dependent kinase kinase beta-AMP-activated protein kinase-alpha1 pathway regulates phosphorylation of cytoskeletal targets in thrombin-stimulated human platelets. J Thromb Haemost. 2014;12:973–986. doi: 10.1111/jth.12568. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42:D1098-106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins RC. Antithrombogenic properties of a hexamethoxylated flavonoid. Reduction of deaths in rats due to intravascular infusion of adenosine diphosphate (ADP) Atherosclerosis. 1973;18:73–82. doi: 10.1016/0021-9150(73)90118-4. [DOI] [PubMed] [Google Scholar]
- Robbins RC. Flavones in citrus exhibit antiadhesive action on platelets. Int J Vitam Nutr Res. 1988;58:418–421. [PubMed] [Google Scholar]
- Sempinska E, Kostka B, Krolikowska M, Kalisiak E. Effect of flavonoids on the platelet adhesiveness in repeatedly bred rats. Pol J Pharmacol Pharm. 1977;29:7–10. [PubMed] [Google Scholar]
- Shattil SJ, Brass LF. Induction of the fibrinogen receptor on human platelets by intracellular mediators. J Biol Chem. 1987;262:992–1000. [PubMed] [Google Scholar]
- Shi MD, Liao YC, Shih YW, Tsai LY. Nobiletin attenuates metastasis via both ERK and PI3K/Akt pathways in HGF-treated liver cancer HepG2 cells. Phytomedicine. 2013;20:743–752. doi: 10.1016/j.phymed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Vaiyapuri S, Ali MS, Moraes LA, Sage T, Lewis KR, Jones CI, et al. Tangeretin regulates platelet function through inhibition of phosphoinositide 3-kinase and cyclic nucleotide signaling. Arterioscler Thromb Vasc Biol. 2013;33:2740–2749. doi: 10.1161/ATVBAHA.113.301988. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Walle T. Methoxylated flavones, a superior cancer chemopreventive flavonoid subclass? Semin Cancer Biol. 2007;17:354–362. doi: 10.1016/j.semcancer.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B, Moraes LA, Kemp CF, Mullen W, Crozier A, Lovegrove JA, et al. A structural basis for the inhibition of collagen-stimulated platelet function by quercetin and structurally related flavonoids. Br J Pharmacol. 2010;159:1312–1325. doi: 10.1111/j.1476-5381.2009.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B, Spencer JP, Lovegrove JA, Gibbins JM. Insights into dietary flavonoids as molecular templates for the design of anti-platelet drugs. Cardiovasc Res. 2012;97:13–22. doi: 10.1093/cvr/cvs304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YQ, Zhou CH, Tao J, Li SN. Antagonistic effects of nobiletin, a polymethoxyflavonoid, on eosinophilic airway inflammation of asthmatic rats and relevant mechanisms. Life Sci. 2006;78:2689–2696. doi: 10.1016/j.lfs.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Yoshimura Y, Yabuki H, Nakazawa T, Ohsawa K, Mimaki Y, et al. Urinary metabolites of nobiletin orally administered to rats. Chem Pharm Bull (Tokyo) 2003;51:1426–1428. doi: 10.1248/cpb.51.1426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Representative histograms show the level of fibrinogen binding (A) or P-selectin exposure (B) on the platelet surface in the absence and presence of various concentrations of nobiletin upon stimulation with 1 μg·mL−1 CRP-XL as measured by flow cytometry.
Appendix S1 Methods.