Abstract
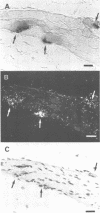
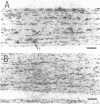
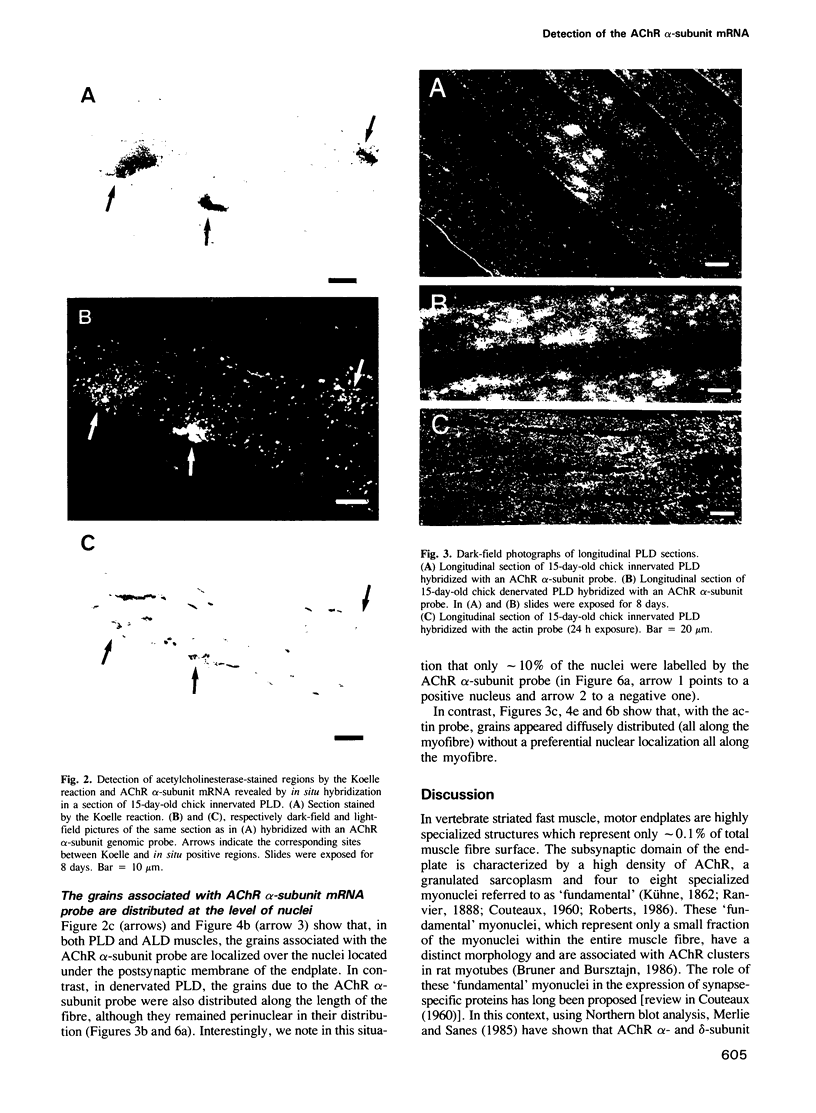
In adult vertebrate striated muscle, the nicotinic acetylcholine receptor (AChR) is almost exclusively localized in the postsynaptic membrane of the neuromuscular junction. Using in situ hybridization, we show that, in two different chicken muscles [the slow multi-innervated anterior latissimus dorsi (ALD) and the fast singly innervated posterior latissimus dorsi (PLD)], the AChR alpha-subunit mRNA is detected at discrete regions on myofibres and that these regions co-localize (80% correspondence) with neuromuscular junctions identified by histochemical staining for acetylcholinesterase. Moreover, autoradiographic grains densely accumulate on and around subsynaptic nuclei. In contrast, hybridization with an actin probe results in a strong signal distributed over the entire length of the myofibres. Denervation increases the level of AChR alpha-subunit mRNA both in the PLD and to a lesser extent in the ALD. By in situ hybridization we observe that, although a perinuclear pattern is maintained, the labelled nuclei appear randomly distributed among approximately 10% of the nuclei. These results are discussed in a model of AChR gene expression in vertebrate striated muscle fibres.
Full text
PDF

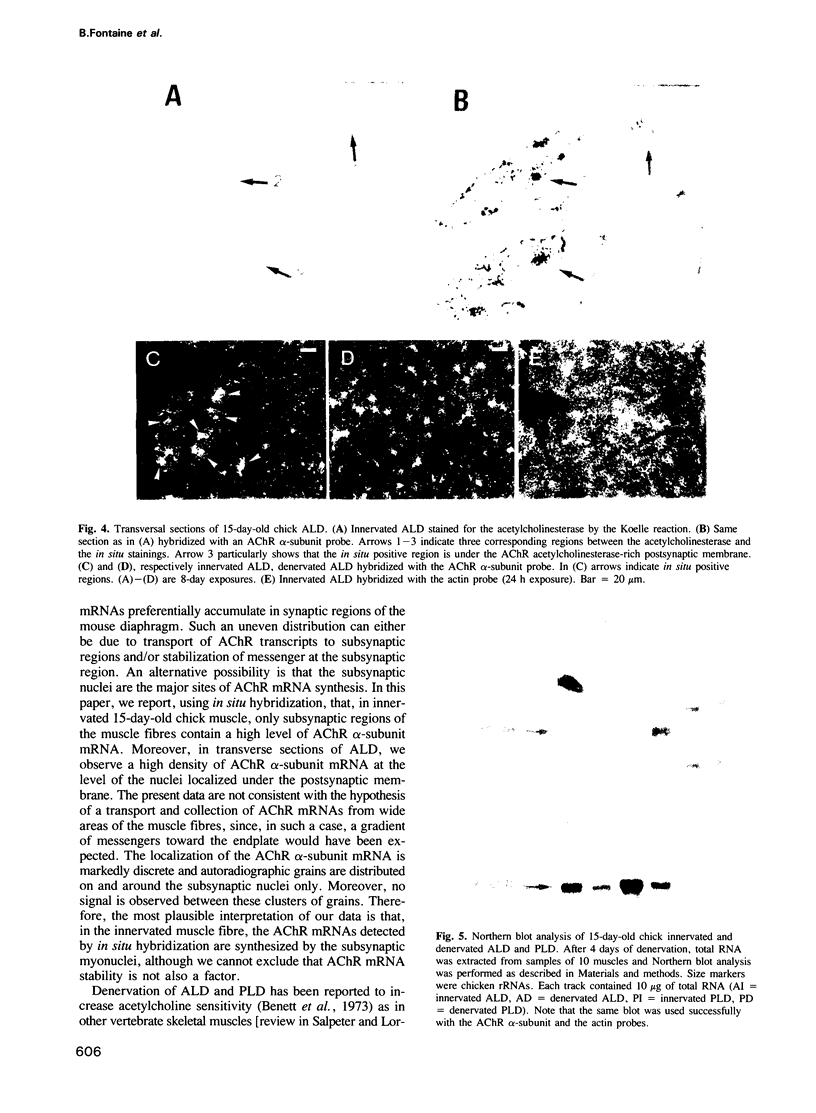
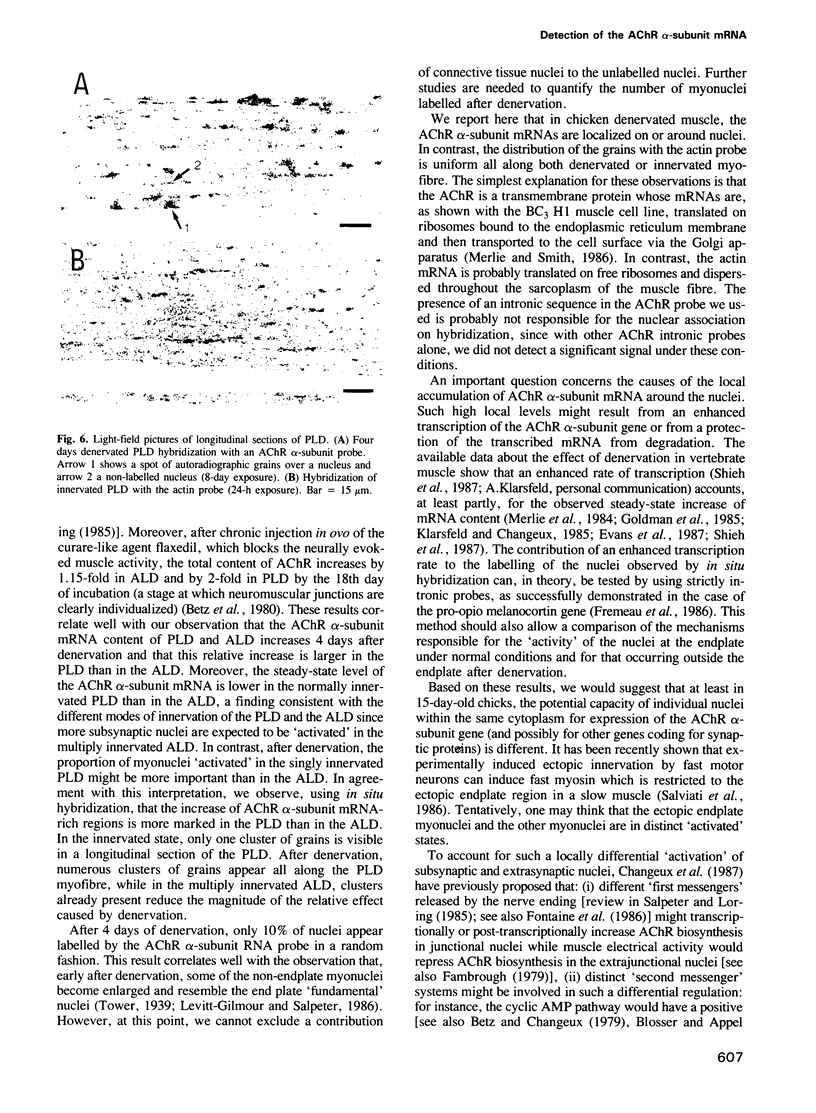


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso S., Minty A., Bourlet Y., Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23(1):11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Ballivet M., Nef P., Stalder R., Fulpius B. Genomic sequences encoding the alpha-subunit of acetylcholine receptor are conserved in evolution. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):83–87. doi: 10.1101/sqb.1983.048.01.011. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G., Taylor R. S. The formation of synapses in reinnervated and cross-reinnervated adult avian muscle. J Physiol. 1973 Apr;230(2):331–357. doi: 10.1113/jphysiol.1973.sp010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H., Bourgeois J. P., Changeux J. P. Evolution of cholinergic proteins in developing slow and fast skeletal muscles in chick embryo. J Physiol. 1980 May;302:197–218. doi: 10.1113/jphysiol.1980.sp013238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H., Changeux J. P. Regulation of muscle acetylcholine receptor synthesis in vitro by cyclic nucleotide derivatives. Nature. 1979 Apr 19;278(5706):749–752. doi: 10.1038/278749a0. [DOI] [PubMed] [Google Scholar]
- Biggin M., Farrell P. J., Barrell B. G. Transcription and DNA sequence of the BamHI L fragment of B95-8 Epstein-Barr virus. EMBO J. 1984 May;3(5):1083–1090. doi: 10.1002/j.1460-2075.1984.tb01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blosser J. C., Appel S. H. Regulation of acetylcholine receptor by cyclic AMP. J Biol Chem. 1980 Feb 25;255(4):1235–1238. [PubMed] [Google Scholar]
- Boulter J., Luyten W., Evans K., Mason P., Ballivet M., Goldman D., Stengelin S., Martin G., Heinemann S., Patrick J. Isolation of a clone coding for the alpha-subunit of a mouse acetylcholine receptor. J Neurosci. 1985 Sep;5(9):2545–2552. doi: 10.1523/JNEUROSCI.05-09-02545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner J. M., Bursztajn S. Acetylcholine receptor clusters are associated with nuclei in rat myotubes. Dev Biol. 1986 May;115(1):35–43. doi: 10.1016/0012-1606(86)90225-3. [DOI] [PubMed] [Google Scholar]
- Burden S. Acetylcholine receptors at the neuromuscular junction: developmental change in receptor turnover. Dev Biol. 1977 Nov;61(1):79–85. doi: 10.1016/0012-1606(77)90343-8. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Devillers-Thiéry A., Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984 Sep 21;225(4668):1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Evans S., Goldman D., Heinemann S., Patrick J. Muscle acetylcholine receptor biosynthesis. Regulation by transcript availability. J Biol Chem. 1987 Apr 5;262(10):4911–4916. [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Fontaine B., Klarsfeld A., Changeux J. P. Calcitonin gene-related peptide and muscle activity regulate acetylcholine receptor alpha-subunit mRNA levels by distinct intracellular pathways. J Cell Biol. 1987 Sep;105(3):1337–1342. doi: 10.1083/jcb.105.3.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine B., Klarsfeld A., Hökfelt T., Changeux J. P. Calcitonin gene-related peptide, a peptide present in spinal cord motoneurons, increases the number of acetylcholine receptors in primary cultures of chick embryo myotubes. Neurosci Lett. 1986 Oct 30;71(1):59–65. doi: 10.1016/0304-3940(86)90257-0. [DOI] [PubMed] [Google Scholar]
- Fremeau R. T., Jr, Lundblad J. R., Pritchett D. B., Wilcox J. N., Roberts J. L. Regulation of pro-opiomelanocortin gene transcription in individual cell nuclei. Science. 1986 Dec 5;234(4781):1265–1269. doi: 10.1126/science.3775385. [DOI] [PubMed] [Google Scholar]
- Goldman D., Boulter J., Heinemann S., Patrick J. Muscle denervation increases the levels of two mRNAs coding for the acetylcholine receptor alpha-subunit. J Neurosci. 1985 Sep;5(9):2553–2558. doi: 10.1523/JNEUROSCI.05-09-02553.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho F. The nicotinic acetylcholine receptor and its ion channel. Eur J Biochem. 1986 Jul 15;158(2):211–226. doi: 10.1111/j.1432-1033.1986.tb09740.x. [DOI] [PubMed] [Google Scholar]
- KOELLE G. B., FRIEDENWALD J. A. A histochemical method for localizing cholinesterase activity. Proc Soc Exp Biol Med. 1949 Apr;70(4):617–622. doi: 10.3181/00379727-70-17013. [DOI] [PubMed] [Google Scholar]
- Klarsfeld A., Changeux J. P. Activity regulates the levels of acetylcholine receptor alpha-subunit mRNA in cultured chicken myotubes. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4558–4562. doi: 10.1073/pnas.82.13.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A., Daubas P., Bourachot B., Changeux J. P. A 5'-flanking region of the chicken acetylcholine receptor alpha-subunit gene confers tissue specificity and developmental control of expression in transfected cells. Mol Cell Biol. 1987 Feb;7(2):951–955. doi: 10.1128/mcb.7.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordeli E., Cartaud J., Nghiêm H. O., Pradel L. A., Dubreuil C., Paulin D., Changeux J. P. Evidence for a polarity in the distribution of proteins from the cytoskeleton in Torpedo marmorata electrocytes. J Cell Biol. 1986 Mar;102(3):748–761. doi: 10.1083/jcb.102.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPolla R. J., Mayne K. M., Davidson N. Isolation and characterization of a cDNA clone for the complete protein coding region of the delta subunit of the mouse acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7970–7974. doi: 10.1073/pnas.81.24.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer R., Changeux J. P. Calcitonin gene-related peptide elevates cyclic AMP levels in chick skeletal muscle: possible neurotrophic role for a coexisting neuronal messenger. EMBO J. 1987 Apr;6(4):901–906. doi: 10.1002/j.1460-2075.1987.tb04836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt-Gilmour T. A., Salpeter M. M. Gradient of extrajunctional acetylcholine receptors early after denervation of mammalian muscle. J Neurosci. 1986 Jun;6(6):1606–1612. doi: 10.1523/JNEUROSCI.06-06-01606.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J., Sanes J. R., Marshall L. M. Cholinesterase is associated with the basal lamina at the neuromuscular junction. Nature. 1978 Jan 12;271(5641):172–174. doi: 10.1038/271172a0. [DOI] [PubMed] [Google Scholar]
- McManaman J. L., Blosser J. C., Appel S. H. Inhibitors of membrane depolarization regulate acetylcholine receptor synthesis by a calcium-dependent, cyclic nucleotide-independent mechanism. Biochim Biophys Acta. 1982 Feb 10;720(1):28–35. doi: 10.1016/0167-4889(82)90035-0. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Isenberg K. E., Russell S. D., Sanes J. R. Denervation supersensitivity in skeletal muscle: analysis with a cloned cDNA probe. J Cell Biol. 1984 Jul;99(1 Pt 1):332–335. doi: 10.1083/jcb.99.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Sanes J. R. Concentration of acetylcholine receptor mRNA in synaptic regions of adult muscle fibres. Nature. 1985 Sep 5;317(6032):66–68. doi: 10.1038/317066a0. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Smith M. M. Synthesis and assembly of acetylcholine receptor, a multisubunit membrane glycoprotein. J Membr Biol. 1986;91(1):1–10. doi: 10.1007/BF01870209. [DOI] [PubMed] [Google Scholar]
- Nef P., Mauron A., Stalder R., Alliod C., Ballivet M. Structure linkage, and sequence of the two genes encoding the delta and gamma subunits of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7975–7979. doi: 10.1073/pnas.81.24.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Tanabe T., Shimizu S., Kikyotani S., Kayano T., Hirose T., Inayama S. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding alpha-subunit precursor of muscle acetylcholine receptor. 1983 Oct 27-Nov 2Nature. 305(5937):818–823. doi: 10.1038/305818a0. [DOI] [PubMed] [Google Scholar]
- Reist N. E., Magill C., McMahan U. J. Agrin-like molecules at synaptic sites in normal, denervated, and damaged skeletal muscles. J Cell Biol. 1987 Dec;105(6 Pt 1):2457–2469. doi: 10.1083/jcb.105.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M. Sodium channels near end-plates and nuclei of snake skeletal muscle. J Physiol. 1987 Jul;388:213–232. doi: 10.1113/jphysiol.1987.sp016611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M., Loring R. H. Nicotinic acetylcholine receptors in vertebrate muscle: properties, distribution and neural control. Prog Neurobiol. 1985;25(4):297–325. doi: 10.1016/0301-0082(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Salviati G., Biasia E., Aloisi M. Synthesis of fast myosin induced by fast ectopic innervation of rat soleus muscle is restricted to the ectopic endplate region. Nature. 1986 Aug 14;322(6080):637–639. doi: 10.1038/322637a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. J., Rothblum K. N. Gene switching in myogenesis: differential expression of the chicken actin multigene family. Biochemistry. 1981 Jul 7;20(14):4122–4129. doi: 10.1021/bi00517a027. [DOI] [PubMed] [Google Scholar]
- Shieh B. H., Ballivet M., Schmidt J. Quantitation of an alpha subunit splicing intermediate: evidence for transcriptional activation in the control of acetylcholine receptor expression in denervated chick skeletal muscle. J Cell Biol. 1987 May;104(5):1337–1341. doi: 10.1083/jcb.104.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara J., Tsien R. Y., Delay M. Inositol 1,4,5-trisphosphate: a possible chemical link in excitation-contraction coupling in muscle. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6352–6356. doi: 10.1073/pnas.82.18.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. G., Bailes J. A., Champion J. E., McMahon A. P. A molecular analysis of mouse development from 8 to 10 days post coitum detects changes only in embryonic globin expression. Development. 1987 Apr;99(4):493–500. doi: 10.1242/dev.99.4.493. [DOI] [PubMed] [Google Scholar]