Background: The role of AIF redox activity in mitochondrial respiration and redox metabolism was unknown.
Results: AIF has rotenone-sensitive NADH:ubiquinone oxidoreductase activity when reconstituted with bacterial or mitochondrial membranes.
Conclusion: AIF is a previously unidentified mammalian NDH-2-type enzyme that could contribute to NADH oxidation in cells.
Significance: The catalytic function of AIF as an NADH:UQ oxidoreductase was uncovered.
Keywords: apoptosis, complex I, mitochondrial metabolism, mitochondrial respiratory chain complex, nicotinamide adenine dinucleotide (NADH), ubiquinone, apoptosis-inducing factor, rotenone
Abstract
Apoptosis-inducing factor (AIF) and AMID (AIF-homologous mitochondrion-associated inducer of death) are flavoproteins. Although AIF was originally discovered as a caspase-independent cell death effector, bioenergetic roles of AIF, particularly relating to complex I functions, have since emerged. However, the role of AIF in mitochondrial respiration and redox metabolism has remained unknown. Here, we investigated the redox properties of human AIF and AMID by comparing them with yeast Ndi1, a type 2 NADH:ubiquinone oxidoreductase (NDH-2) regarded as alternative complex I. Isolated AIF and AMID containing naturally incorporated FAD displayed no NADH oxidase activities. However, after reconstituting isolated AIF or AMID into bacterial or mitochondrial membranes, N-terminally tagged AIF and AMID displayed substantial NADH:O2 activities and supported NADH-linked proton pumping activities in the host membranes almost as efficiently as Ndi1. NADH:ubiquinone-1 activities in the reconstituted membranes were highly sensitive to 2-n-heptyl-4-hydroxyquinoline-N-oxide (IC50 = ∼1 μm), a quinone-binding inhibitor. Overexpressing N-terminally tagged AIF and AMID enhanced the growth of a double knock-out Escherichia coli strain lacking complex I and NDH-2. In contrast, C-terminally tagged AIF and NADH-binding site mutants of N-terminally tagged AIF and AMID failed to show both NADH:O2 activity and the growth-enhancing effect. The disease mutant AIFΔR201 showed decreased NADH:O2 activity and growth-enhancing effect. Furthermore, we surprisingly found that the redox activities of N-terminally tagged AIF and AMID were sensitive to rotenone, a well known complex I inhibitor. We propose that AIF and AMID are previously unidentified mammalian NDH-2 enzymes, whose bioenergetic function could be supplemental NADH oxidation in cells.
Introduction
Apoptosis-inducing factor (AIF)2 was discovered as a caspase-independent cell death effector. In response to cell death signaling, AIF translocates from the mitochondria to the nucleus (1). Under normal circumstances, AIF is loosely associated with the inner mitochondrial membrane (1). AIF is a ubiquitous flavin adenine dinucleotide (FAD)-containing protein and exhibits low NADH oxidase activity (1). Interestingly, its NADH oxidase activity was found to be independent of its apoptogenic activity (2). Later, however, the low NADH oxidase activity of AIF turned out to be an artifact of the refolding process of apo-AIF with FAD. AIF harboring naturally incorporated FAD forms a tight, air-stable charge transfer complex with NADH, showing literally no NADH oxidation, and it takes 2 days for air oxygen to fully oxidize FAD in charge transfer complex (3).
Mice in which the aif gene had been specifically inactivated in cardiac and skeletal muscle developed severe dilated cardiomyopathy, heart failure, and skeletal muscle atrophy and exhibited high lactate production and enhanced dependence on glycolytic ATP generation (4). These symptoms are consistent with those caused by mitochondrial respiratory chain defects. In fact, impaired activity and decreased complex I expression were found in these aif mutant tissues (4–6). The Harlequin mouse strain, in which the expression of AIF was reduced to 10–20% of the normal value, was found to have reduced complex I activity and complex I subunit expression in both the brain and the retina. These mice developed progressive cerebellar ataxia and blindness due to retinal degeneration (7). Human colon carcinoma cells in which the aif gene had been knocked out displayed lower complex I activity and failed to form tumors (6). Only AIF with intact NADH oxidase activity restored complex I activity and maintained the transformed state of colon cancer via mechanisms that involve complex I function (6).
Therefore, it has been suggested that AIF plays a vital role in mitochondrial respiration and redox metabolism (8, 9). However, the mechanism of how AIF functions to maintain the normal level of complex I activity and its protein expression has remained entirely unknown. AIF does not appear to be associated with complex I, and it does not affect transcription of complex I subunits (8, 10). To date, most studies, including blue native-PAGE analysis, have failed to pinpoint the exact role of AIF in mitochondrial respiration and redox metabolism in relation to complex I functions (10). Additionally, the physiological electron acceptor for AIF remains unidentified.
Meanwhile, it has been noted that AIF and its family member AMID (AIF-homologous mitochondrion-associated inducer of death) are similar to respiratory NADH:ubiquinone (UQ) oxidoreductases type 2 (NDH-2) (Fig. 1A) (11). We confirmed that relatively high sequence similarities exist between human AIF/AMID and the yeast NDH-2 enzyme Ndi1 (designated as internal NADH dehydrogenase), at 17.2 and 32%, respectively. In addition, we found that structural similarity between AIF and Ndi1 was 23.4% using RCSB PDB Protein Comparison Tool. Ndi1 is an essential enzyme in the mitochondria of Saccharomyces cerevisiae, an organism with the rare quality of lacking complex I.
FIGURE 1.

A, phylogenic tree of AIF/AMID proteins and type 2 NADH:quinone oxidoreductases. (The sequences used are as follow: Arabidopsis thaliana (A.t.), AMID (NP_563783); C. elegans (C.e.), Wah-1 (NP_499564); Drosophila melanogaster (D.m.), AIF (NP_001259907); E. coli (E.c.), Ndh (NP_415627); Homo sapiens (H.s.), AIF (NP_001124318); H. sapiens, AMID (NP_001185625); Mus musculus (M.m.), AIF (NP_001277293); M. musculus, AMID (NP_001034283); S. cerevisiae (S.c.), Ndi1 (NP_013586); S. cerevisiae, Nde1 (NP_013865); S. cerevisiae, AIF (NP_014472); and Y. lipolytica (Y.l.), Nde1 (XP_504691). B, NADH-O2 activities of wild-type, ΔnuoB, Δndh, and Ndi1- or AMID-overexpressed membranes in Δndh in the presence (solid bars) or absence (open bars) of piericidin A. 5 μl of membrane vesicles were used for the assay. Values are shown as means ± S.D. (n = 3).
NDH-2 enzymes are widely present in archaea, bacteria, and eukaryotic organisms from the fungal and plant kingdoms (11). NDH-2 catalyzes the same reaction as complex I, but it does not translocate protons (11). There are two types of NDH-2 enzymes. Ndi (designated internal NADH dehydrogenase) faces the matrix like complex I, whereas the other Nde (designated external NADH dehydrogenase) faces the intermembrane space. Impaired NADH oxidation in cells leads to high NADH/NAD+ ratio, increased production of reactive oxygen species (ROS), and eventually cell death. Maintenance of proper NADH/NAD+ ratio is so central to cellular energy metabolism. Upon sudden environmental changes, both internal and external NDH-2 enzymes, which have much higher turnover numbers of NADH:UQ oxidase activity than complex I, play a significant role in quickly restoring the altered NADH/NAD+ balance. Interestingly, however, NDH-2 has not previously been identified in mammalian mitochondria.
Therefore, we hypothesized that human AIF and/or AMID might function as NDH-2 in mammalian mitochondria. In this study, we investigated the redox properties of human AIF and/or AMID as NADH:UQ oxidoreductases in comparison with yeast Ndi1. We found that both human AIF and AMID display NADH:UQ oxidoreductase activities when they are reconstituted with the bacterial or mitochondrial membranes, and they are capable of establishing the NADH-linked respiratory activity in bovine submitochondrial particles (SMP) as well as in Escherichia coli membranes, almost as efficiently as Ndi1. Our data provide novel insight into the bioenergetic function of AIF and AMID in mitochondrial respiration and redox metabolism.
Experimental Procedures
Materials
The pCRScript Cloning kit was from Stratagene (La Jolla, CA). Materials for PCR product purification, gel extraction, and plasmid preparation were obtained from Qiagen (Valencia, CA). The BCA protein assay kit and SuperSignal West Pico chemiluminescent substrate were from Pierce. The pKO3 vector was a generous gift from Dr. George M. Church (Harvard Medical School, Boston). Bovine heart SMP were prepared as described previously (12). Antibodies against AIF and AMID were purchased from Santa Cruz Biotechnology (Dallas, TX).
Generation of Single Knock-out ΔnuoB and Δndh and Double Knock-out (DKO) E. coli Strains
The E. coli strain MC4100 (F−, araD139, Δ(arg F-lac)U169, ptsF25, relA1, flb5301, rpsL 150.λ−) was used to generate single knock-out strains devoid of either complex I (NDH-1) or NDH-2, and a DKO strain devoid of both NDH-1 and NDH-2. E. coli ΔnuoB and Δndh were constructed by replacement of the nuoB gene (in the nuo operon encoding NDH-1) or the ndh gene (encoding E. coli NDH-2) with the nuoB or ndh fragment disrupted spectinomycin gene, using a gene replacement technique with the gene replacement vector pKO3 (13). To generate DKO strains, first, the cluster N1b knock-out (ΔN1b strain), in which all four cysteine residues ligating to cluster N1b were replaced with alanine (C36A, C47A, C50A, and C69A), was generated from the nuoG knock-out strain [Δ(nuoG::Spc)] as described previously (14) using the same gene replacement technique with pKO3 (13). This ΔN1b strain showed no complex I activities, and no dehydrogenase subcomplex (NuoEFG subcomplex) of NDH-1 was detected in the membranes as well as in the supernatant. This ΔN1b strain was further used to knock out the ndh gene, yielding a DKO strain. The presence of the spectinomycin cassette and its location in the genomic gene were verified by PCR and DNA sequencing.
Cloning and Site-directed Mutagenesis of Human AIF and AMID Genes
Human AIF gene (AIFM1) and AMID (AIFM2) coding sequences have been amplified by PCR from human heart cDNA libraries (BioChain Institute). Because AIF contains the mitochondrial localization signal and inner membrane sorting signal sequences (8), we obtained cDNA fragments for both full-length (613AA) and two forms, Δ1–53AA and Δ1–101AA (15, 16). AIF and AMID cDNA fragments were subsequently ligated into the E. coli expression vector pET21b and pET16b, respectively. Later, to generate N-terminally tagged AIF, the AIF cDNA fragments were ligated into pET28b, and the stop codon was inserted. Site-directed mutagenesis was performed by QuikChange®II XL (Stratagene). We generated NADH-binding site mutants AIF-D444E/D444N and AMID-D285E/D285N, and AIF-ΔR201, which was the first disease-causing mutation identified in human AIF (17). The presence of mutations was confirmed by DNA sequencing. The oligonucleotides used for cloning and site-directed mutagenesis are listed in Table 1.
TABLE 1.
Oligonucleotides used in this study
The boldface bases were altered from the genomic DNA. The italicized bases represent the introduced restriction sites. F indicates forward and R indicates reverse. Mutated nucleotides are in boldface.
| Oligonucleotides | Sequences |
|---|---|
| hAIF-full-F (SalI) | 5′-GGGCTTATCGCCAGCTGCTTTACAAGGCCACACCTCCG-3′ |
| hAIFΔ1–53-F (SalI) | 5′-CTCCAGATGACAAGAGTCGACGCTAGCTCTGGTGCATCAGGG-3′ |
| hAIFΔ1–101-F (SalI) | 5′-AATGAAAGAATTTCAGTCGACGGGCTGACACCAGAACAG-3′ |
| hAIF-R (NotI) | 5′-CAATTCCACTGTGGCGGCCGCGTCTTCATGAATGTTGAATAGTTTGGC-3′ |
| hAIF-R (NotI)-stop-F | 5′-CAACATTCATGAAGACTAGGCCGCCACAGTGGAATTG-3′ |
| hAIF-R (NotI)-stop-R | 5′-CAATTCCACTGTGGCGGCCTAGTCTTCATGAATGTTG-3′ |
| hAMID-F (NdeI) | 5′-GACAGTGCCTGATTTCATATGGGGTCCCAGGTCTCG-3′ |
| hAMID-R (BamHI) | 5′-GCCAGGCGGGATCCATGCGCCAAGCAGTCCGTACGCCCACC-3′ |
| hAIF-D444E-F | 5′-GATGCTGCATGCTTCTACGAAATAAAGTTGGGAAGGAGGCGG-3′ |
| hAIF-D444E-R | 5′-CCGCCTCCTTCCCAACTTTATTTCGTAGAAGCATGCAGCATC-3′ |
| hAIF-D444N-F | 5′-GATGCTGCATGCTTCTACAATATAAAGTTGGGAAGGAGGCGG-3′ |
| hAIF-D444N-R | 5′-CCGCCTCCTTCCCAACTTTATATTGTAGAAGCATGCAGCATC-3′ |
| hAIF-ΔR201-F | 5′-CAGTGGAATGGAAAAGAGAGCATATATTTCCAGCCACC-3′ |
| hAIF-ΔR201-R | 5′-GGTGGCTGGAAATATATGCTCTCTTTTCCATTCCACTG-3′ |
| hAMID-D285E-F | 5′-GTCTACGCCATTGGTGAATGTGCCGACGTG-3′ |
| hAMID-D285E-R | 5′-CGTCCTCACGTCGGCACATTCACCAATGGCGTAGAC-3′ |
| hAMID-D285N-F | 5′-GTCTACGCCATTGGTAACTGTGCCGACGTG-3′ |
| hAMID-D285N-R | 5′-CGTCCTCACGTCGGCACAGTTACCAATGGCGTAGAC-3′ |
| NuoB-F (SalI) | 5′-GGGATATCGAGTAGTCGACGGACGATAG-3′ |
| NuoB-R (NcoI) | 5′-CCCCAGTTAACCATGGCATTGAGCTTGCCC-3′ |
| NuoB-F (NcoI) | 5′-CGCCGACAGTCACCATGGACCATTTGCAATG-3′ |
| NuoB-R (BamHI) | 5′-CGCGAATGACGTTAACGGGATCCGGCACGGG-3′ |
| Ndh-F (BamHI) | 5′-CACGAGAAAGGGATCCAATTGCAGTTTATTGACCCGG-3′ |
| Ndh-R (SalI) | 5′-CCTGCACCGGTCGACGACGGGTCACTGTGACG-3′ |
Overexpression and Membrane Preparation
C41 (DE3)-, ΔnuoB-, and Δndh-competent cells were transformed with pET16b/Ndi1, pET16b/AMID, pET21b/AIFΔ1–53, and pET21b/AIFΔ1–101, pET28b/AIFΔ1–53, or pET28b/AIFΔ1–101 vectors. Cells were then precultured at 37 °C overnight in 2× YT media with ampicillin (100 μg/ml) for pET16b and pET21b vectors or kanamycin (30 μg/ml) for pET28b vector. These cells were then transferred to Terrific Broth media also containing the same concentrations of ampicillin/kanamycin. For Ndi1 and AMID overexpression, the cells were grown at 250 rpm and 37 °C to the late stationary stage (typically an OD of 6–7), then 0.5 mm IPTG was added and cultured for an additional 2–3 h at 250 rpm and at 37 °C. For AIFΔ1–53 and AIFΔ1–101 overexpression, 0.5 mm IPTG was added at an OD of 0.8, and the cells were grown for an additional 10–15 h at 80 rpm and 30 °C. The cells were harvested and suspended in 50 mm Tris-HCl (pH 8.0), 1 mm EDTA, and 1 mm phenylmethylsulfonyl fluoride (PMSF). The cells were then broken by French press at 16,000 p.s.i. Unbroken cells and inclusion bodies were removed by centrifugation at 23,400 × g for 20 min, and the supernatant was centrifuged at 256,600 × g for 60 min. The pellet (membrane fraction) was suspended at 30 mg/ml in 50 mm Tris-HCl (pH 8.0 at 4 °C), 0.1 mm EDTA, 1 mm PMSF, and 10% glycerol. The supernatant fraction was also collected. The orientation of membrane vesicles was determined from the ratio of NADH:ferricyanide reductase assay measured in the absence and in the presence of 1% dodecyl β-d-maltoside. Our preparation of membrane vesicles was >95% inside out.
Growth Curves
DKO cells were transformed with pET16b/Ndi1, pET16b/AMID, pET16b/AMID(D285N), pET21b/AIFΔ1–53, pET21b/AIFΔ1–101, pET28b/AIFΔ1–53, pET28b/AIFΔ1–53(D444N), pET28b/AIFΔ1–53(ΔR201), pET28b/AIFΔ1–101, and empty pET21b and pET28b vectors. Transformed cells were precultured overnight in 2× YT media containing 100 μg/ml ampicillin or 30 μg/ml kanamycin (depending on the vectors) and 20 μg/ml of spectinomycin. The appropriate volume of preculture to give the same amount of cells was then added to 100 ml of 50% LB media containing the same concentrations of appropriate antibiotics in a 250-ml flask. The cell growth was monitored for 72–96 h, and 0.5 mm IPTG was added at OD = 0.1 to 0.5 at 600 nm. The growth of cells was monitored for 72 h.
Protein Purification
Proteins overexpressed in C41(DE3) cells were purified basically according to Ref. 18. AMID, C-terminally tagged AIFΔ1–53, and AIFΔ1–101 were purified from the supernatant, whereas Ndi1, and N-terminally tagged AIFΔ1–53 and AIFΔ1–101 were purified from the membrane. Dodecyl β-d-maltoside at a final concentration of 0.3% (w/v) instead of Triton X-100 was used to extract AIF proteins from the membrane. To elute AIF and AMID proteins, 500 mm imidazole was used instead of 200 mm histidine. To avoid aggregation, 1 m NaCl was added to all buffers during the purification process for AIF proteins. The enzyme fraction was then quickly frozen in liquid nitrogen and stored at −80 °C until further use.
Preparation of Liver Mitoplasts
Liver mitochondria from C57BL/6 mice were isolated in a similar manner as described previously (19, 20). Liver mitochondria were first diluted to 20 mg/ml in 10 mm HEPES buffer (pH 7.8) containing 320 mm sucrose and 0.1 mm EDTA. The suspension was then treated with 0.1 mg of digitonin/mg of mitochondrial protein for 10 min on ice before being centrifuged at 16,000 × g for 10 min. The pellet was then suspended in the same buffer.
Membrane Reconstitution
Isolated proteins Ndi1, AMID, and AIF were mixed with DKO membrane vesicles or bovine heart SMP at a 1:10 ratio (typically 1:10 mg/ml) by vortex (DKO) or sonication (SMP) for up to 5 s and then placed on ice for 5 min before use. To increase flavin incorporation, N-terminally tagged AIF proteins were incubated with 500 μm FAD for 5 min prior to the mixing with membranes. Mitoplasts were reconstituted with Ndi1 and AIF proteins at a 1:5 ratio by vortex.
Proton Pumping Activity and Membrane Potential Measurements
20 μl of the 1:10 mg/ml of reconstituted membranes was then added in 1 ml of 50 mm MOPS buffer (pH 7.0) containing 50 mm KCl, 10 mm MgCl2, and 0.2 μm 9-amino-6-chloro-2-methoxyacridine (ACMA) fluorescent dye. The reaction was initiated upon the addition of 200 μm NADH, and proton pumping activities were measured at 30 °C by ACMA (λex = 430 nm, λem = 480 nm) fluorescence quenching using Fluomax-4 spectrofluorometer (Horiba). When necessary, the channel-former gramicidin D was added as an uncoupler. The same buffer for proton pumping assay was used for membrane potential measurements, except ACMA was replaced with 2 μm oxonol VI was used. The reaction was started with the addition of 200 μm NADH, and the absorbance changes at 634 to 604 nm were monitored for 100 s with a diode array spectrophotometer 8452A (Hewlett Packard) as described previously (21, 22).
Other Analytical Procedures
NADH oxidase activity was spectrophotometrically measured with 5 μl of reconstituted membrane at 340 nm in 10 mm potassium phosphate (pH 7.0) containing 1 mm EDTA and 100 μm NADH at 30 °C. For NADH-ubiquinone 1 (UQ1) activity measurements, 10 mm KCN, 0.15 mm NADH, and 100 μm UQ1 were added to the assay mixture. The extinction coefficient used for activity calculations was ϵ340 = 6.22 mm−1 cm−1 for NADH. For NADH oxidase assays for the reconstituted SMP, the reaction mixture was incubated with/without rotenone for 3 min at 30 °C prior to the addition of NADH. Protein estimation was routinely done by the method of Bradford. SDS-PAGE was carried out according to Ref. 23. Any variations from the procedures and other details are described in the figure legends.
Results
Overexpression and Purification of AIF and AMID Proteins
We first attempted to overexpress Ndi1, AMID, AIFΔ1–53 (the mature form of AIF), and AIFΔ1–101 (the truncated cytosolic/nuclear form of AIF) in the E. coli NDH-2 knock-out strain (Δndh), in which overlapping NADH oxidase activity of NDH-2 from the host E. coli cells was removed. Although we successfully obtained E. coli cells overexpressing Ndi1 and AMID, AIF proteins were not overexpressed in the Δndh strain under the conditions we tried. Ndi1- or AMID-overexpressed membranes exhibited significantly higher NADH oxidase activity than the host Δndh membranes by 3.9 and 3.3 times, respectively (Fig. 1B). In the presence of the complex I inhibitor piericidin A at 5 μm, the NADH oxidase activity in the Δndh membranes that contain only complex I drastically decreased to 16% of the control. In contrast, the inhibitory effect of piericidin A was partial in Ndi1- or AMID-overexpressed Δndh membranes, similar to the effect seen in the membranes prepared from the wild type, which contains both complex I and E. coli NDH-2. This suggests that the remaining NADH oxidase activity is coming from overexpressed Ndi1 or AMID. Because ∼30% of NADH oxidase activity was also inhibited in the ΔnuoB membranes, which contain only E. coli NDH-2, it is highly likely that NADH oxidase activities by Ndi1 or AMID are also partially sensitive to piericidin A, similar to E. coli NDH-2.
By using E. coli C41(DE3) cells, which are known to be very effective in expressing toxic proteins (24), we finally succeeded in overexpressing N- and C-terminally tagged AIFΔ1–53 (the mature form of AIF) and AIFΔ1–101 (the truncated cytosolic/nuclear form of AIF) proteins as well as Ndi1 and AMID. Ndi1 and AMID were overexpressed mostly in the membrane fraction, whereas C-terminally tagged AIF proteins were mostly overexpressed in soluble fractions. N-terminally tagged AIF proteins were overexpressed in both soluble and membrane fractions. We purified AIF proteins from both fractions. As shown in Fig. 2A, C-terminally tagged AIFΔ1–53 was very susceptible to proteases even though it was purified in the presence of PMSF, EDTA, and a protease inhibitor mixture. More than half of AIFΔ1–53 was cleaved down to a similar size of AIFΔ1–101 (lane 4 in Fig. 2A). The absorption spectra of these purified proteins showed typical features of oxidized flavoproteins, with λmax at 380 and 450 nm, and a shoulder at ∼475 nm (Fig. 2B). According to the literature (3, 25), if there is a tag attached to the N terminus of AIF, which is close to the FAD-binding site, AIF is overexpressed as an apoprotein. Indeed, our N-terminally tagged AIF proteins purified from soluble fractions had low FAD content. However, AIF proteins purified from membrane fractions retained a significant amount of FAD (Fig. 2B). The FAD content in purified N-terminally tagged AIF was ∼30% lower than that in purified C-terminally tagged AIF. The spectra of Ndi1 and AIF proteins were very similar to previously published results, although our spectra for AMID were very different. According to Ref. 26, human AMID binds 6-hydroxy-FAD (a cofactor that accumulates only adventitiously and is in a low abundance in other flavoproteins), which has a green color with a sharp peak at 430 nm and a broad long-wavelength feature with a peak at ∼600 nm. These spectral features were completely different from those we obtained for our purified human AMID. We tried to express AMID using different conditions, including the exact same conditions as described in the aforementioned study (26), but we did not find any sign of 6-hydroxy-FAD features in our purified AMID.
FIGURE 2.

A, SDS-PAGE analysis of purified Ndi1, AMID, and AIF proteins. Ndi1 (20 μg, lane 1), AMID (15 μg, lane 2), C-terminally tagged AIFΔ1–101 and AIFΔ1–53 (20 μg, lane 3; 15 μg, lane 4), and N-terminally tagged AIF Δ1–101 and Δ1–53 (10 μg, lane 5; 10 μg, lane 6) were loaded onto a 10% Laemmli SDS-polyacrylamide gel. B, UV-visible absorption spectra of purified Ndi1, AMID, C-terminally tagged AIFΔ1–53 and AIFΔ1–101, and N-terminally tagged AIFΔ1–53 and AIFΔ1–101. The UV-visible spectra were taken in 50 mm Tris-HCl (pH 8.0) containing 10% glycerol and 200 μm EDTA. All the data were shown normalized at 1 mg/ml (black, Ndi1; red, AMID; thick blue, C-terminally tagged AIFΔ1–53; thin blue, C-terminally tagged AIFΔ1–101; broken blue, N-terminally tagged AIFΔ1–53; dotted blue, N-terminally tagged AIFΔ1–101).
NADH Oxidase and Proton Pumping Activities in DKO Membranes Reconstituted with AIF and AMID Proteins
First, we tried to measure NADH oxidase activities of purified Ndi1, AMID, and C- and N-terminally tagged AIFΔ1–53. All of them, including Ndi1, showed very low or almost no NADH oxidase activity (Table 2). The extremely low or no NADH oxidase activity of AIF is known, which is caused by the strong binding of NADH that leads to form an air-stable charge-transfer complex (3). NADH:ferricyanide activities of AMID and C- and N-terminally tagged AIFΔ1–53 were clearly detected, but they were 8–10 times lower than that of Ndi1. However, AMID and C-and N-terminally tagged AIFΔ1–53 showed very low NADH:ubiquinone-1 (UQ1, a hydrophilic quinone analog) activities, whereas Ndi1 displayed 50–100 times higher NADH:ubiquinone-1 (UQ1, a hydrophilic quinone analog) activities (Table 2). Therefore, we hypothesized that the quinone-binding sites in AMID and AIF might be different from that in Ndi1 and are not easily accessible from the hydrophilic outside. We decided to check AIF/AMID activities by reconstitution with E. coli membrane vesicles.
TABLE 2.
NADH:UQ1, NADH:O2, and NADH:ferricyanide oxidoreductase activities of purified Ndi1, AMID, and N- and C-terminally tagged AIFΔ1–53 proteins
| NADH:UQ1 | NADH:O2 | NADH:ferricyanide | |
|---|---|---|---|
| Ndi1 | 108 ± 28a | 3.58 ± 0.50 | 79.7 ± 2.9 |
| AMID | 3.72 ± 0.34 | 1.04 ± 0.061 | 10.6 ± 1.3 |
| C-terminally tagged AIFΔ1–53 | 0.395 ± 0.10 | 0.401 ± 0.03 | 6.67 ± 0.63 |
| N-terminally tagged AIFΔ1–53 | 0.713 ± 0.017 | 0.400 ± 0.044 | 7.95 ± 1.4 |
a Values are shown as means ± S.D. in micromoles of NADH/min/mg of purified protein (n = 3).
We reconstituted Ndi1, AMID, and AIF proteins into E. coli double knock-out (DKO) membrane vesicles and measured NADH oxidase activities (Fig. 3A). In the DKO membrane vesicles, only a trace amount of background NADH oxidase activity was detected. After the DKO membrane vesicles were reconstituted with Ndi1 or AMID, NADH oxidase activity increased by factors of 10.24 or 8.25, respectively. N-terminally tagged AIFΔ1–53 and AIFΔ1–101 also showed significant amounts of NADH oxidase activity. In contrast, the NADH-binding site mutants AMID-D285N and AIFΔ1–53-D444N completely lost their activities, whereas the human AIF disease mutant AIFΔ1–53-ΔR201 retained 60% of the wild-type AIFΔ1–53. Unexpectedly, no measurable NADH oxidase activities were detected in the DKO membrane vesicles reconstituted with C-terminally tagged AIF proteins (AIFΔ1–53 and AIFΔ1–101).
FIGURE 3.
A, NADH-O2 activities of Ndi1, AMID, and AIF proteins reconstituted in double knock-out (DKO) membranes. 5 μl of reconstituted membranes were used for the assay. Values are means ± S.D. (n = 3). B, proton pumping activities in the DKO membrane vesicles reconstituted with isolated Ndi1 (panel a), AMID (panel b), C-terminally tagged (panel c), and N-terminally tagged (panel d) AIF proteins. Generation of a proton gradient across the membranes was monitored by the quenching of ACMA fluorescence. At the time indicated by arrowheads, 200 μm NADH or 1 μm gramicidin was added to the assay mixture. Line 1, DKO membrane alone; line 2, Ndi1-reconstituted; line 3, AMID-D285N; line 4, AMID-D285E; line 5, AMID; line 6, C-terminally tagged AIFΔ1–53; line 7, C-terminally tagged AIFΔ1–101; line 8, N-terminally tagged AIFΔ1–53; line 9, N-terminally tagged AIFΔ1–101; line 10, N-terminally tagged AIFΔ1–53-ΔR201; line 11, N-terminally tagged AIFΔ1–53-D444N; line 12, DKO membrane.
In the aerobic E. coli respiratory chain, after UQ is reduced by complex I or NDH-2, cytochrome bo3 directly oxidizes ubiquinol and also proton pumps. Because the DKO membranes do not contain complex I, any proton pumping activity in reconstituted DKO membranes is solely operated by cytochrome bo3 that requires ubiquinol as a substrate. Thus, if proton pumping activities initiated by NADH are observed in the reconstituted DKO membranes, this indirectly indicates an NADH:UQ oxidoreductase activity by the reconstituted proteins. Proton translocation activity in these reconstituted membrane vesicles was measured using ACMA as a ΔpH indicator. As shown in Fig. 3B, a proton gradient was generated by the addition of NADH in the DKO membrane vesicles reconstituted with Ndi1 or AMID (lines 2 or 5, respectively). This proton gradient was immediately dissipated by gramicidin, an uncoupler. In contrast, no proton pumping activity was detected in nonreconstituted DKO membrane vesicles (Fig. 3B, line 1), nor in those reconstituted with the AMID-D285N (line 3) or AMID-D285E (line 4) mutants, nor in the C-terminally tagged AIFΔ1–53 (line 6) and AIFΔ1–101 (line 7). Proton pumping activity was detected in the DKO membrane vesicles reconstituted with the N-terminally tagged AIF proteins (Fig. 3B, panel d). Compared with Ndi1- or AMID-reconstituted samples, the proton pumping activity of N-terminally tagged AIFΔ1–53 was slower, reflecting its lower NADH oxidase activity. In fact, the proton pumping activity was higher in AIFΔ1–53 (line 8 in Fig. 3B, panel d), AIFΔ1–101 (line 9), AIFΔ1–53-ΔR201 (line 10), and AIFΔ1–53-D444N (line 11), in that order, and their proton pumping activity levels were consistent with their NADH oxidase activity levels. These results strongly suggest that similar to Ndi1, AMID and N-terminally tagged AIF proteins oxidize NADH and reduce ubiquinone in the DKO membranes, providing ubiquinol for the major terminal oxidase cytochrome bo3, which pumps protons. In other words, AMID and N-terminally tagged AIF proteins are capable of associating with the membrane and are integrated with the host respiratory chain. It is highly likely that AMID and N-terminally tagged AIF can function as respiratory NADH:UQ oxidoreductases.
HQNO Inhibits NADH:UQ1 Activity of AMID and N-terminally Tagged AIFΔ1–53
If AMID and N-terminally tagged AIF are NADH:UQ oxidoreductases, their physiological electron acceptors should be UQ (UQ10 for mammalian cells and UQ8 for E. coli cells). Therefore, we decided to investigate the inhibitory effect of 2-n-heptyl-4-hydroxyquinoline-N-oxide (HQNO), a well known quinone-binding inhibitor. UQ10 and UQ8 are extremely hydrophobic and cannot be used in aqueous assay reaction mixtures. We used UQ1, a hydrophilic UQ substrate, and measured NADH:UQ1 activities in the reconstituted DKO membranes.
NADH-UQ1 activities by Ndi1, AMID, and N-terminally tagged AIF reconstituted in DKO membrane vesicles were 23.4, 7.52, and 6.77 μmol/min/mg, respectively. In each case, the activity was strongly inhibited by HQNO at submicromolar levels (Fig. 4). The concentrations of half-maximal inhibition (IC50) were 1.34, 1.04, and 0.75 μm for Ndi1, AMID, and N-terminally tagged AIF, respectively. These values are comparable with the reported values for NDH-2 enzymes and other quinone oxidoreductases (27–30). These data strongly suggest that UQ is the physiological electron acceptor for AIF and AMID, as it is for Ndi1.
FIGURE 4.

Effect of the quinone analog inhibitor HQNO on NADH-UQ1 activities by Ndi1 (A), AMID (B), and N-terminally tagged AIFΔ1–53 (C) in the reconstituted DKO membranes. The control values (n = 3) for Ndi1, AMID, and N-terminally tagged AIFΔ1–53, were 23.4, 7.52, and 6.77 μmol/min/mg, respectively.
Deamino-NADH (dNADH) Does Not Serve as an Efficient Substrate for AMID or N-terminally Tagged AIFΔ1–53
The NADH analog dNADH has been used to discriminate complex I activity from NDH-2 in E. coli membranes (31), and NDH-2 has been believed to react exclusively with NADH. We measured proton pumping activity in DKO membranes reconstituted with Ndi1, AMID, or N-terminally tagged AIF. It is known that Ndi1 is highly specific for the electron donor NADH and that dNADH and NADPH yield more than 100-fold lower rates in terms of NADH:UQ2 redox activity (32). Interestingly, however, the difference between NADH- and dNADH-linked proton pumping activities in DKO membrane reconstituted with Ndi1 was not very large (Fig. 5A). The dNADH-linked initial pumping rate was 38.5% of the control (NADH-linked initial rate). This could be due to the possibility that Ndi1-linked proton pumping activity with NADH was limited by the turnover rate of cytochrome bo3 in DKO membranes. In contrast, no NADPH-linked pumping activity was observed in the Ndi1-reconstituted DKO membranes. Compared with Ndi1, the dNADH-linked initial pumping rates in DKO membranes reconstituted with AMID or N-terminally tagged AIFΔ1–53 were much less: 4.3 and 0% of the control, respectively (Fig. 5, B and C). Our data suggest that dNADH is a poor substrate for both AMID and N-terminally tagged AIF.
FIGURE 5.

Deamino-NADH is a poor substrate for Ndi1, AMID, and N-terminally tagged AIF-mediated proton pumping activities. The DKO membrane vesicles were reconstituted with isolated Ndi1 (A), AMID (B), and AIF (C) proteins. The reaction was started with the addition of 200 μm NADH (black), 100 μm dNADH (red), or 100 μm NADPH (blue). 5, 20, and 20 μl of 1:10 reconstituted mixture of Ndi1, AMID, and N-terminally tagged AIF, respectively, were added to the reaction mixture.
Expression of AMID or N-terminally Tagged AIF Enhances the Growth of DKO Cells
To further support the idea that AMID and/or AIF proteins have respiratory NADH:UQ oxidoreductase activity in vivo, we compared the growth curves of E. coli DKO cells transformed with pET16b/Ndi1, pET16b/AMID, pET16b/AMID-D285N, pET21b/AIFΔ1–53, pET28b/AIFΔ1–53, pET28b/AIFΔ1–53-D444N, pET28b/AIFΔ1–53-ΔR201, pET28b/AIFΔ1–101, and corresponding empty vectors. After the addition of IPTG, the cells overexpressing AMID (red in Fig. 6A), N-terminally tagged AIFΔ1–53 (red in Fig. 6, B–D), or Ndi1 (blue in Fig. 6, A, B, and D) grew faster than the control cells carrying an empty vector (broken line in Fig. 6, A–D). However, neither overexpression of NADH-binding deficient mutants, AMID-D258N (green in Fig. 6A) and AIF-D444N (green in Fig. 6C), nor that of C-terminally tagged AIF (green in Fig. 6B) had this growth-enhancing effect. N-terminally tagged AIFΔ1–101 (red open circles in Fig. 6D) and the human AIF disease mutant AIFΔ1–53-ΔR201 (green open circles in Fig. 6C) showed intermediate growth-enhancing effect. These results are all consistent with NADH oxidase and proton pumping activities of their corresponding reconstituted purified proteins (Fig. 3). It is especially important to state that the growth-enhancing effect of AIF is the same level as that of Ndi1 or AMID. In addition, the absence of a growth-enhancing effect with C-terminally tagged AIF supports our hypothesis that the C-terminal region of AIF is important for membrane association. Thus, it is highly likely that N-terminally tagged AIF and AMID are respiratory NADH:UQ oxidoreductases similar to Ndi1.
FIGURE 6.

Growth curves of E. coli DKO cells overexpressing Ndi1, AMID, or AIF proteins. A, blue, Ndi1; red, AMID; green, AMID-D285N; dashed line, empty pET16b. B, blue, Ndi1; red, N-terminally tagged AIFΔ1–53; green, C-terminally tagged AIFΔ1–53; dashed line, empty pET16b. C, red, N-terminally tagged AIFΔ1–53; green, N-terminally tagged AIFΔ53-D444N; green with open circles, N-terminally tagged AIFΔ1–53-ΔR201; dashed line, empty pET28b. D, blue, Ndi1; red, N-terminally tagged AIFΔ1–53; red with open circles, N-terminally tagged AIFΔ1–101; dashed line, empty pET28b. The arrows indicate the addition of 0.5 mm IPTG.
AMID and N-terminally Tagged AIF Enzyme Can be Integrated into the Respiratory Chain of Bovine Heart SMP
To gain insight into the physiological role of AIF/AMID protein in the mitochondrial respiratory chain, we performed a series of reconstitution experiments using bovine heart SMP. To see their enzyme activities, we mixed Ndi1, AIF, and AMID proteins with bovine heart SMP at a 1:10 ratio, which is beyond the physiological stoichiometry. The SMP preparation we used for these experiments had an NADH oxidase activity of 2.78 μmol/min/mg. As shown in Table 3, when Ndi1, AMID, and N-terminally tagged AIF were incorporated into the SMP, the reconstituted SMP exhibited basically similar levels of NADH oxidase activities. However, in the presence of 0.1 μm rotenone, the NADH oxidase activity in nonreconstituted, intact SMP was completely inhibited, whereas significant NADH oxidase activity remained in SMP reconstituted with Ndi1-, AMID-, and N-terminally tagged AIF. These activities were completely inhibited by KCN (a complex IV inhibitor) and antimycin A (a complex III inhibitor). These results suggest that NADH oxidase activity of Ndi1, AMID, and N-terminally tagged AIF are resistant to 0.1 μm rotenone and that these activities are linked to the downstream of the mitochondrial respiratory chain in SMP.
TABLE 3.
NADH:O2 activities (μmol of NADH/min/mg of reconstituted) of reconstituted SMP
| SMP | +Ndi1 | +AMID | +N-terminally tagged AIFΔ1–53 | ||
|---|---|---|---|---|---|
| Rotenone (0.1 μm) | − | 2.78 ± 0.27a | 3.11 ± 0.46 | 3.28 ± 0.19 | 2.58 ± 0.19 |
| + | 0.012 ± 0.01 (0.46%)b | 1.62 ± 0.07 (52.1%) | 0.91 ± 0.07 (27.6%) | 0.91 ± 0.03 (36.4%) |
a Values are shown as means ± S.D. (n = 3).
b Percentage of the remaining activity after the addition of rotenone is shown.
To ascertain this point, we also measured the proton pumping activity in these reconstituted SMP. Similarly, reconstitution of these proteins did not increase the total proton pumping activities (red lines in Fig. 7A, panels a–c), but it reduced the rotenone sensitivity of SMP. In the presence of 0.1 μm rotenone, no proton pumping activity was observed in SMP-only samples (thin black lines in Fig. 7A, panels a–c), whereas proton pumping activity largely remained in the SMP reconstituted with Ndi1, AMID, or N-terminally tagged AIF (red thick lines in Fig. 7A, panels a–c, respectively). In addition, no difference in the level of the remaining proton pumping activity was observed between Ndi1-, AMID-, and N-terminally tagged AIF-reconstituted SMP, in contrast with the proton pumping data with reconstituted DKO membranes (Fig. 3B). This could be due to the stronger association of N-terminally tagged AIF with SMP than that with E. coli DKO membrane vesicles. However, a higher 0.5 μm of rotenone drastically decreased the proton pumping activities in the SMP reconstituted with AMID or N-terminally tagged AIF, whereas the proton pumping activity remained at the same level in the SMP reconstituted with Ndi1 (red thin lines in Fig. 7A, panels a–c).
FIGURE 7.
A, proton pumping activities in bovine heart SMP reconstituted with isolated Ndi1 (panel a), AMID (panel b), or N-terminally tagged AIFΔ1–53 (panel c). 10 μl of 1:10 reconstituted mixture of Ndi1, AMID, or AIF were added to the reaction mixture. At the time indicated by arrowheads, 200 μm NADH or 1 μm gramicidin were added to the assay mixture. Thick black line, SMP; thin black line, SMP + 0.1 μm rotenone; blue line, SMP + isolated proteins (panel a, Ndi1; panel b, AMID; panel c, N-terminally tagged AIFΔ1–53); red thick line, SMP + 0.1 μm rotenone + isolated proteins (panel a, Ndi1; panel b, AMID; panel c, N-terminally tagged AIFΔ1–53); red thin line, SMP + 0.5 μm rotenone + isolated proteins (panel a, Ndi1; panel b, AMID; panel c, N-terminally tagged AIFΔ1–53). B, membrane potentials generated by the addition of NADH in bovine heart SMP reconstituted with Ndi1, AMID, and N-terminally tagged AIFΔ1–53. SMP alone (panel a) SMP + Ndi1 (panel b) SMP + AMID (panel c) SMP + N-terminally tagged AIFΔ1–53 (panel d). Membrane potentials were measured in the absence (blue dotted line) or presence (red dotted line) of 0.1 μm rotenone. 10 μl of 1:10 reconstituted mixture of Ndi1, AMID, and AIF were added in the reaction mixture. At the time indicated by arrowheads, 200 μm NADH was added to the assay mixture. The representative of three measurements is shown in the figure.
We also monitored membrane potentials (ΔΨ) of those reconstituted SMP by following the absorbance change of oxonol VI (22, 33). As shown in Fig. 7B, the addition of NADH to the nonreconstituted SMP increased the absorbance, indicating the inside positive ΔΨ formation (blue dotted lines). As expected, preincubation of the SMP with 0.1 μm rotenone resulted in a complete loss of the ΔΨ formation (red dotted line, Fig. 7B, panel a). However, a significant ΔΨ was still generated in the SMP reconstituted with Ndi1, AMID, and N-terminally tagged AIF after preincubation with 0.1 μm rotenone (red dotted line, Fig. 7B, panels b and c). These results are consistent with the proton pumping activity data (Fig. 7A, panels a–c, red thick lines). These data strongly suggest that AMID and N-terminally tagged AIF could function as NADH:UQ oxidoreductases in the host respiratory chain, when complex I is inhibited by rotenone, and their activity levels are comparable with those of Ndi1.
N-terminally Tagged AIF Can Be Associated with Mitoplasts and Integrated into the Respiratory Chain of Liver Mitochondria
Reconstitution experiments with inside-out SMP do not mimic the physiological location of AIF in mammalian cells, as it is believed that AIF is loosely attached to the cytosolic side of the mitochondrial inner membranes. So, we prepared mitoplasts from mouse liver tissue, which are more likely to provide physiological membrane association of AIF. We measured the NADH:O2 activity of AIF reconstituted with this mitoplast, and we studied its rotenone sensitivity. As expected, there was no basal NADH:O2 activity due to complex I in the mitoplast (Fig. 8). The activity was detected only after the mitoplasts were reconstituted with AIF. Interestingly, compared with the data with SMP, much higher NADH:O2 activity was observed in AIF-reconstituted mitoplasts. This activity was sensitive to rotenone as seen in the AIF-reconstituted SMP, and it was completely inhibited by KCN, an inhibitor of cytochrome c oxidase (Fig. 8). These data directly support the notion that AIF can function as a respiratory NADH:ubiquinone oxidoreductase (NDH-2).
FIGURE 8.
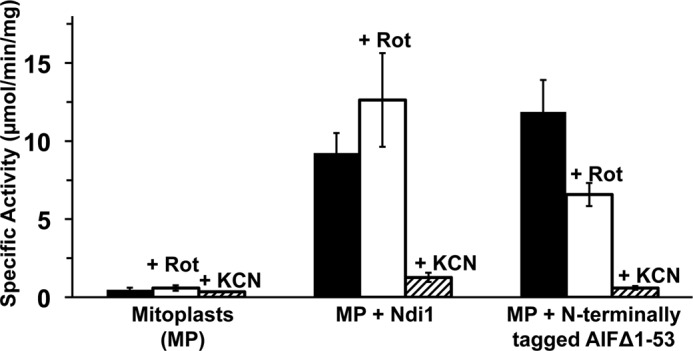
NADH:O2 activities of Ndi1 and N-terminally tagged AIFΔ1–53 proteins reconstituted in mouse liver mitoplasts. 2.5 μl of reconstituted mitoplasts were used for the assay. Values are means ± S.D. (n = 3). NADH:O2 activities were measured without inhibitors (black bars), in the presence of 0.1 μm rotenone (Rot, open bars), or 1 mm KCN (hatched bars).
AMID and N-terminally Tagged AIF Activities Are Inhibited at Submicromolar Rotenone Concentrations
Because we noticed that AMID- and AIF-associated proton pumping activities in SMP and the mitoplasts were sensitive to higher concentrations of rotenone, we investigated rotenone's effects on AMID- or AIF-associated NADH oxidase and proton pumping activities using DKO membranes. As expected, Ndi1-associated NADH oxidase and proton pumping activity levels were not affected by rotenone at 0.1 and 0.5 μm (Fig. 9, A, panel a, and B, panel a). In contrast, AMID- and AIF-associated NADH oxidase activities drastically decreased to 61.5 and 37.7% for AMID and to 65.8 and 39.5% for AIF at 0.1 and 0.5 μm rotenone, respectively (Fig. 9A, panels b and c). This decreased NADH oxidase activity in AMID and AIF-reconstituted DKO membranes was parallel with the decreased proton pumping activity (Fig. 9B, panels b and c). Their rotenone sensitivity was more enhanced in DKO membranes than that in SMP, where 0.1 μm rotenone did not significantly inhibit AMID and AIF activities. These results strongly suggest that AMID and AIF are rotenone-sensitive, although NDH-2 enzymes, including Ndi1 and E. coli NDH-2, are generally considered to be rotenone-resistant (11).
FIGURE 9.

Effect of rotenone on NADH:O2 (A) and proton pumping activities (B) in DKO reconstituted with Ndi1 (panel a), AMID (panel b), or N-terminally tagged AIFΔ1–53 (panel c). Black (bars and lines), red, (bars and lines), and blue (bars and lines) indicated that NADH-O2 or proton pumping activities were measured in the presence of 0, 0.1, or 0.5 μm rotenone, respectively. The measurements were repeated at least three times.
Discussion
Various studies on in vivo phenotypes associated with AIF deficiency have revealed the importance of AIF in the maintenance of mitochondrial energy metabolism (25, 34). This bioenergetic role of AIF is also conserved in lower organisms. AIF-deficient yeast failed to grow on nonfermentable carbon sources, such as galactose and lactate (35). AIF deficiency also led to growth arrest in fruit flies, which experienced defective complex I function and reduced ATP levels (36). The slower growth rate was reported for AIF knockdown in Caenorhabditis elegans (37). The first case of disease-causing mutations in human AIF (Arg-201 deletion in the AIFM1 gene) was identified (17). Again, multiple respiratory chain-defective activities were observed in infant monozygotic twin patients with a mitochondrial early onset encephalomyopathy (17). Despite the many experiments that have been conducted to investigate whether AIF is required for optimal biogenesis/assembly of multiprotein respiratory chain complexes, there has been no indication of a physical interaction of AIF with specific subunits from respiratory chain complexes (8, 34, 38). It has been shown that AIF contains a FAD, but, over a decade later, many mysteries still surround bioenergetics roles of AIF, including the identity of the physiological electron acceptor of AIF. Therefore, our study demonstrating that “AIF is an NDH-2 enzyme” is highly significant. This means that the physiological electron acceptor for AIF is ubiquinone. If AIF could function like an external NDH-2 (which is attached to inner mitochondrial membrane facing the intermembrane space), we can expect that AIF might work as an antioxidant enzyme and contribute to decreasing the rate of ROS production by oxidizing excessive cytosolic NADH, otherwise higher NADH levels disturb the cellular redox balance, leading to cytosolic ROS production. In fact, the antioxidant roles for external NDH-2 enzymes have been experimentally proven previously (39, 40). In addition, there is a report demonstrating with AIF-silenced cell experiments that the loss of AIF leads to an increase in ROS production and impairment of complex I respiration, which were restored by adding antioxidants (41). This supports the idea that free radicals play an important role in the observed respiration defect. Therefore, it is highly likely that AIF deficiency causes complex I defects through increased ROS production. In that respect, our finding that AIF is an NDH-2 and can be integrated into the mitochondrial respiratory chain provides a significant step toward understanding the mechanisms linking AIF deficiency with ROS production and complex I defects. Although the mechanism of how AIF deficiency increases ROS production remains unknown, one possible explanation is that AIF perhaps helps maintain the cytosolic NAD+/NADH ratio by its NADH:UQ oxidoreductase activity. This function can also be reinforced by the redox potential of AIF. The reported midpoint redox potential of FAD/FADH2 in AIFΔ1–120 is −308 mV at pH 7.5 obtained by spectral titration with dithionite (2). This value is within the range of efficient oxidation of NADH, −320 mV, which is slightly higher compared with the midpoint redox potential of FMN/FMNH2 (−376 mV) in complex I by monitoring flavosemiquinone radicals with EPR (42).
If AIF is catalytically active in mitochondria, why has its activity thus far gone undetected? According to previous literature, AIF is ubiquitously expressed, but a relatively small amount is expressed in mitochondria as compared with complex I. Because complex I and AIF catalyze the same reaction, it is necessary to use a specific inhibitor for complex I to distinguish between their activities. However, rotenone, a commonly used complex I inhibitor, was found to inhibit AIF. Based on our study, 0.1 μm or slightly smaller concentrations of rotenone is enough to completely inhibit complex I, providing a possibility to measure AIF activity. In most experiments, NADH oxidase activities were measured in the presence of 1–10 μm rotenone, so there was no possibility to detect AIF activity even if it did exist. It was also reported, however, that low level expression of internal alternative NADH dehydrogenase, even when the activity was below the detection limit, is still sufficient to rescue complex I deficiency in Yarrowia lipolytica (43). Therefore, even though no NADH oxidase activity of AIF was observed after complex I was inhibited, it is still possible that NADH-dependent oxidoreductase activity of AIF is physiologically relevant.
One of our unexpected results was that C-terminally tagged AIF was expressed only in soluble fractions and did not show any NADH oxidase activity after being reconstituted into DKO membranes (Fig. 3A) nor any growth-enhancing effect (Fig. 6B), which is in striking contrast to N-terminally tagged AIF. These results prompted us to consider the possibility that the C-terminal polyhistidine tag might inhibit AIF association to the membrane. To explore this idea, we compared the x-ray crystal structures of mouse AIF (44) with that of Ndi1 from S. cerevisiae (45, 46) and NDH-2 from Caldalkalibacillus thermarum (47). In both the Ndi1 and NDH-2 enzymes, the C-terminal regions are essential for membrane anchoring. We found a short α-helix segment in the C-terminal region of AIF, which could be involved in membrane association, although the length of the α-helix is much shorter in AIF than in Ndh and Ndi1. The previous report by Otera et al. (16) has suggested that the mature AIF is anchored to the mitochondrial inner membrane with the N terminus facing the intermembrane space through the N-terminal region at a cluster of hydrophobic residues 66–84. However, based on this study, it seems that the C-terminal amphipathic helix region is required for AIF's membrane association to establish its NADH:UQ oxidoreductase activity in vivo (Fig. 6D). We also found that the truncated cytosolic AIFΔ1–101 form is still capable of being associated with membranes and showed its redox activities (Fig. 3). Our results do not completely exclude the possibility that AIF could also be associated with the membrane through the N-terminal putative transmembrane region. Further experiments will be needed to clarify how AIF is associated with the mitochondrial inner membrane.
Our result that AIF and AMID were sensitive to rotenone (shown in Figs. 7A, 8, and 9) was totally unexpected. Although AMID also seems to have some sensitivity toward the other common complex I inhibitor piericidin A (Fig. 1B), this feature can easily be expected. Piericidin A is regarded as a quinone analog, and in fact, a higher concentration of piericidin A (5 μm) partially inhibited E. coli NDH-2 and seemingly Ndi1 as well (Fig. 1B). However, the rotenone sensitivity of AIF and AMID seems to be a very unusual feature for NDH-2, as Ndi1 and E. coli NDH-2 are completely rotenone-insensitive. We found that rotenone inhibits the NADH oxidase activities of AIF or AMID with half-maximal inhibitory concentrations (IC50) of ∼0.15 μm, which is still much higher than the IC50 = 17.3 nm reported for mammalian complex I (48). It has been reported that rotenone also depolymerizes cellular microtubules and inhibits the binding of colchicine to tubulin (49). The rotenone inhibitory effect against AIF and AMID might be due to the difference between AIF/AMID and other NDH-2 enzymes' respective quinone-binding sites, possibly caused by their conformational states and unique membrane association fashions. Further study will be needed to clarify the inhibitory mechanism of rotenone in AIF and AMID.
Our other unexpected result was that in contrast to the previous report by Marshall et al. (26), our purified AMID contained FAD and not 6-hydroxy-FAD. Although we tried several different expression conditions and used the same host strain as described previously (26), our purified AMID contained only FAD. This difference could be caused by a difference in the N-terminal His tag sequence (MHHHHHHSSGLVPRGSH versus MGHHHHHHHHHHSSGHIEGRH), which might affect the microenvironment of the FAD-binding site. Considering the fact that these authors observed a partial conversion from FAD to 6-hydroxy-FAD following reduction by NADPH in FAD-reconstituted AMID and the other published fact that 6-hydroxy-FAD was found in a minor inactive form of d-aspartate oxidase (50) and electron transferring flavoprotein purified from beef liver (51), it can be speculated that 6-hydroxy-FAD results from oxidative modification of FAD under certain conditions after its incorporation.
Our data clearly showed that AMID could function as an NDH-2. However, compared with AIF, there is almost no evidence that AMID is expressed in mitochondria and is physiologically active, as AMID lacks a mitochondrial localization sequence. The role of AMID in apoptosis is also unclear. By in silico methods and image analysis, the localization of AMID was predicted to be cytoplasmic, most probably incorporated into the cytoplasmic side of the cellular membranes (52, 53). It was also suggested that AMID in human leukemia Jurkat T-cells after apoptosis induction is associated with the plasma membrane by immunohistochemical study (54). It will be interesting to determine whether AMID is associated with the mitochondrial inner membrane facing the matrix side under normal conditions.
Interestingly, Ndi1 and AIF seem to share a similar story, despite their different orientations as follows: Ndi1 faces the matrix, and AIF faces the intermembrane space. It was reported that overexpression of Ndi1 induces a caspase-independent apoptosis-like cell death in yeast when it is cultured in glucose-rich media (55). Similar to AIF, its pro-apoptotic property is separable from its NADH:UQ oxidoreductase activity. Under normal physiological conditions, Ndi1 and probably AIF assimilate electrons into the electron transport chain, whereas upon apoptotic signaling, AIF and probably Ndi1 are transformed into apoptotic proteins.
In summary, for the first time, we successfully showed the following: 1) AIF and AMID can catalyze NADH:UQ oxidoreductase reactions, and UQ is the physiological electron acceptor for AIF and AMID. 2) AIF and AMID are both capable of being integrated as a member of the host respiratory chain when they are associated with membrane vesicles. 3) AIF and AMID are highly likely associated with membranes via their C-terminal regions. 4) AIF and AMID activities are HQNO-sensitive, a common quinone-binding inhibitor. 5) dNADH is not an efficient substrate for AIF and AMID. All of these results support that AIF and AMID are alternative NADH dehydrogenases (NDH-2). It was remarkable that both AIF and AMID can easily be associated with the matrix side of the inner membrane of SMP and show NADH:UQ activities, even though AIF is thought be localized in the intermembrane space. Although further experiments will be necessary to clarify the physiological role of AIF in the mitochondria, this study will help clarify the relationship between complex I and AIF in the mitochondria, and it could explain the “enigma” related to the bioenergetic function of AIF, including why AIF deficiency results in complex I defects.
Author Contributions
E. N. O. designed the study and wrote the paper. M. M. E. and E. N. O. designed, performed the experiments, and analyzed the results. All authors reviewed and approved the final version of the manuscript.
Acknowledgments
We thank Dr. Madhavan Narayanan and Michael Happ for discussion and technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1GM097409 (to E. N.-O.). This work was also supported by American Heart Association Grant 11SDG5560001 (to E. N.-O.). The authors declare that they have no conflicts of interest with the contents of this article.
- AIF
- apoptosis-inducing factor
- ACMA
- 9-amino-6-chloro-2-methoxyacridine
- AMID
- AIF-homologous mitochondrion-associated inducer of death
- DKO
- double knock-out
- ROS
- reactive oxygen species
- SMP
- submitochondrial particles
- UQ
- ubiquinone
- IPTG
- isopropyl 1-thio-β-d-galactopyranoside
- dNADH
- deamino-NADH.
References
- 1. Susin S. A., Lorenzo H. K., Zamzami N., Marzo I., Snow B. E., Brothers G. M., Mangion J., Jacotot E., Costantini P., Loeffler M., Larochette N., Goodlett D. R., Aebersold R., Siderovski D. P., Penninger J. M., Kroemer G. (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397, 441–446 [DOI] [PubMed] [Google Scholar]
- 2. Miramar M. D., Costantini P., Ravagnan L., Saraiva L. M., Haouzi D., Brothers G., Penninger J. M., Peleato M. L., Kroemer G., Susin S. A. (2001) NADH oxidase activity of mitochondrial apoptosis-inducing factor. J. Biol. Chem. 276, 16391–16398 [DOI] [PubMed] [Google Scholar]
- 3. Churbanova I. Y., Sevrioukova I. F. (2008) Redox-dependent changes in molecular properties of mitochondrial apoptosis-inducing factor. J. Biol. Chem. 283, 5622–5631 [DOI] [PubMed] [Google Scholar]
- 4. Joza N., Oudit G. Y., Brown D., Bénit P., Kassiri Z., Vahsen N., Benoit L., Patel M. M., Nowikovsky K., Vassault A., Backx P. H., Wada T., Kroemer G., Rustin P., Penninger J. M. (2005) Muscle-specific loss of apoptosis-inducing factor leads to mitochondrial dysfunction, skeletal muscle atrophy, and dilated cardiomyopathy. Mol. Cell. Biol. 25, 10261–10272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown D., Yu B. D., Joza N., Bénit P., Meneses J., Firpo M., Rustin P., Penninger J. M., Martin G. R. (2006) Loss of AIF function causes cell death in the mouse embryo, but the temporal progression of patterning is normal. Proc. Natl. Acad. Sci. U.S.A. 103, 9918–9923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Urbano A., Lakshmanan U., Choo P. H., Kwan J. C., Ng P. Y., Guo K., Dhakshinamoorthy S., Porter A. (2005) AIF suppresses chemical stress-induced apoptosis and maintains the transformed state of tumor cells. EMBO J. 24, 2815–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klein J. A., Longo-Guess C. M., Rossmann M. P., Seburn K. L., Hurd R. E., Frankel W. N., Bronson R. T., Ackerman S. L. (2002) The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature 419, 367–374 [DOI] [PubMed] [Google Scholar]
- 8. Hangen E., Blomgren K., Bénit P., Kroemer G., Modjtahedi N. (2010) Life with or without AIF. Trends Biochem. Sci. 35, 278–287 [DOI] [PubMed] [Google Scholar]
- 9. Joza N., Pospisilik J. A., Hangen E., Hanada T., Modjtahedi N., Penninger J. M., Kroemer G. (2009) AIF: not just an apoptosis-inducing factor. Ann. N.Y. Acad. Sci. 1171, 2–11 [DOI] [PubMed] [Google Scholar]
- 10. Vahsen N., Candé C., Brière J. J., Bénit P., Joza N., Larochette N., Mastroberardino P. G., Pequignot M. O., Casares N., Lazar V., Feraud O., Debili N., Wissing S., Engelhardt S., Madeo F., et al. (2004) AIF deficiency compromises oxidative phosphorylation. EMBO J. 23, 4679–4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Melo A. M., Bandeiras T. M., Teixeira M. (2004) New insights into type II NAD(P)H:quinone oxidoreductases. Microbiol. Mol. Biol. Rev. 68, 603–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsuno-Yagi A., Hatefi Y. (1985) Studies on the mechanism of oxidative phosphorylation. Catalytic site cooperativity in ATP synthesis. J. Biol. Chem. 260, 11424–11427 [PubMed] [Google Scholar]
- 13. Link A. J., Phillips D., Church G. M. (1997) Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179, 6228–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakamaru-Ogiso E., Yano T., Yagi T., Ohnishi T. (2005) Characterization of the iron-sulfur cluster N7 (N1c) in the subunit NuoG of the proton-translocating NADH-quinone oxidoreductase from Escherichia coli. J. Biol. Chem. 280, 301–307 [DOI] [PubMed] [Google Scholar]
- 15. Wilkinson J. C., Wilkinson A. S., Galbán S., Csomos R. A., Duckett C. S. (2008) Apoptosis-inducing factor is a target for ubiquitination through interaction with XIAP. Mol. Cell. Biol. 28, 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Otera H., Ohsakaya S., Nagaura Z., Ishihara N., Mihara K. (2005) Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J. 24, 1375–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghezzi D., Sevrioukova I., Invernizzi F., Lamperti C., Mora M., D'Adamo P., Novara F., Zuffardi O., Uziel G., Zeviani M. (2010) Severe X-linked mitochondrial encephalomyopathy associated with a mutation in apoptosis-inducing factor. Am. J. Hum. Genet. 86, 639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamashita T., Nakamaru-Ogiso E., Miyoshi H., Matsuno-Yagi A., Yagi T. (2007) Roles of bound quinone in the single subunit NADH-quinone oxidoreductase (Ndi1) from Saccharomyces cerevisiae. J. Biol. Chem. 282, 6012–6020 [DOI] [PubMed] [Google Scholar]
- 19. Hoppel C., DiMarco J. P., Tandler B. (1979) Riboflavin and rat hepatic cell structure and function. Mitochondrial oxidative metabolism in deficiency states. J. Biol. Chem. 254, 4164–4170 [PubMed] [Google Scholar]
- 20. Peng M., Falk M. J., Haase V. H., King R., Polyak E., Selak M., Yudkoff M., Hancock W. W., Meade R., Saiki R., Lunceford A. L., Clarke C. F., Gasser D. L. (2008) Primary coenzyme Q deficiency in Pdss2 mutant mice causes isolated renal disease. PLoS Genet. 4, e1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sinha P. K., Torres-Bacete J., Nakamaru-Ogiso E., Castro-Guerrero N., Matsuno-Yagi A., Yagi T. (2009) Critical roles of subunit NuoH (ND1) in the assembly of peripheral subunits with the membrane domain of Escherichia coli NDH-1. J. Biol. Chem. 284, 9814–9823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghelli A., Benelli B., Esposti M. D. (1997) Measurement of the membrane potential generated by complex I in submitochondrial particles. J. Biochem. 121, 746–755 [DOI] [PubMed] [Google Scholar]
- 23. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 24. Miroux B., Walker J. E. (1996) Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260, 289–298 [DOI] [PubMed] [Google Scholar]
- 25. Sevrioukova I. F. (2011) Apoptosis-inducing factor: structure, function, and redox regulation. Antioxid. Redox Signal. 14, 2545–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marshall K. R., Gong M., Wodke L., Lamb J. H., Jones D. J., Farmer P. B., Scrutton N. S., Munro A. W. (2005) The human apoptosis-inducing protein AMID is an oxidoreductase with a modified flavin cofactor and DNA binding activity. J. Biol. Chem. 280, 30735–30740 [DOI] [PubMed] [Google Scholar]
- 27. Mogi T., Matsushita K., Murase Y., Kawahara K., Miyoshi H., Ui H., Shiomi K., Omura S., Kita K. (2009) Identification of new inhibitors for alternative NADH dehydrogenase (NDH-II). FEMS Microbiol. Lett. 291, 157–161 [DOI] [PubMed] [Google Scholar]
- 28. Jeuken L. J., Connell S. D., Henderson P. J., Gennis R. B., Evans S. D., Bushby R. J. (2006) Redox enzymes in tethered membranes. J. Am. Chem. Soc. 128, 1711–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim M. S., Kim Y. J. (2004) Enzymatic properties of the membrane-bound NADH oxidase system in the aerobic respiratory chain of Bacillus cereus. J. Biochem. Mol. Biol. 37, 753–756 [DOI] [PubMed] [Google Scholar]
- 30. Tokuda H., Unemoto T. (1984) Na+ is translocated at NADH:quinone oxidoreductase segment in the respiratory chain of Vibrio alginolyticus. J. Biol. Chem. 259, 7785–7790 [PubMed] [Google Scholar]
- 31. Matsushita K., Ohnishi T., Kaback H. R. (1987) NADH-ubiquinone oxidoreductases of the Escherichia coli aerobic respiratory chain. Biochemistry 26, 7732–7737 [DOI] [PubMed] [Google Scholar]
- 32. Duarte M., Peters M., Schulte U., Videira A. (2003) The internal alternative NADH dehydrogenase of Neurospora crassa mitochondria. Biochem. J. 371, 1005–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kao M. C., Di Bernardo S., Nakamaru-Ogiso E., Miyoshi H., Matsuno-Yagi A., Yagi T. (2005) Characterization of the membrane domain subunit NuoJ (ND6) of the NADH-quinone oxidoreductase from Escherichia coli by chromosomal DNA manipulation. Biochemistry 44, 3562–3571 [DOI] [PubMed] [Google Scholar]
- 34. Polster B. M. (2013) AIF, reactive oxygen species, and neurodegeneration: a “complex” problem. Neurochem. Int. 62, 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wissing S., Ludovico P., Herker E., Büttner S., Engelhardt S. M., Decker T., Link A., Proksch A., Rodrigues F., Corte-Real M., Fröhlich K. U., Manns J., Candé C., Sigrist S. J., Kroemer G., Madeo F. (2004) An AIF orthologue regulates apoptosis in yeast. J. Cell Biol. 166, 969–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Joza N., Galindo K., Pospisilik J. A., Benit P., Rangachari M., Kanitz E. E., Nakashima Y., Neely G. G., Rustin P., Abrams J. M., Kroemer G., Penninger J. M. (2008) The molecular archaeology of a mitochondrial death effector: AIF in Drosophila. Cell Death Differ. 15, 1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang X., Yang C., Chai J., Shi Y., Xue D. (2002) Mechanisms of AIF-mediated apoptotic DNA degradation in Caenorhabditis elegans. Science 298, 1587–1592 [DOI] [PubMed] [Google Scholar]
- 38. Ye H., Cande C., Stephanou N. C., Jiang S., Gurbuxani S., Larochette N., Daugas E., Garrido C., Kroemer G., Wu H. (2002) DNA binding is required for the apoptogenic action of apoptosis inducing factor. Nat. Struct. Biol. 9, 680–684 [DOI] [PubMed] [Google Scholar]
- 39. Luttik M. A., Overkamp K. M., Kötter P., de Vries S., van Dijken J. P., Pronk J. T. (1998) The Saccharomyces cerevisiae NDE1 and NDE2 genes encode separate mitochondrial NADH dehydrogenases catalyzing the oxidation of cytosolic NADH. J. Biol. Chem. 273, 24529–24534 [DOI] [PubMed] [Google Scholar]
- 40. Verner Z., Skodová I., Poláková S., Durišová-Benkovičová V., Horváth A., Lukeš J. (2013) Alternative NADH dehydrogenase (NDH2): intermembrane-space-facing counterpart of mitochondrial complex I in the procyclic Trypanosoma brucei. Parasitology 140, 328–337 [DOI] [PubMed] [Google Scholar]
- 41. Apostolova N., Cervera A. M., Victor V. M., Cadenas S., Sanjuan-Pla A., Alvarez-Barrientos A., Esplugues J. V., McCreath K. J. (2006) Loss of apoptosis-inducing factor leads to an increase in reactive oxygen species, and an impairment of respiration that can be reversed by antioxidants. Cell Death Differ. 13, 354–357 [DOI] [PubMed] [Google Scholar]
- 42. Sled V. D., Rudnitzky N. I., Hatefi Y., Ohnishi T. (1994) Thermodynamic analysis of flavin in mitochondrial NADH:ubiquinone oxidoreductase (complex I). Biochemistry 33, 10069–10075 [DOI] [PubMed] [Google Scholar]
- 43. Garofano A., Eschemann A., Brandt U., Kerscher S. (2006) Substrate-inducible versions of internal alternative NADH: ubiquinone oxidoreductase from Yarrowia lipolytica. Yeast 23, 1129–1136 [DOI] [PubMed] [Google Scholar]
- 44. Maté M. J., Ortiz-Lombardía M., Boitel B., Haouz A., Tello D., Susin S. A., Penninger J., Kroemer G., Alzari P. M. (2002) The crystal structure of the mouse apoptosis-inducing factor AIF. Nat. Struct. Biol. 9, 442–446 [DOI] [PubMed] [Google Scholar]
- 45. Iwata M., Lee Y., Yamashita T., Yagi T., Iwata S., Cameron A. D., Maher M. J. (2012) The structure of the yeast NADH dehydrogenase (Ndi1) reveals overlapping binding sites for water- and lipid-soluble substrates. Proc. Natl. Acad. Sci. U.S.A. 109, 15247–15252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Feng Y., Li W., Li J., Wang J., Ge J., Xu D., Liu Y., Wu K., Zeng Q., Wu J. W., Tian C., Zhou B., Yang M. (2012) Structural insight into the type-II mitochondrial NADH dehydrogenases. Nature 491, 478–482 [DOI] [PubMed] [Google Scholar]
- 47. Heikal A., Nakatani Y., Dunn E., Weimar M. R., Day C. L., Baker E. N., Lott J. S., Sazanov L. A., Cook G. M. (2014) Structure of the bacterial type II NADH dehydrogenase: a monotopic membrane protein with an essential role in energy generation. Mol. Microbiol. 91, 950–964 [DOI] [PubMed] [Google Scholar]
- 48. Nadanaciva S., Bernal A., Aggeler R., Capaldi R., Will Y. (2007) Target identification of drug induced mitochondrial toxicity using immunocapture based OXPHOS activity assays. Toxicol In Vitro 21, 902–911 [DOI] [PubMed] [Google Scholar]
- 49. Marshall L. E., Himes R. H. (1978) Rotenone inhibition of tubulin self-assembly. Biochim. Biophys. Acta 543, 590–594 [DOI] [PubMed] [Google Scholar]
- 50. Negri A., Massey V., Williams C. H., Jr. (1987) d-Aspartate oxidase from beef kidney. Purification and properties. J. Biol. Chem. 262, 10026–10034 [PubMed] [Google Scholar]
- 51. Mayhew S. G., Whitfield C. D., Ghisla S., Schuman-Jörns M. (1974) Identification and properties of new flavins in electron-transferring flavoprotein from Peptostreptococcus elsdenii and pig-liver glycolate oxidase. Eur. J. Biochem. 44, 579–591 [DOI] [PubMed] [Google Scholar]
- 52. Varecha M., Amrichová J., Zimmermann M., Ulman V., Lukásová E., Kozubek M. (2007) Bioinformatic and image analyses of the cellular localization of the apoptotic proteins endonuclease G, AIF, and AMID during apoptosis in human cells. Apoptosis 12, 1155–1171 [DOI] [PubMed] [Google Scholar]
- 53. Varecha M., Zimmermann M., Amrichová J., Ulman V., Matula P., Kozubek M. (2009) Prediction of localization and interactions of apoptotic proteins. J. Biomed Sci. 16, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bilyy R., Kit Y., Hellman U., Stoika R. (2008) AMID: new insights on its intracellular localization and expression at apoptosis. Apoptosis 13, 729–732 [DOI] [PubMed] [Google Scholar]
- 55. Li W., Sun L., Liang Q., Wang J., Mo W., Zhou B. (2006) Yeast AMID homolog Ndi1p displays respiration-restricted apoptotic activity and is involved in chronological aging. Mol. Biol. Cell 17, 1802–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]




