Background: Caspase activation triggers apoptotic cell death.
Results: Chelerythrine triggers necrotic-like cell death by an early and pronounced activation of caspases.
Conclusion: The rate and level of caspase activation dictate whether cells die displaying apoptotic or necrotic features.
Significance: Most antitumor drugs prompt concomitant apoptotic and necrotic morphological deaths; therefore understanding how the apoptosis-necrosis continuum occurs should shed light on anticancer treatment efficacy.
Keywords: apoptosis, caspase, cell death, necrosis (necrotic death), oxidative stress, apoptotic nuclear morphology, chelerythrine, thiolic oxidative stress
Abstract
Apoptosis is triggered by the activation of caspases and characterized by chromatin condensation and nuclear fragmentation (type II nuclear morphology). Necrosis is depicted by a gain in cell volume (oncosis), swelling of organelles, plasma membrane leakage, and subsequent loss of intracellular contents. Although considered as different cell death entities, there is an overlap between apoptosis and necrosis. In this sense, mounting evidence suggests that both processes can be morphological expressions of a common biochemical network known as “apoptosis-necrosis continuum.” To gain insight into the events driving the apoptosis-necrosis continuum, apoptotically proficient cells were screened facing several apoptotic inducers for the absence of type II apoptotic nuclear morphologies. Chelerythrine was selected for further studies based on its cytotoxicity and the lack of apoptotic nuclear alterations. Chelerythrine triggered an early plasma membrane leakage without condensed chromatin aggregates. Ultrastructural analysis revealed that chelerythrine-mediated cytotoxicity was compatible with a necrotic-like type of cell death. Biochemically, chelerythrine induced the activation of caspases. Moreover, the inhibition of caspases prevented chelerythrine-triggered necrotic-like cell death. Compared with staurosporine, chelerythrine induced stronger caspase activation detectable at earlier times. After using a battery of chemicals, we found that high concentrations of thiolic antioxidants fully prevented chelerythrine-driven caspase activation and necrotic-like cell death. Lower amounts of thiolic antioxidants partially prevented chelerythrine-mediated cytotoxicity and allowed cells to display type II apoptotic nuclear morphology correlating with a delay in caspase-3 activation. Altogether, these data support that an early and pronounced activation of caspases can drive cells to undergo a form of necrotic-like regulated cell death.
Introduction
Old, damaged, or superfluous cells from multicellular organisms undergo regulated cell death to preserve the correct homeostasis. Alterations in the intracellular lethal pathways can lead cells toward proliferative diseases, such as cancer. Accordingly, the identification and classification of the existing subroutines of cell death have drawn a great amount of attention (1). Research in the field has focused on apoptosis and necrosis and the identification of their biochemical and morphological differences. Apoptotic dying cells undergo cellular shrinkage and subsequent fragmentation into apoptotic bodies surrounded by plasma membrane, reducing the occurrence of proinflammatory responses (2, 3). Nuclear apoptotic changes involve a partial chromatin condensation around the nuclear envelope (type I apoptotic nuclear morphology) and/or fragmentation of the nuclei into highly packaged round masses of condensed chromatin (type II apoptotic nuclear morphology) with this last stage one of the most distinctive morphological characteristics of apoptosis (4, 5). One of the most studied biochemical hallmarks of apoptosis is the precise activation in both space and time of a family of cysteine proteases called caspases. Caspases are subdivided into initiator or instigator (caspase-8, -9, and -10) and effector or executioner (caspase-3, -6, and -7) proteases (6). Upon activation by initiator caspases, effector caspases will dismantle in an orderly manner the injured cell by specifically cleaving a series of intracellular substrates (7). Conversely, necrotic cells display cellular swelling that culminates with the plasma membrane rupture, allowing the release of the intracellular content and the subsequent inflammatory responses (8). The nuclear alterations observed in necrotic cell death are depicted by the absence of chromatin condensation or in some cases the presence of passive DNA clumps (5, 9, 10).
Despite apoptosis and necrosis being considered different cell death entities, mounting evidence suggests that both can be morphological expressions of a common biochemical network termed “apoptosis-necrosis continuum” (11). Genetic and/or pharmacological manipulations of the intracellular events leading to apoptosis, such as chemical inhibition of caspases (12) or redox system alterations through depletion of intracellular glutathione by buthionine sulfoximine or co-incubation with pro-oxidant compounds, such as hydrogen peroxide (H2O2) (13–15), can shift the type of cell death to necrotic-like. Nonetheless, the possibility of changing the phenotype of a dying cell from necrotic to caspase-dependent apoptotic remains poorly understood.
Here, we describe that chelerythrine, a PKC inhibitor and BH36-mimetic compound, triggers necrotic-like cell death in human-derived neuroblastoma cells. This cytotoxicity relies on a reactive oxygen species (ROS)-elicited early overactivation of caspases and is prevented by high concentrations of thiolic antioxidants or pan-caspase inhibitors. Alternatively, moderate concentrations of thiolic antioxidants can switch death to apoptotic-like, showing type II chromatin condensation. To our best knowledge, this is the first time reporting that an early and pronounced activation of caspases drives a cell death that phenotypically resembles necrosis.
Materials and Methods
Reagents
All chemicals were obtained from Sigma unless otherwise indicated. Thapsigargin, nocodazole, rotenone, and colchicine were purchased from Merck KGaA. The inhibitors of caspases Z-VAD(OMe)-fmk and q-VD-OPh were obtained from MP Biomedicals Europe (Illkirch, France). BH3 mimetics and PKC inhibitors were purchased from Selleckchem (Munich, Germany). The caspase substrate Ac-DEVD-afc was obtained from Calbiochem. Antibodies against caspase-3 (9662; 1:2000) and caspase-7 (9492; 1:2000) were purchased from Cell Signaling Technology (Beverly, MA). Antibody against α-fodrin (clone AA6) (MAB1622; 1:40,000) was obtained from Millipore Iberica S.A.U. (Madrid, Spain). Antibodies against caspase-9 (clone 5B4) (M054-3; 1:5000) and caspase-6 (clone 3E8) (M070-3; 1:2,000) were from MBL International Corp. (Naka-ku Nagoya, Japan). Anti-caspase-2 (clone 11B4) (ALX-804-356; 1:5000) was purchased from Enzo Life Sciences (Lausen, Switzerland). Anti-lamin A/C (clone JOL2) (Ab40567; 1:2000) was from Abcam (Cambridge, UK). Anti-p23 antibody (clone JJ3) (NB300-576; 1:10,000) was obtained from Novus Biological Europe, Inc. (Cambridge, UK). Horseradish peroxidase-conjugated secondary antibodies against mouse IgG (A9044; 1:10,000), rabbit IgG (A0545; 1:20,000), and rat IgG (A9037; 1:20,000) were purchased from Sigma.
Cell Lines and Culture Procedures
Human neuroblastoma-derived SH-SY5Y, SK-N-AS, SK-N-SH, IMR-5, IMR-32, LAN-1, SK-N-BE(2), and SK-N-JD cell lines were routinely grown in 100-mm culture dishes (BD Falcon, Madrid, Spain) containing 10 ml of Dulbecco's modified Eagle's medium (DMEM) supplemented with penicillin/streptomycin (100 units/ml and 100 μg/ml, respectively) and 10% heat-inactivated fetal bovine serum (Invitrogen S.A.). Medium was routinely changed every 3 days. Cells were maintained at 37 °C in a saturating humidity atmosphere containing 95% air and 5% CO2. For the different experiments, cells were grown at the adequate cell densities in culture dishes or multiwell plates (BD Falcon) using the same culture conditions as described above.
Cell Treatments
The following cytotoxic drugs were used: staurosporine (1 μm), rotenone (100 μm), chelerythrine (40 μm for experiments in Fig. 1 and 10 μm for the rest), camptothecin (20 μm), etoposide (100 μm), oxaliplatin (50 μm), colchicine (10 μg/ml), nocodazole (50 μm), thapsigargin (50 μm), Ac-LLnL-CHO (MG101; 50 μm), Ac-LLM-CHO (50 μm), Z-FL-COCHO (50 μm), MG132 (Ac-LLL-CHO; 25 μm), Z-LLF-CHO (20 μm), and Z-LLnV-CHO (MG115; 20 μm). The following chemical compounds were used in the presence or not of 10 μm chelerythrine: q-VD-OPh (20 μm), pepstatin A (100 μm), tosyl-l-phenylalanyl chloromethane (10 μm), 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (150 μm), Z-LLF-CHO (20 μm), Z-LLnV-CHO (20 μm), Z-VAD(OMe)-fmk (50 μm), Z-FA-fmk (100 μm), leupeptin (100 μm), Z-FL-COCHO (20 μm), Ac-LLnL (20 μm), Ac-LLM-CHO (20 μm), dithiothreitol (DTT; 2 mm), methylated glutathione (Me-GSH; 5 mm), N-acetylcysteine (NAC; 5 mm), ascorbate (100 μm), α-tocopherol (1 mm), and Trolox (100 μm). Each one of these inhibitors was individually added to the culture medium 1 h before chelerythrine treatment.
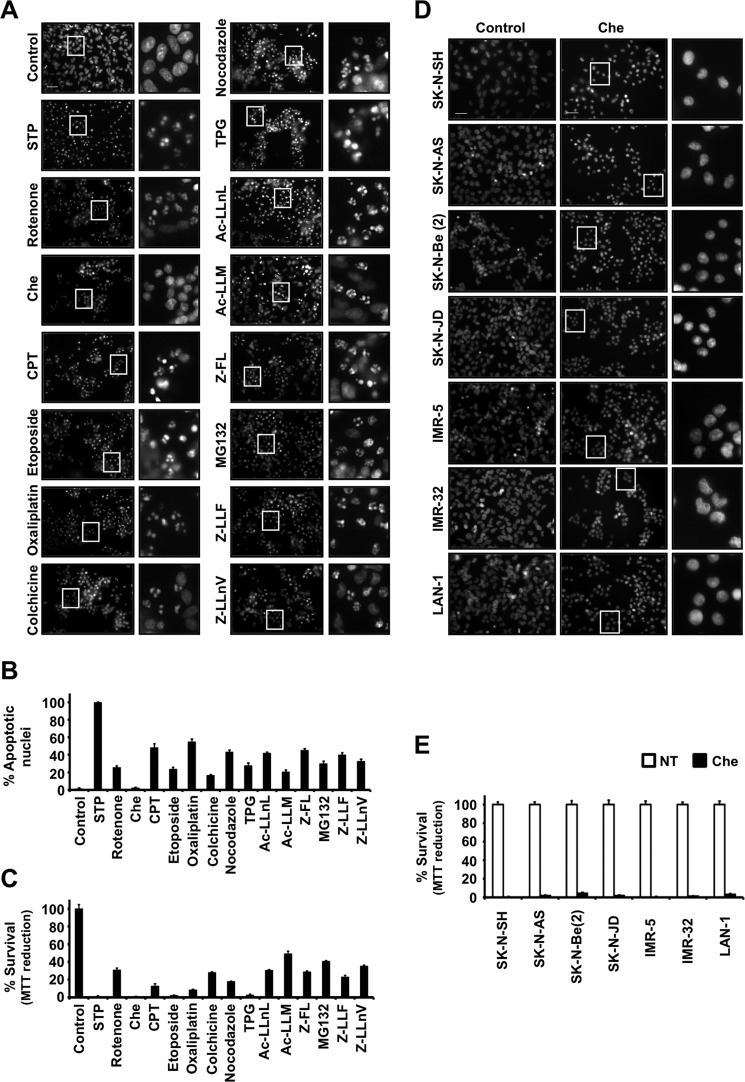
FIGURE 1.
Absence of nuclear alterations in chelerythrine-treated human neuroblastoma cell lines undergoing cell death. A, B, and C, SH-SY5Y cells were treated for 24 h as described under “Cell Treatments” or left untreated (Control). A, nuclear morphology was analyzed after staining nuclei with Hoechst 33258. The insets are higher magnifications of the cells framed in the images. The scale bar indicates 50 μm. B, the number of apoptotic nuclei was scored as described under “Materials and Methods.” The values are expressed as mean ± S.E. (n = 3). C, cell viability was determined by MTT assay. Data represent mean ± S.E. (n = 3). D and E, different human neuroblastoma-derived cell lines (SK-N-SH, SK-N-AS, SK-N-BE(2), SK-N-JD, IMR-5, IMR32, and LAN-1) were treated for 24 h with 40 μm chelerythrine (Che) or left untreated (Control). D, nuclear morphology was assessed after staining the nuclei with Hoechst 33258. The insets are higher magnifications of the cells framed in the images. The scale bar equals 50 μm. E, cell viability was analyzed by MTT assay. Data shown are the mean ± S.E. (n = 3). Error bars represent S.E. STP, staurosporine; TPG, thapsigargin; CPT, camptothecin; NT, not treated.
Cell Viability Assays: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Reduction, Lactate Dehydrogenase Release, and Double Direct Staining of Nuclei with Propidium Iodide (PI) and Cell-permeable Bisbenzimide Hoechst 33342
3.5 × 105 cells/ml were seeded in 96-multiwell plates to carry out different viability assays. The MTT survival assay was performed as established previously (16). The lactate dehydrogenase release assay was carried out by its enzymatic detection in the extracellular medium as described (17). Cell death was also determined by PI/Hoechst double staining (18). PI and cell-permeable Hoechst 33342 were added to the culture medium at a final concentration of 1 and 2 μg/ml, respectively. After 10 min of cold incubation, fluorescence images were taken, and the percentage of cell death was determined by counting PI-positive cells over the total nuclei stained with Hoechst 33342. Images were obtained with a Nikon ECLIPSE TE2000-E microscope equipped with epifluorescence optics under UV illumination and a Hamamatsu ORCA-ER photographic camera.
Nuclear Morphology Analysis by Chromatin Staining with a Non-cell-permeable Bisbenzimide (Hoechst 33258)
3.5 × 105 cells/ml were seeded in 96-multiwell plates, and we proceeded as established previously (19). The images were acquired through the Nikon ECLIPSE TE2000-E microscope as mentioned above. The percentages of apoptotic nuclei were obtained by counting bisbenzimide-stained nuclei with condensed chromatin and/or fragmented nuclei versus total bisbenzimide-stained nuclei.
Morphology Analysis by Transmission Electron Microscopy
5 × 105 cells/ml were seeded in 60-mm culture dishes. After treatment, cells were detached, pelleted at 500 × g for 5 min, and washed gently with PBS. Transmission electron microscopy was performed as reported previously (17).
Protein Extractions and Western Blotting
Cells were detached from 35-mm culture dishes, pelleted at 500 × g for 5 min, and washed once with PBS. Cells were lysed in 10 volumes of 10 mm Tris-HCl, pH 6.8, 150 mm NaCl, 1 mm EDTA, 1% sodium dodecyl sulfate (SDS) extraction buffer and heated at 95 °C until a non-viscous transparent extract was obtained. The protein concentration in the supernatants was quantified by a modified Lowry assay (DC protein assay, Bio-Rad), and 20–50 μg of total protein extracts were loaded in SDS-polyacrylamide gels. The proteins were electrophoresed and electrotransferred onto polyvinylidene difluoride (PVDF) Immobilon-P membrane (Millipore Ibérica S.A.U) or Protran nitrocellulose transfer membrane (Whatman GmbH). After blocking with Tris-buffered saline (TBS) with 0.1% Tween 20 and 5% nonfat dry milk, the membranes were probed with the appropriate specific primary antibodies and incubated with the adequate secondary antibodies conjugated with horseradish peroxidase. Finally, immunoblots were developed with the EZ-ECL chemiluminescence detection kit (Biological Industries, Kibbutz Beit-Haemek, Israel). After blotting with the specific antibodies, the membranes were stained for 5 min in a solution containing 10% methanol, 2% acetic acid, and 0.1% naphthol blue and destained in a 10% methanol and 2% acetic acid solution for 10 min. Dry membranes were scanned and used as a loading control.
DEVD-directed Caspase Activity
Caspase activity was measured after incubating 20 μg of protein with the fluorogenic substrate of caspases, Ac-DEVD-afc, for 12 h at 35 °C in 96-multiwell microplates (17). Fluorescence intensity was obtained by using a BIO-TEK Synergy HT fluorometer under an excitation filter of 360 nm (40-nm bandwidth) and an emission filter of 530 nm (25-nm bandwidth) and expressed as arbitrary units of fluorescence.
Annexin V/PI Double Staining Assay
SH-SY5Y cells were seeded into 12-multiwell plates the day before in DMEM supplemented with 20% FBS and antibiotics. Then cells were treated with hydrogen peroxide (1 mm), chelerythrine (10 μm), or staurosporine (1 μm) for 1, 3, 6, and 24 h or left untreated. Cells were detached by using ⅓ diluted trypsin/EDTA (0.025% trypsin and 0.01% EDTA; Life Technologies), pelleted at 500 × g for 2 min, and washed once with binding buffer (10 mm HEPES, 140 mm NaCl, 4 mm KCl, 0.75 mm MgCl2, 1.8 mm CaCl2). Pellets were resuspended by vortexing in 100 μl of binding buffer containing 0.5 μg/ml PI (Sigma) and 5 μl of annexin V-allophycocyanin (obtained from Annexin V Apoptosis Detection kit APC, 88-8007-72, eBioscience). After 15 min of incubation at room temperature, 300 μl of binding buffer were added to each tube, and a minimum of 5,000 events were analyzed by flow cytometry using a BD FACSCanto flow cytometer. PI and annexin V staining were detected under a 670LP or 660/20BP filter, respectively. Data were acquired and analyzed with FlowJo software.
Results
Chelerythrine Is a Potent Cytotoxic Compound That Induces a Synchronic Necrotic-like Cell Death
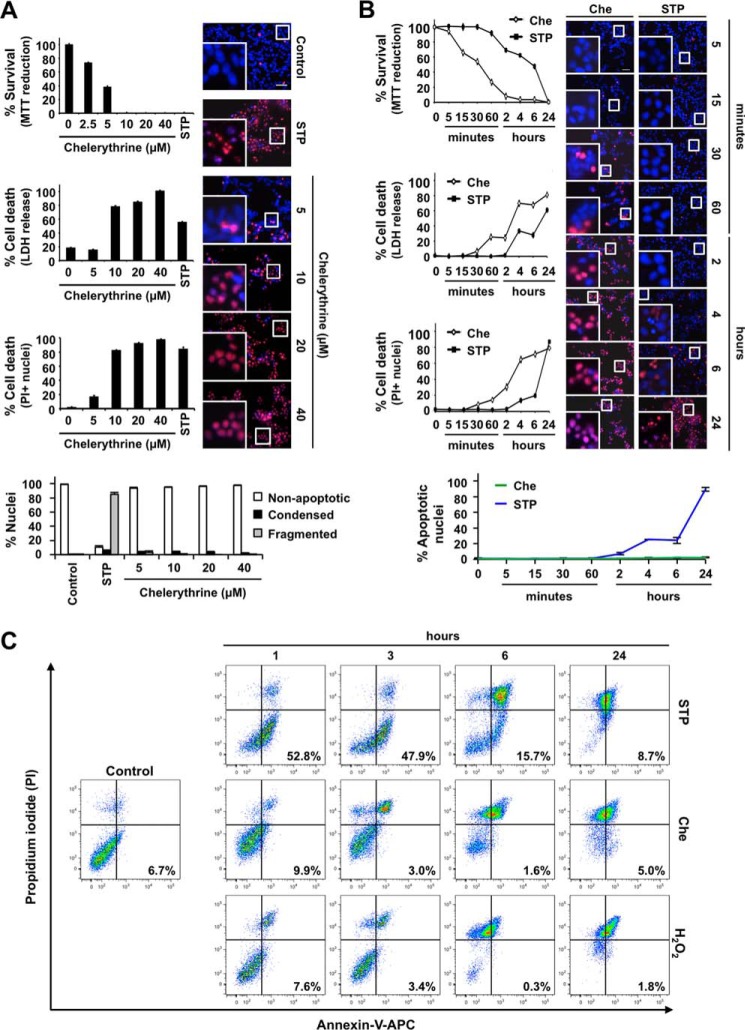
We have previously focused on the molecular and biochemical mechanisms of non-canonical apoptotic cell death in human neuroblastoma-derived cell lines in response to different apoptotic insults. Indeed, we have described that human neuroblastoma-derived IMR-5 and SK-N-AS cells do not show complete apoptosis during caspase-dependent cell death (20–22). In contrast, human neuroblastoma-derived SH-SY5Y cells subjected to apoptotic triggers display nuclear alterations of canonical apoptotic cell death (type II nuclear morphology) (16, 21). According to this behavior, we selected SH-SY5Y cells to screen for cytotoxic drugs triggering the appearance of a homogenous population of non-apoptotic nuclei. We first evaluated the apoptotic behavior of SH-SY5Y cells facing a broad battery of different cytotoxic insults. Among them, we used staurosporine (broad kinase inhibitor); rotenone (inhibitor of mitochondrial electron transport chain complex I); chelerythrine (protein kinase C inhibitor and BH3 mimetic); camptothecin and etoposide (topoisomerase I and II inhibitors, respectively); oxaliplatin (DNA cross-linker agent); colchicine and nocodazole (microtubule-destabilizing compounds); thapsigargin (sarcoendoplasmic reticulum calcium transport ATPase inhibitor); Ac-LLnL (or MG101) and Ac-LLM (calpain inhibitors); Z-FL (cathepsin S inhibitor); and MG132, Z-LLF, and Z-LLnV (or MG115) (proteasome inhibitors). The percentages of cell viability obtained by MTT assay after treating cells with the different compounds were as follows: staurosporine, 0.15 ± 0.10%; rotenone, 30.77 ± 2.16%; chelerythrine, 0.08 ± 0.05%; camptothecin, 12.83 ± 2.26%; etoposide, 2.14 ± 0.48%; oxaliplatin, 8.37 ± 0.58%; colchicine, 27.90 ± 0.59%; nocodazole, 43.19 ± 2.23%; thapsigargin, 2.52 ± 1.21%; Ac-LLnL, 30.33 ± 0.96%; Ac-LLM, 49.03 ± 2.82%; Z-FL, 28.51 ± 1.36%; MG132, 40.57 ± 0.77%; Z-LLF, 22.98 ± 1.64%; and Z-LLnV, 35.03 ± 1.26% (Fig. 1C). Most of the compounds induced the appearance of both apoptotic and non-apoptotic nuclei. The percentage of apoptotic nuclear morphologies varied depending on the specific treatment as follows: staurosporine, 99.72 ± 0.20%; rotenone, 25.63 ± 1.97; chelerythrine, 2.35 ± 0.49%; camptothecin, 48.20 ± 4.69%; etoposide, 23.42 ± 2.40%; oxaliplatin, 54.83 ± 3.14%; colchicine, 16.43 ± 1.12%; nocodazole 43.19 ± 2.23%; thapsigargin, 27.59 ± 3.40%; Ac-LLnL, 41.92 ± 1.31%; Ac-LLM, 20.42 ± 2.35; Z-FL, 45.13 ± 1.97%; MG132, 29.83 ± 3.02; Z-LLF, 39.98 ± 2.58; and Z-LLnV, 32.49 ± 2.67% (Fig. 1, A and B). According to these results, staurosporine was the most effective compound at inducing apoptotic nuclear morphology (Fig. 1, A and B). Others, such as chelerythrine, camptothecin, etoposide, oxaliplatin, and thapsigargin, induced lower percentages of apoptotic nuclei despite the high cytotoxicity provoked (Fig. 1C). Among them, we selected chelerythrine for further studies because it exhibited high cytotoxicity without inducing the appearance of apoptotic nuclei (Fig. 1, A and B). The nuclei from chelerythrine-treated SH-SY5Y cells shrank without signs of apoptotic nuclear changes (chromatin condensation and/or nuclear fragmentation) (Fig. 1A). Most of the drugs used triggered a heterogeneous cell death except for staurosporine and chelerythrine, which induced a prominent cytotoxicity characterized by the presence of homogeneous populations of apoptotic and non-apoptotic nuclear morphologies, respectively. The same behavior was observed in different human neuroblastoma-derived cell lines. Although 40 μm chelerythrine induced loss of cell viability (SK-N-SH, 0.32 ± 0.10%; SK-N-AS, 1.93 ± 0.84%; SK-N-BE(2), 4.54 ± 0.98%; SK-N-JD, 1.97 ± 1.01%; IMR-5, 0.20 ± 0.16%; IMR-32, 1.34 ± 0.69%; and LAN-1, 3.30 ± 0.96%) (Fig. 1E), the alkaloid was unable to induce apoptotic nuclear changes in any of the cell lines used (Fig. 1D). This cytotoxic response was not shared by other BH3-mimetic compounds, such as ABT-737 (50 μm), gossypol (25 μm), TW-37 (50 μm), and obatoclax (5 μm), or PKC inhibitors, such as calphostin C (100 μm), sotrastaurin (50 μm), Go 6983 (25 μm), Go 6976 (100 μm), and GF109203X (12.5 μm), which after 24 h of treatment caused apoptotic cell death with type II nuclear morphology (data not shown). To avoid uncontrolled cell responses due to excessive concentrations of chelerythrine in the culture medium, we checked the minimal concentration inducing the maximal cytotoxicity. The presence of 10 μm chelerythrine in the culture medium was already highly cytotoxic (0.10 ± 0.05% survival by MTT assay, 78.33 ± 1.24% cytotoxicity by lactate dehydrogenase release assay, and 82.51 ± 0.69% PI-positive nuclei) because the percentages of cell death reached were comparable with those obtained after treating cells with 40 μm chelerythrine (0.08 ± 0.06% survival by MTT assay, 99.93 ± 0.99% cytotoxicity by lactate dehydrogenase release assay, and 97.86 ± 0.80% PI-positive nuclei) (Fig. 2A). Nevertheless, none of the different concentrations used induced the presence of apoptotic nuclei (Fig. 2A). According to these results and unless otherwise stated, we used 10 μm chelerythrine henceforth. We then compared the cell death profile and nuclear alterations induced by chelerythrine and staurosporine through a time course analysis. As shown in Fig. 2B, chelerythrine-induced cytotoxicity occurred at earlier times when compared with staurosporine. For example, whereas staurosporine needed 6 h to induce 50% cell viability loss (MTT assay), chelerythrine provoked the same biological effect after 30 min of treatment (Fig. 2B). In the same sense, although 30 min of chelerythrine proved to be enough to induce plasma membrane leakage (as shown by either lactate dehydrogenase release assay or PI staining), staurosporine-treated cells maintained their plasma membrane integrity up to 2 h (Fig. 2B). Annexin V/PI co-staining coupled with flow cytometry can distinguish live, early apoptotic, late apoptotic/early necrotic, and late necrotic cell populations (23, 24). As shown in Fig. 2C, chelerythrine did not raise annexin V-positive/PI-negative (early apoptotic) cells at different time points of treatment. Indeed, similarly to H2O2 but contrarily to staurosporine, chelerythrine increased the percentage of annexin V/PI-double stained (late apoptotic/early necrotic) cells from early times (between 1 and 3 h) of treatment (Fig. 2C). Finally, at longer times (6 h for chelerythrine or H2O2 and 24 h for staurosporine), injured cells partially shifted to an annexin V-negative/PI-positive (late necrosis) population irrespective of the treatment used (Fig. 2C). Therefore, chelerythrine-treated cells showed necrotic-like (annexin V-positive/PI-positive or annexin V-negative/PI-positive) features without exhibiting apoptotic-like (annexin V-positive/PI-negative) features.
FIGURE 2.
Chelerythrine provokes an early and non-apoptotic strong cytotoxicity compared with staurosporine. A, SH-SY5Y cells were treated for 24 h with the indicated concentrations of chelerythrine (Che) or 1 μm staurosporine (STP) or left untreated (0 or Control). B, SH-SY5Y cells were left untreated (0) or treated with 10 μm chelerythrine or 1 μm staurosporine at the indicated times. A and B, cell viability was analyzed by MTT reduction, lactate dehydrogenase (LDH) release, and PI staining. Results are expressed as mean ± S.E. (n = 3). Nuclear morphology was assessed by double staining of the nuclei with PI and Hoechst 33342. The insets are higher magnifications of the cells framed in the images. The scale bar equals 50 μm. Nuclei were counted and scored as apoptotic (condensed or fragmented) or non-apoptotic. Graphs represent the means ± S.E. from more than 1,000 nuclei for each condition (n = 3). C, SH-SY5Y cells were treated with 10 μm chelerythrine, 1 μm staurosporine, or 1 mm H2O2 or left untreated (Control). Double staining with annexin-V and PI was assessed through flow cytometry. More than 5,000 events were acquired in each condition. Error bars represent S.E. APC, allophycocyanin.
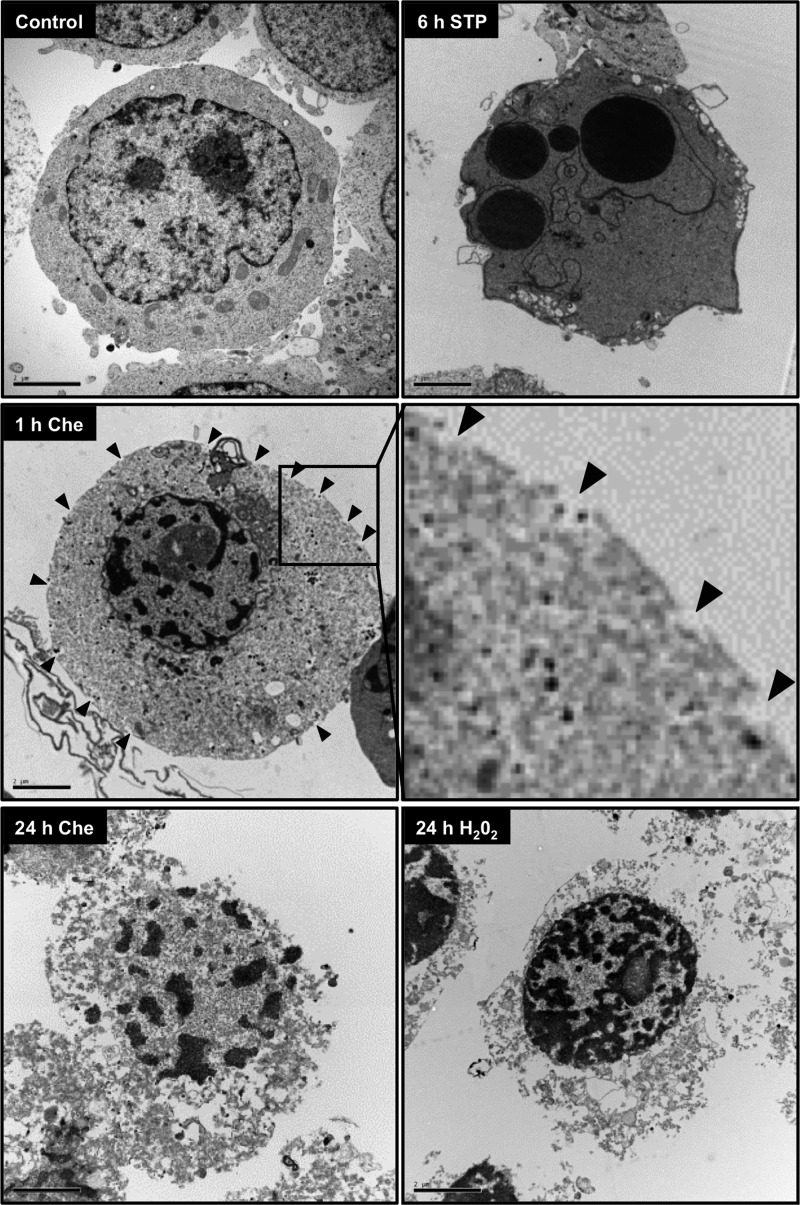
Regarding the nuclear aspect, apoptotic nuclei (condensed or fragmented) were not detected at any concentration or time of chelerythrine treatment (Fig. 2, A and B). Indeed, electron microscopy revealed a nuclear coarse pattern of irregular non-interconnected chromatin masses scattered throughout the nucleus in chelerythrine-challenged cells just 1 h after treatment (Fig. 3, middle left image). These chromatin clumps were homogeneously distributed inside the nucleus and some were located beneath the nuclear envelope, which was preserved although it had a misshapen contour (Fig. 3, middle left image). After 6 h of chelerythrine treatment, the previously observed chromatin alterations were preserved even though the nuclear envelope was barely detected (data not shown). These ultrastructural nuclear alterations were in contrast with those observed in staurosporine-challenged cells, which displayed aggregates of highly condensed chromatin distributed under the nuclear envelope as dense rounded caps (Fig. 3, upper right image). Besides these nuclear changes, cells cultured in the presence of chelerythrine showed multiple breaks at the plasma membrane, and their cytoplasm became disorganized and vacuolized (Fig. 3, middle image). These alterations were more prominent after 6 h of treatment (data not shown). Moreover, chelerythrine-treated cells displayed a cytoplasm characterized by a more electron-lucent pattern compared with untreated or staurosporine-treated cells (Fig. 3). The ultrastructural traits observed in chelerythrine-injured cells were compatible with the cellular changes described for necrotic cell death (cell swelling, early loss of plasma membrane integrity, electron-lucent and vacuolized cytoplasm, cell rupture, and irregular chromatin destruction) (25–27). We then compared the ultrastructural changes from chelerythrine-treated cells with those induced by a necrotic stimulus such as hydrogen peroxide (28). After 24 h of treatment, the cytoplasm of hydrogen peroxide-exposed cells appeared completely disintegrated, and the chromatin was slightly packaged into irregular patches (Fig. 3, lower right image). This aspect was highly similar to what we observed after 24 h of chelerythrine treatment (Fig. 3, lower left image). Therefore, the chelerythrine-induced demise occurred without signs of apoptosis but shared necrotic morphological traits.
FIGURE 3.
The ultrastructural morphology of chelerythrine-damaged cells is similar to that observed in H2O2-injured necrotic cells. Shown are electron microscopy images of SH-SY5Y cells left untreated (Control) or treated with 10 μm chelerythrine (Che), 1 μm staurosporine (STP), or 1 mm H2O2 at the indicated times. The arrowheads indicate the break sites at the plasma membrane. The inset is a higher magnification of the plasma membrane region framed in the image of chelerythrine-treated cells at 1 h. The scale bars equal 2 μm.
Necrotic-like Cell Death Induced by Chelerythrine Is a Caspase-dependent Process
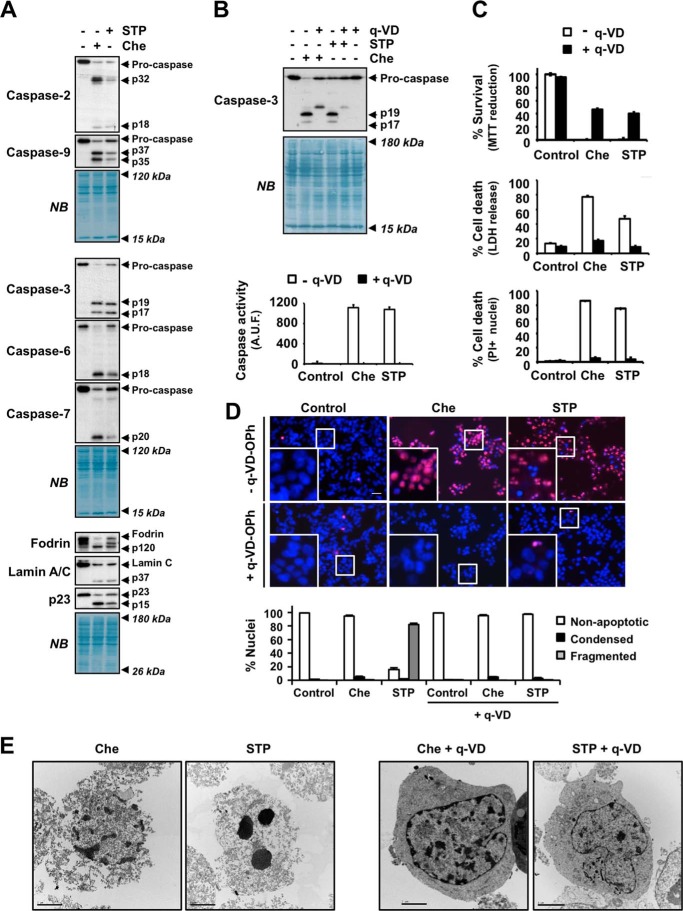
Because a defect in the activation of caspases could explain the lack of apoptotic morphological traits, we analyzed the activation of these proteases after chelerythrine challenge. After 24 h, chelerythrine induced the cleavage of initiator caspase-2 and -9 to their respective active fragments, p32/p18 and p37/p35 (Fig. 4A, first panel). We also observed the processing of the executioner caspases, i.e. caspase-3, -6, and -7, into their p19/p17, p18, and p20 active fragments, respectively (Fig. 4A, second panel). Finally, we confirmed the activation of executioner caspases by assessing the cleavage of different specific substrates. As shown in Fig. 4A, chelerythrine triggered the cleavage of α-fodrin to the p120 fragment (caspase-3-mediated) (29), lamin C into the p37 fragment (caspase-6-mediated) (30, 31), and p23 co-chaperone to the p15 fragment (caspase-7-mediated) (32). These results highlighted the similarities between staurosporine and chelerythrine regarding the activation of caspases and the ensuing cascade of proteolytic events (Fig. 4A). We then wanted to ascertain whether caspases were involved in the necrotic-like cell death induced by chelerythrine. For that, we treated cells with chelerythrine for 24 h in the presence or absence of the broad pan-caspase inhibitor q-VD-OPh. We also co-treated cells with staurosporine and q-VD-OPh as a positive control of caspase-dependent apoptosis (22). First, we corroborated the proper q-VD-OPh-mediated inhibition of caspases by Western blotting and a caspase activity assay. As shown in Fig. 4B, when q-VD-OPh was present in the culture medium, caspase-3 was not processed into its respective active fragments (p19 and p17). Accordingly, q-VD-OPh abolished DEVD-directed caspase activity triggered by chelerythrine (Fig. 4B, lower panel). Subsequently, we examined the cytotoxicity induced by chelerythrine in the presence of q-VD-OPh. By means of an MTT reduction assay, we showed that chelerythrine-mediated loss of cell viability was partially prevented by q-VD-OPh (from 0.10 ± 0.08 to 46.49 ± 1.67%). Similar results were reported when cells were co-treated with q-VD-OPh and staurosporine (from 0.34 ± 0.21 to 40.33 ± 1.93%) (Fig. 4C, upper graph). We also analyzed the integrity of the plasma membrane by the lactate dehydrogenase release assay or by counting PI-positive nuclei (Fig. 4C, middle and lower graphs). As shown in Fig. 4C, the addition of q-VD-OPh to the culture medium of chelerythrine-treated cells reduced cytotoxicity from 76.92 ± 1.82 to 17.20 ± 1.51% (by lactate dehydrogenase release assay) and from 85.83 ± 2.05 to 4.98 ± 0.73% (PI-positive nuclei counting). Taking advantage of the double PI/Hoechst nuclear staining, we found that q-VD-OPh also prevented the mild nuclear changes observed after chelerythrine challenge (Fig. 4D). Indeed, electron microscopy analysis confirmed that q-VD-OPh blocked the formation of the chromatin irregular masses observed in chelerythrine-treated cells (Fig. 4E). Additionally, chelerythrine/q-VD-OPh-co-treated cells recovered their cytoplasmic integrity. Hence, our results showed that cell death and necrotic-like alterations induced by chelerythrine required the proper activation of caspases.
FIGURE 4.
Chelerythrine-driven cell death and nuclear alterations rely on a proper activation of caspases. SH-SY5Y cells were left untreated (−) or treated for 24 h with 10 μm chelerythrine (+Che) or 1 μm staurosporine (+STP) in the presence (+) or absence (−) of 20 μm pan-caspase inhibitor q-VD-OPh. A, initiator caspases (caspase-2 and caspase-9; upper panel), effector caspases (caspase-3, caspase-6, and caspase-7; middle panel), and their specific substrates (α-fodrin, lamin C, and p23 co-chaperone; lower panel) were analyzed by Western blotting. Caspase-2 (p32 and p18), caspase-9 (p37 and p35), caspase-3 (p19 and p17), caspase-6 (p18), and caspase-7 (p20) processed fragments are indicated. The activation of caspase-3, -6, or -7 was corroborated by the detection of p120 (α-fodrin), p37 (lamin C), and p15 (p23 co-chaperone) proteolytic fragments, respectively. Naphthol blue (NB) staining served as a loading control. B, Western blot of caspase-3 and its active fragments p19 and p17 (upper panel). Naphthol blue staining served as a loading control. Caspase activity was quantified by a DEVD-directed activity assay (lower panel). Activity was measured as arbitrary units of fluorescence (A.U.F.) and expressed as mean ± S.E. (n = 3). C, cell viability was analyzed by MTT reduction, lactate dehydrogenase (LDH) release, and PI staining. The values are shown as mean ± S.E. (n = 3). D, nuclear morphology was assessed by double staining of nuclei with PI and Hoechst 33342. The insets are higher magnifications of the cells framed in the images. The scale bar equals 50 μm. Nuclei were counted and scored as apoptotic (condensed or fragmented) or non-apoptotic. Graphs represent the means ± S.E. from more than 1,000 nuclei for each condition (n = 3). E, electron microscopy images from cells subjected to the above mentioned conditions. All scale bars equal 2 μm. Error bars represent S.E.
Necrotic-like Cell Death Prompted by Chelerythrine Is Biochemically Characterized by an Early and Prominent Activation of Caspases
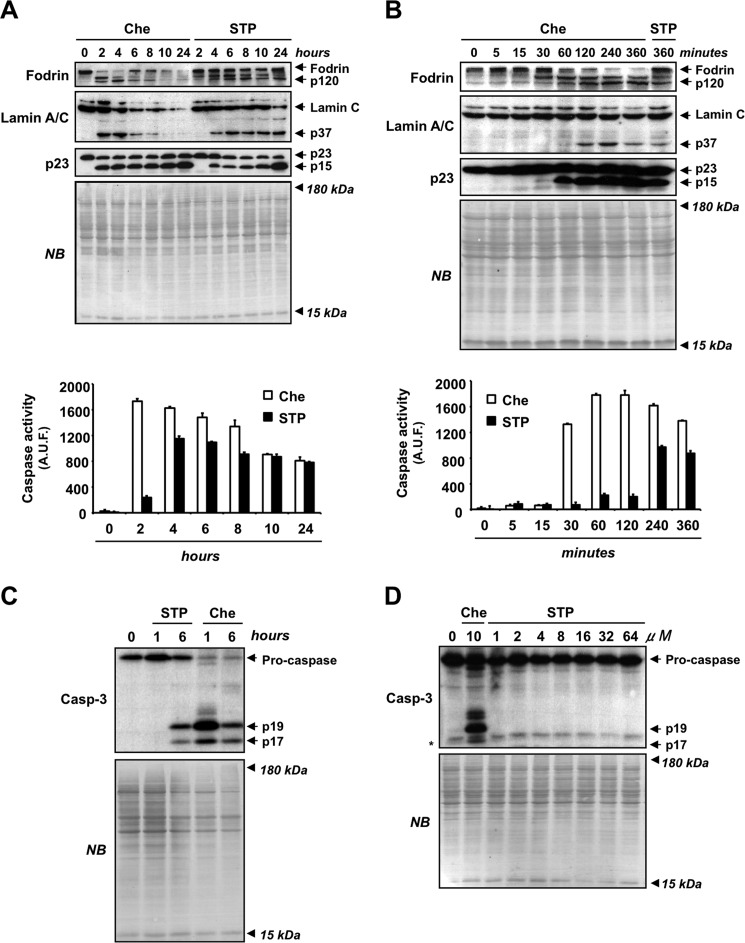
Despite that both staurosporine- and chelerythrine-driven cytotoxicity relied on caspase activation, these alkaloids triggered apoptotic and necrotic-like phenotypes, respectively. To identify dissimilarities in the activation of these enzymes, we performed a time course analysis of caspase activation in both chelerythrine- and staurosporine-treated cells. By Western blotting, we observed that both drugs induced the processing of fodrin, lamin C, and p23 co-chaperone to p120, p37, and p15 fragments, respectively (Fig. 5A). However, these fragments were already evidenced after 2 h of chelerythrine treatment, whereas they were not detected until 4 h of staurosporine treatment. Similarly, a DEVD-directed caspase-like activity assay proved that after 2 h chelerythrine-injured cells displayed 8 times higher caspase activity than staurosporine-treated cells (Fig. 5A, lower graph). We then wanted to identify the earliest time point, prior to 2 h, at which chelerythrine induced the activation of caspases. The p120 and p15 fragments from fodrin and p23 co-chaperone processing, respectively, were already detected after 30 min, being fully evident at 1 h of chelerythrine treatment (Fig. 5B). Accordingly, the enzymatic assay revealed a marked increase in caspase activation at 30 min and a maximal activity 1 h after the treatment. In contrast, staurosporine barely induced the activation of caspases for the first 2 h of treatment (Fig. 5B). When looking at caspase-3, the main executioner protease involved in the apoptotic hallmarks, we observed an early activation in chelerythrine-injured cells compared with staurosporine-treated cells. Indeed, after 1 h of chelerythrine, cells displayed a robust processing of caspase-3 into its p19/p17 active fragments that was not observed in staurosporine-injured cells (Fig. 5C). Based on these results, we explored whether higher concentrations of staurosporine could reproduce the early processing of caspase-3 driven by chelerythrine. As shown in Fig. 5D, at 1 h of treatment, staurosporine was unable to induce the processing of caspase-3 into its active p19/p17 fragments even at very high concentrations (64 μm). Therefore, our results indicated that the early activation of caspases was a biochemical peculiarity of chelerythrine not shared by staurosporine, suggesting that the early activation of caspases could be a key event orchestrating necrotic-like cell death.
FIGURE 5.
Chelerythrine induces an early and prominent activation of caspases not mimicked by high concentrations of staurosporine. A, B, and C, SH-SY5Y cells were left untreated (0) or treated with 10 μm chelerythrine (Che) or 1 μm staurosporine (STP) at the indicated times. A and B, after obtaining the protein extracts, the activation of caspases was analyzed by Western blotting (upper panels) and a DEVD-directed caspase-like activity assay (lower panels). Activation of effector caspases (caspase-3, -6, and -7) was assessed by the detection of p120 (α-fodrin), p37 (lamin C), and p15 (p23 co-chaperone) proteolytic fragments, respectively. In the DEVD-directed activity assay, data are expressed as the mean of arbitrary units of fluorescence (A.U.F.) ±S.E. (n = 3). C, after obtaining the protein extracts, the processing of caspase-3 to its active fragments (p19 and p17) was assessed through Western blotting. D, SH-SY5Y cells were left untreated (0) or treated with 10 μm chelerythrine or 1, 2, 4, 8, 16, 32, or 64 μm staurosporine for 1 h. Caspase-3 (Casp-3) and its active fragments (p19 and p17) were analyzed by Western blotting. * indicates an unspecific band. Naphthol blue (NB) staining served as a loading control in Western blots from A, B, C, and D. Error bars represent S.E.
Caspase-dependent Necrotic-like Cell Death Induced by Chelerythrine Is Prompted by Thiolic Oxidative Stress
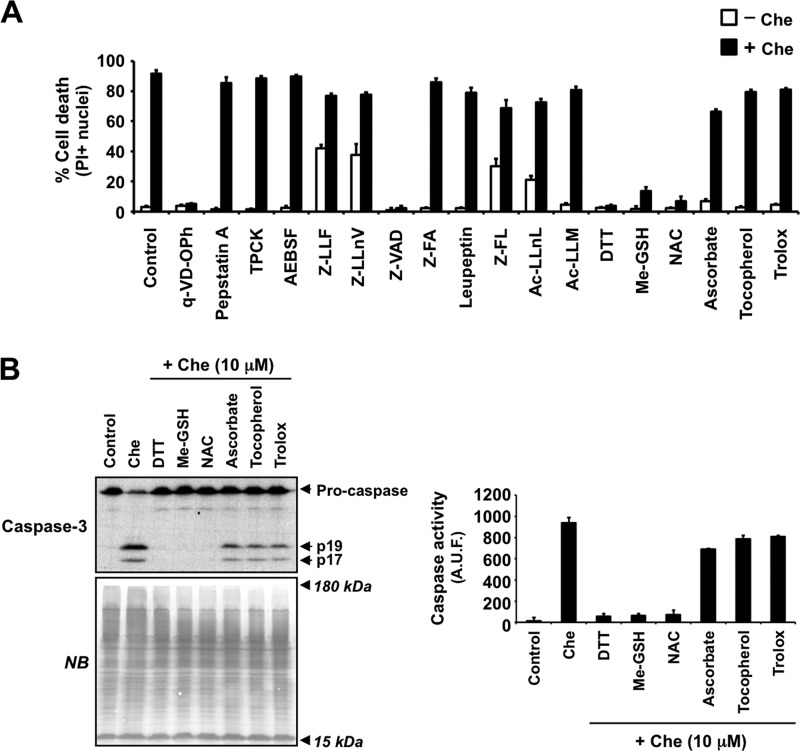
Next, we wanted to identify the upstream key event provoking the early activation of caspases triggered by chelerythrine. In addition to q-VD-OPh, we used a broad battery of inhibitors, including pepstatin A (aspartic protease inhibitor, such as cathepsin D); tosyl-l-phenylalanyl chloromethane (inhibitor of α-chymotrypsin-like and some non-caspase cysteine proteases); 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (broad hydrophilic PMSF-like serine protease inhibitor); Z-LLF and Z-LLnV (or MG115) (proteasome inhibitors); Z-VAD (broad caspase inhibitor with additional ability to interfere with calpain and cathepsin activities); Z-FA (inhibitor of cysteine proteases, such as cathepsins B, L, and S); leupeptin (inhibitor of cysteine proteases, such as calpains, papain, and cathepsins B, H, and L, and some serine proteases, such as plasmin, kallikrein, and trypsin); Z-FL (cathepsin S inhibitor); Ac-LLnL (or MG101) and Ac-LLM (calpain inhibitors); DTT, Me-GSH, and NAC (thiolic antioxidant agents); and ascorbate, α-tocopherol, and Trolox (antioxidant compounds with a major role in the prevention of lipid peroxidation). We tested their ability to prevent cytotoxicity after 24 h of chelerythrine treatment by means of PI/Hoechst double staining. Among the inhibitors tested and similarly to q-VD-OPh, Z-VAD reduced chelerythrine-triggered cytotoxicity from 91.56 ± 2.43 to 2.25 ± 0.05%. These data reasserted that chelerythrine induced a caspase-dependent cell death. Interestingly, all thiolic antioxidants used (DTT, Me-GSH, and NAC) fully prevented chelerythrine-induced cytotoxicity (from 91.56 ± 2.43 to 5.79 ± 0.88% (DTT), to 10.26 ± 2.09% (Me-GSH), and to 7.90 ± 2.80% (NAC)). In contrast, the other antioxidants tested displayed only a minor effect on chelerythrine-triggered cell death (Fig. 6A). Moreover, the nuclear morphological analysis corroborated that only caspase inhibitors and thiolic antioxidants prevented the nuclear alterations provoked by chelerythrine (data not shown). Thus, our results suggested a key role of thiol oxidative stress processes in chelerythrine-induced cytotoxicity. We then asked whether thiol oxidative stress was involved in the activation of caspases triggered by chelerythrine. For this purpose, we assessed by Western blotting the cleavage of caspase-3 in cells treated for 24 h with chelerythrine in the presence or absence of the above mentioned antioxidants. Chelerythrine-induced caspase-3 p19/p17 active fragments were not detected when thiolic antioxidants were added to the culture medium (Fig. 6B). Similarly, the DEVD-directed activity assay revealed that chelerythrine-mediated activation of caspases was inhibited by any of the above mentioned thiolic antioxidants (Fig. 6B). Altogether, our results showed that chelerythrine-mediated necrotic-like cell death required strong thiolic oxidative damage, which seemed an upstream pivotal event to activate caspases in a very short period after cytotoxic insult.
FIGURE 6.
Chelerythrine-mediated caspase-dependent cell death is regulated by thiolic antioxidants. A and B, SH-SY5Y cells were left untreated (Control) or treated for 24 h with different inhibitors in the absence (−) or presence (+) of chelerythrine (Che) as described under “Cell Treatments.” A, cell death was analyzed by PI staining. Data shown are the mean ± S.E. (n = 3). B, the processing of caspase-3 to its active fragments (p19 and p17) was examined by Western blotting (left panel). Naphthol blue (NB) staining served as a loading control. Caspase activity was assessed by a DEVD-directed activity assay (right panel). Data are represented as arbitrary units of fluorescence (A.U.F.) and expressed as mean ± S.E. (n = 3). Error bars represent S.E. TPCK, tosyl-L-phenylalanyl chloromethane; AEBSF, 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride.
Necrotic-like Cell Death Induced by Chelerythrine Can Be Shifted to Apoptosis by the Fine-tuning of the Thiol Oxidative Intracellular Status
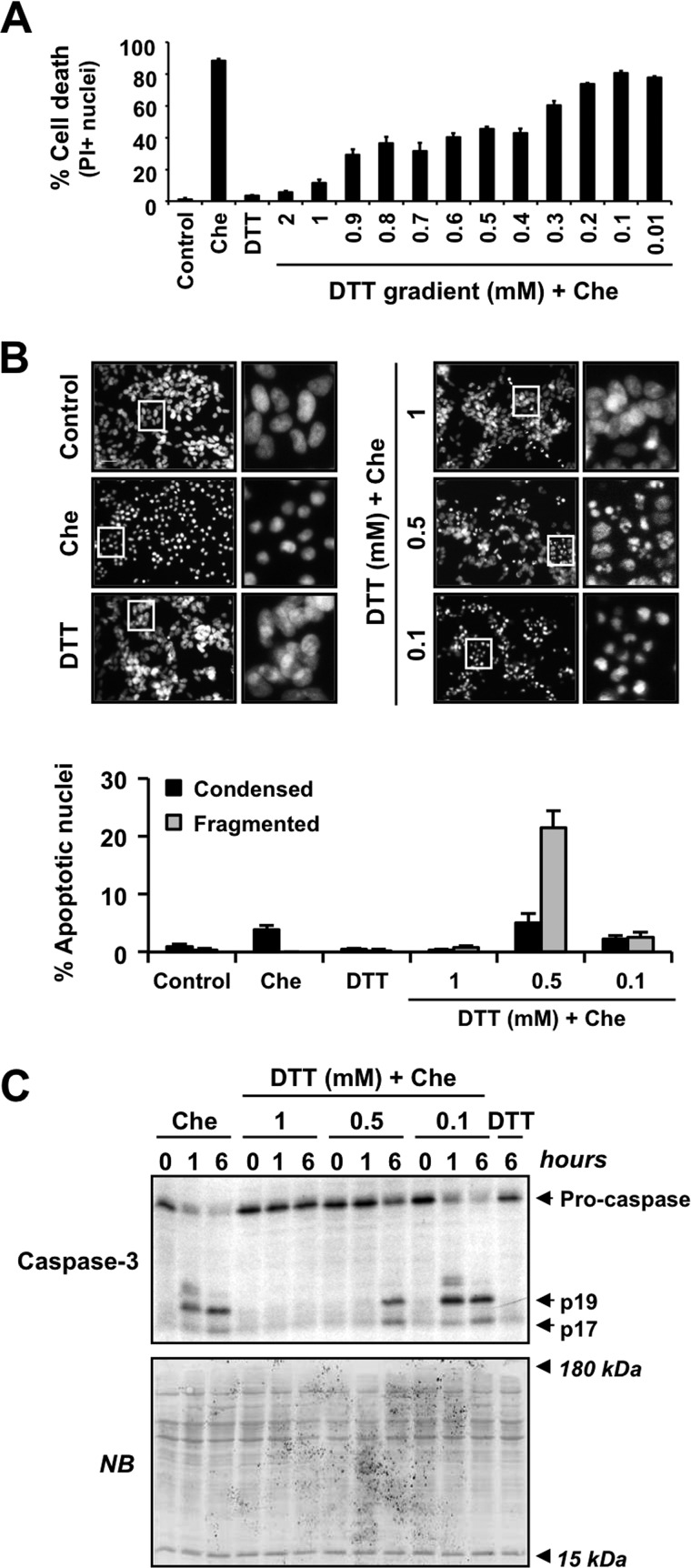
Taking into account the results obtained, we established that necrotic-like cell death induced by chelerythrine depended on the intracellular oxidative stress and subsequently on the activation of caspases. Because caspases played a key role in chelerythrine-triggered necrotic-like cytotoxicity, we wondered whether a gentle modulation of the thiol oxidative status could switch the cell death to one with apoptotic nuclear traits. We then evaluated cell viability and nuclear morphology after chelerythrine treatment in the presence of different concentrations of DTT. As shown in Fig. 7A, cells cultured in the presence of high concentrations of DTT (1 or 2 mm) were fully protected from chelerythrine-triggered cell death. Moreover, 1 mm DTT prevented the mild nuclear alterations induced by chelerythrine (Fig. 7B). At the other end, the lowest concentrations of DTT used (from 0.01 to 0.3 mm) did not alter chelerythrine-mediated cytotoxicity (Fig. 7A). Taking 0.1 mm as a representative concentration, we observed that DTT did not prevent the nuclear alterations induced by chelerythrine (Fig. 7B). When we used intermediate concentrations of DTT (from 0.4 to 0.9 mm), we observed a partial reduction of the cytotoxicity induced by chelerythrine (Fig. 7A). To analyze the nuclear morphology under these conditions, we chose 0.5 mm as the representative concentration of DTT. Interestingly, 0.5 mm DTT allowed chelerythrine-treated cells to display classical type II apoptotic nuclear morphology (Fig. 7B). As shown before in Fig. 5, caspases were processed early in chelerythrine-injured necrotic-like cells compared with staurosporine-treated apoptotic cells. To ensure that the rate of caspase activation is the mandatory biochemical event determining apoptotic- or necrotic-like outcomes, we assessed the activation of caspase-3, the key executioner caspase allowing the apoptotic hallmarks, after chelerythrine and DTT co-treatment. High concentrations of DTT (1 mm), which preserves cell viability (Fig. 7A), fully prevented caspase-3 processing (Fig. 7C). However, the presence of intermediate concentrations of DTT (0.5 mm) in the culture medium, which allowed the presence of apoptotic-like nuclei (Fig. 7B), delayed chelerythrine-triggered activation of caspase-3 (Fig. 7C). Indeed, in chelerythrine/DTT-co-treated cells, the activation of caspase-3 was not detected at 1 h but after 6 h of treatment, similarly to that observed in staurosporine-injured apoptotic cells (Figs. 5C and 7C). Altogether, our results show that chelerythrine can trigger necrotic-like or apoptotic cytotoxic fates (both caspase-dependent) driven by particular intracellular thiol oxidative stress.
FIGURE 7.
A delay in chelerythrine-triggered activation of caspase-3 prompted by intermediate concentrations of DTT allows cells to display apoptotic-like nuclear alterations. A, SH-SY5Y cells were left untreated (Control) or treated for 6 h with 2 mm DTT or 10 μm chelerythrine (Che) in the absence or presence of the indicated concentrations of DTT. Cell death was measured by PI staining. Data are represented as the mean ± S.E. (n = 3). B and C, cells were treated with 1 mm DTT, 10 μm chelerythrine, or a combination of 10 μm chelerythrine and different concentrations of DTT (1, 0.5, and 0.1 mm). B, after 24 h, nuclear morphology was assessed by nuclei staining with Hoechst. The insets are higher magnifications of the cells framed in the images. The scale bar indicates 50 μm. More than 1,000 nuclei from each condition were counted and scored as apoptotic (condensed or fragmented) or non-apoptotic nuclei. Graphs represent the means ± S.E. (n = 3). C, after the indicated times, cells were collected, and protein extracts were obtained. Caspase-3 cleavage into its p19/p17 active fragments was assessed by Western blotting. Naphthol blue (NB) staining served as a loading control. Note that in the presence of 0.5 mm DTT chelerythrine does not promote caspase-3 cleavage at 1 h but after 6 h of treatment, similar to the pattern observed in cells challenged with staurosporine (Fig. 5C). Error bars represent S.E.
Discussion
Here, we report that chelerythrine is a highly cytotoxic compound that synchronously triggers cell death in different human neuroblastoma-derived cells. SH-SY5Y cells treated with chelerythrine die without any apoptotic morphological traits by undergoing a fast cytoplasmic disintegration and loss of plasma membrane integrity. Chelerythrine-driven cell death features a suite of traits of canonical necrotic (oncotic) cell death. In this sense, ultrastructural analysis demonstrates that chelerythrine-injured cells show electron-lucent vacuolized cytoplasm, early loss of plasma membrane integrity, and irregular and circumscribed DNA patches, which are typical morphological changes described in necrotic dying cells (9, 25, 26, 33–35). Intriguingly, chelerythrine triggers a prominent and early activation of caspases, which is necessary for the necrotic-like cell death to occur. These findings prove that an early activation of caspases can lead to cell demise with morphological traits resembling those observed in classical necrotic cell death. Chelerythrine-mediated cell death is not prevented by the action of several chemical inhibitors of calpains, cathepsins, proteasome, or other non-caspase proteases. However, high concentrations of thiolic antioxidants, such as DTT, methylated glutathione, and N-acetylcysteine, block the activation of caspases and the subsequent cell death triggered by chelerythrine. These results indicate that a thiolic oxidative stress is eliciting the activation of caspases. Interestingly, cells co-cultured in the presence of chelerythrine and low concentrations of DTT display type II apoptotic nuclear morphologies, proving that the sole modulation of the thiolic oxidative stress allows the shift from chelerythrine-driven necrosis-like to apoptosis-like cell death.
The switch of apoptotic to necrotic paradigms of cell death has been profusely addressed (13–15, 36–39). In this regard, reducing the content of ATP or glutathione is a reported strategy to convert apoptotic to necrotic cell death (13, 15, 38, 39). Conversely, only a scarce number of publications have demonstrated that necrosis can be transformed into an apoptotic cell death; among them, we highlight the findings on prothymosin-α1 as a molecular switch of necrotic to apoptotic cell death (40, 41). Moreover, no reports have approached the possibility of turning a caspase-dependent necrotic-like cell death into an apoptotic-like process of cell demise. In this context, we prove that the fine-tuning of thiolic oxidative processes is a successful strategy to modulate the shape of chelerythrine-mediated cell death by promoting a proper (delayed on time) activation of caspases. Indeed, we demonstrate for the first time that modulating the intracellular thiolic oxidative environment by co-culturing cells with DTT switches chelerythrine-elicited necrotic-like cell death into an apoptotic-like process. This fact is of capital relevance because most antitumor drugs used in the clinics prompt a continuum of apoptotic and necrotic morphological deaths, thus proving the same trigger is able to induce different types of cell demise (42). Our data support the notion that the apoptotic-necrotic continuum could rely on particular environmental cues, such as the thiolic oxidative status of the cell.
Many insults have the capacity to induce apoptosis and necrosis when used at low and high concentrations, respectively (36, 37, 43). Alternatively, some chemicals trigger concomitant signs of necrotic and apoptotic cell death modalities (44). This fact was evidenced in our initial drug screening whereby a series of chemical compounds was selected according to their ability to trigger a homogenous cytotoxic phenotype. All the tested drugs, with the exception of staurosporine and chelerythrine, trigger mixed subroutines of cell death as proven by the high cytotoxicity and the dissimilar nuclear morphologies observed. Cytotoxic concentrations of chelerythrine provoke the appearance of a pure population of dead cells displaying a homogenous nuclear phenotype different from either healthy or apoptotic nuclei irrespectively of the cytotoxic concentration used. In contrast with staurosporine, chelerythrine triggers an abrupt form of cytotoxicity. It is well established that cellular necrosis can occur in a regulated way triggered by different signal transduction cascades provided that caspases remain inactive (12). At the same time, it is well known that this family of cysteine proteases represents the main molecular machinery involved in the apoptotic cell dismantling (45, 46). Although mounting data support that caspase-dependent necrotic-like processes can arise under particular circumstances (38, 39, 47–54), there is no explanation on how caspases can lead an injured cell to die by necrosis. Here, we shed light on this issue, and we report for the first time that the necrotic-like features evidenced during chelerythrine-mediated caspase-dependent cell death rely on a non-canonical activation of these proteases. Notably, our findings show that a great and prompt activation of caspases provoked by cytotoxic concentrations of chelerythrine will not lead to apoptotic cell death but on the contrary is detrimental for the appearance of canonical apoptotic features. In other words, as long as caspases are the main cytotoxic machinery switched on, the time taken by the injured cell to activate them seems critical to display either apoptotic or necrotic cell death. In SH-SY5Y cells, chelerythrine elicits an early burst of caspase activity not mimicked by extreme concentrations of staurosporine. In sum, we show that caspases can lead cells to undergo apoptotic- or necrotic-like cell death depending on the timing and strength of activation. Moreover, these findings suggest that, after receiving a caspase-activating insult, cells require a preparatory time to set up the appropriate intracellular context for the caspases to trigger an ordered and canonical apoptotic cell death. Of note, such a situation has been reported before in a more physiological context. Prior to the maternal-zygotic transition, zebrafish embryos can activate caspases but lyse almost immediately after, whereas in older embryos, death is delayed until the cells can undergo proper apoptosis (54).
Activation of caspases in response to chemical compounds is mostly the consequence of the release of cytochrome c from the mitochondrial intermembrane space to the cytoplasm and the subsequent activation of the intrinsic pathway of apoptosis via apoptosome (55). In this sense, at merely 1 h of chelerythrine treatment, cytochrome c translocates to the cytoplasm in wild type and Bax(−/−)/Bak(−/−) double knock-out mouse embryonic fibroblast cells (56). Accordingly and contrarily to H2O2 (57), chelerythrine induces cell death in Bax(−/−)/Bak(−/−) double knock-out mouse embryonic fibroblasts (Ref. 56 and data not shown). Similarly, in primary chronic lymphocytic leukemia cells, chelerythrine triggers an early release of cytochrome c after 30 min of treatment, correlating with a quick activation of caspase-3 (58). Thus, it seems that the early translocation of cytochrome c and/or other mitochondrial proapoptogenic factors could be playing a pivotal role in the early activation of caspases induced by chelerythrine. Our data also show that chelerythrine-driven caspase activation is mediated by thiolic oxidative events. Overall, it is reasonable to assume that thiolic oxidative processes are at the origin of the mitochondrial permeabilization and the subsequent early release of mitochondrial proapoptogenic factors, such as cytochrome c, to the cytoplasm. In this regard, we show that the presence of a thiolic antioxidant, such as DTT, Me-GSH, or NAC, in the culture medium prevents the activation of caspases and the necrotic-regulated cell death induced by chelerythrine. Interestingly, most of the cells treated with chelerythrine (∼95%) display a marked increase of intracellular ROS at 1 h of treatment. In contrast, only 8.2% of staurosporine-treated cells show an increase in the ROS content after 1 h.7 According to our data, the early increase of intracellular ROS elicited by chelerythrine parallels the activation of caspases. Furthermore, the mere attenuation of thiolic oxidative injury, which delays chelerythrine-driven caspase-3 activation, is sufficient to revert from the necrotic-like to apoptotic-like mode of cell demise.
Overall, our findings have uncovered an intriguing form of regulated necrosis, which relies on the activation of caspases in response to an intracellular thiolic oxidative stress. In the same line, a limited amount of work has intended to address the molecular pathways involved in different modes of caspase-dependent regulated necrosis. These studies identify several key factors for caspase-dependent necrotic-like cell death, such as the acidic pH (39) and the developmental stage of a specific cell type (52). However, none of those studies address how caspases can elicit necrotic-like cell death. Our findings establish that thiolic oxidative stress is another element to consider in caspase-dependent necrotic-like cell death and more importantly that the rate and level of caspase activity seem to be determinants to promote cell death with apoptotic-like or necrotic-like morphological traits. Notably, most of those elements, including the oxidative status, are naturally found in the tumor context, and thus they could influence the final mode of cell death elicited by a chemotherapeutic drug. In this sense, the molecular pathways leading to different subroutines of cell death are not trivial issues for anticancer drug applicability. On the contrary, they are central events determining the potential therapeutic and side effects of a drug. Malignant cells are often characterized by a lower extracellular pH (59) and a higher intrinsic oxidative stress than healthy cells (60, 61). In addition, escalated generation of ROS promotes chemotherapeutic resistance processes because it enhances multiple genetic alterations, which are usually detected in a more advanced tumoral stage (60, 61). Then, under these environmental circumstances and after an apoptotic insult, naturally apoptosis-resistant malignant cells could be especially susceptible to necrotic-like forms of cell death.
Author Contributions
M. G.-B., M. S.-O., L. M.-E., C. G.-C., S. P.-G., V. I.-G., E. C., and J. R. performed the experiments. M. G.-B. and V. J. Y. analyzed the initial data. M. S.-O. and V. J. Y. reorganized, analyzed, and interpreted the data in the final version of the manuscript. M. S.-O. and V. J. Y. coordinated, analyzed, and interpreted the data shown in Fig. 2C. M. S.-O., J. R., and V. J. Y. wrote the manuscript. V. J. Y. conceived, designed, and coordinated the study. All authors performed the critical reading of the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We are grateful to the personnel of Servei de Microscopia and Unitat de Citometria (Institut de Biotecnologia i de Biomedicina) from Universitat Autònoma de Barcelona. We also thank all other members of the laboratory for helpful criticisms.
This work was supported in part by Ministerio de Ciencia e Innovación/Fondo Europeo de Desarrollo Regional (MICINN/FEDER) Grant SAF2011-24081, Ministerio de Economía y Competitividad/FEDER Grant SAF2012-31485, and Generalitat de Catalunya Grants 2009-SGR-346 and 2014-SGR-1609. The authors declare that they have no conflicts of interest with the contents of this article.
M. Garcia-Belinchón and V. J. Yuste, unpublished results.
- BH3
- Bcl-2 homology 3
- ABT-737
- 4-[4-[[2-(4-chlorophenyl)phenyl]methyl]piperazin-1-yl]-N-[4-[[(2R)-4-(dimethylamino)-1-phenylsulfanylbutan-2-yl]amino]-3-nitrophenyl]sulfonylbenzamide
- Ac-DEVD-afc
- acetyl-Asp[OMe]-Glu[OMe]-Val-Asp[OMe]-7-amino-4-trifluoromethylcoumarin
- Ac-LLM
- N-acetyl-l-leucyl-l-leucyl-l-methioninal
- Ac-LLnL
- N-acetyl-l-leucyl-l-leucyl-l-norleucinal
- Me-GSH
- methylated glutathione
- MG132
- Z-Leu-Leu-Leu-al
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NAC
- N-acetylcysteine
- PI
- propidium iodide
- q-VD-OPh
- N-(2-quinolyl)valyl-aspartyl-(2,6-difluorophenoxy)methyl ketone
- ROS
- reactive oxygen species
- TW-37
- N-[4-[[2-(1,1-dimethylethyl)phenyl]sulfonyl]phenyl]-2,3,4-trihydroxy-5-[[2-(1-methylethyl)phenyl]methyl]benzamide
- Z-FA
- Z-Phe-Ala fluoromethyl ketone
- Z-FL
- Z-Phe-leucinal
- Z-LLF
- Z-Leu-Leu-phenylalaninal
- Z-LLnV
- Z-Leu-Leu-norvalinal
- Z-VAD(OMe)-fmk
- Z-Val-Ala-Asp[OMe]-fluoromethyl ketone
- Z
- benzyloxycarbonyl.
References
- 1. Galluzzi L., Vitale I., Abrams J. M., Alnemri E. S., Baehrecke E. H., Blagosklonny M. V., Dawson T. M., Dawson V. L., El-Deiry W. S., Fulda S., Gottlieb E., Green D. R., Hengartner M. O., Kepp O., Knight R. A., Kumar S., Lipton S. A., Lu X., Madeo F., Malorni W., Mehlen P., G., Peter M. E., Piacentini M., Rubinsztein D. C., Shi Y., Simon H. U., Vandenabeele P., White E., Yuan J., Zhivotovsky B., Melino G., Kroemer G. (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 19, 107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. I., Schild H. (2003) Apoptosis: the complex scenario for a silent cell death. Mol. Imaging Biol. 5, 2–14 [DOI] [PubMed] [Google Scholar]
- 3. Beyer C., Pisetsky D. S. (2013) Modeling nuclear molecule release during in vitro cell death. Autoimmunity 46, 298–301 [DOI] [PubMed] [Google Scholar]
- 4. Susin S. A., Daugas E., Ravagnan L., Samejima K., Zamzami N., Loeffler M., Costantini P., Ferri K. F., Irinopoulou T., M. C., Brothers G., Mak T. W., Penninger J., Earnshaw W. C., Kroemer G. (2000) Two distinct pathways leading to nuclear apoptosis. J. Exp. Med. 192, 571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. M., Tschopp J. (2003) Caspase-independent cell death in T lymphocytes. Nat. Immunol. 4, 416–423 [DOI] [PubMed] [Google Scholar]
- 6. Riedl S. J., Shi Y. (2004) Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 5, 897–907 [DOI] [PubMed] [Google Scholar]
- 7. Pop C., Salvesen G. S. (2009) Human caspases: activation, specificity, and regulation. J. Biol. Chem. 284, 21777–21781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kroemer G., Galluzzi L., Vandenabeele P., Abrams J., Alnemri E. S., Baehrecke E. H., Blagosklonny M. V., El-Deiry W. S., Golstein P., Green D. R., Hengartner M., Knight R. A., Kumar S., Lipton S. A., Malorni W., G., Peter M. E., Tschopp J., Yuan J., Piacentini M., Zhivotovsky B., Melino G.; (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 16, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vandenabeele P., Galluzzi L., Vanden Berghe T., Kroemer G. (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 11, 700–714 [DOI] [PubMed] [Google Scholar]
- 10. Vanden Berghe T., Grootjans S., Goossens V., Dondelinger Y., Krysko D. V., Takahashi N., Vandenabeele P. (2013) Determination of apoptotic and necrotic cell death in vitro and in vivo. Methods 61, 117–129 [DOI] [PubMed] [Google Scholar]
- 11. Zeiss C. J. (2003) The apoptosis-necrosis continuum: insights from genetically altered mice. Vet. Pathol. 40, 481–495 [DOI] [PubMed] [Google Scholar]
- 12. Galluzzi L., Kepp O., Krautwald S., Kroemer G., Linkermann A. (2014) Molecular mechanisms of regulated necrosis. Semin. Cell Dev. Biol. 35, 24–32 [DOI] [PubMed] [Google Scholar]
- 13. Fernandes R. S., Cotter T. G. (1994) Apoptosis or necrosis: intracellular levels of glutathione influence mode of cell death. Biochem. Pharmacol. 48, 675–681 [DOI] [PubMed] [Google Scholar]
- 14. Troyano A., C., Sancho P., de Blas E., Aller P. (2001) Effect of glutathione depletion on antitumor drug toxicity (apoptosis and necrosis) in U-937 human promonocytic cells. The role of intracellular oxidation. J. Biol. Chem. 276, 47107–47115 [DOI] [PubMed] [Google Scholar]
- 15. Sancho P., C., Yuste V. J., D., Ramos A. M., de Blas E., Susin S. A., Aller P. (2006) Regulation of apoptosis/necrosis execution in cadmium-treated human promonocytic cells under different forms of oxidative stress. Apoptosis 11, 673–686 [DOI] [PubMed] [Google Scholar]
- 16. Boix J., Llecha N., Yuste V. J., Comella J. X. (1997) Characterization of the cell death process induced by staurosporine in human neuroblastoma cell lines. Neuropharmacology 36, 811–821 [DOI] [PubMed] [Google Scholar]
- 17. Sánchez-Osuna M., Garcia-Belinchón M., Iglesias-Guimarais V., Gil-Guiñón E., Casanelles E., Yuste V. J. (2014) Caspase-activated DNase is necessary and sufficient for oligonucleosomal DNA breakdown, but not for chromatin disassembly during caspase-dependent apoptosis of LN-18 glioblastoma Cells. J. Biol. Chem. 289, 18752–18769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ribas J., Yuste V. J., X., Meijer L., Esquerda J. E., Boix J. (2008) 7-Bromoindirubin-3′-oxime uncovers a serine protease-mediated paradigm of necrotic cell death. Biochem. Pharmacol. 76, 39–52 [DOI] [PubMed] [Google Scholar]
- 19. Casanelles E., Gozzelino R., F., Iglesias-Guimarais V., M., M., C., Moubarak R. S., Comella J. X., Yuste V. J. (2013) NF-κB activation fails to protect cells to TNFα-induced apoptosis in the absence of Bcl-xL, but not Mcl-1, Bcl-2 or Bcl-w. Biochim. Biophys. Acta 1833, 1085–1095 [DOI] [PubMed] [Google Scholar]
- 20. Iglesias-Guimarais V., E., M., Casanelles E., M., Comella J. X., Yuste V. J. (2013) Chromatin collapse during caspase-dependent apoptotic cell death requires DNA fragmentation factor, 40-kDa subunit-/caspase-activated deoxyribonuclease-mediated 3′-OH single-strand DNA breaks. J. Biol. Chem. 288, 9200–9215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yuste V. J., Bayascas J. R., Llecha N., I., Boix J., Comella J. X. (2001) The absence of oligonucleosomal DNA fragmentation during apoptosis of IMR-5 neuroblastoma cells: disappearance of the caspase-activated DNase. J. Biol. Chem. 276, 22323–22331 [DOI] [PubMed] [Google Scholar]
- 22. Iglesias-Guimarais V., E., Gabernet G., M., M., Casanelles E., Comella J. X., Yuste V. J. (2012) Apoptotic DNA degradation into oligonucleosomal fragments, but not apoptotic nuclear morphology, relies on a cytosolic pool of DFF40/CAD endonuclease. J. Biol. Chem. 287, 7766–7779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peña F. J., Johannisson A., Wallgren M., Rodríguez-Martínez H. (2003) Assessment of fresh and frozen-thawed boar semen using an annexin-V assay: a new method of evaluating sperm membrane integrity. Theriogenology 60, 677–689 [DOI] [PubMed] [Google Scholar]
- 24. Moubarak R. S., Yuste V. J., Artus C., Bouharrour A., Greer P. A., Menissier-de Murcia J., Susin S. A. (2007) Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol. Cell. Biol. 27, 4844–4862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yasuhara S., Zhu Y., Matsui T., Tipirneni N., Yasuhara Y., Kaneki M., Rosenzweig A., Martyn J. A. (2003) Comparison of comet assay, electron microscopy, and flow cytometry for detection of apoptosis. J. Histochem. Cytochem. 51, 873–885 [DOI] [PubMed] [Google Scholar]
- 26. Zong W. X., Thompson C. B. (2006) Necrotic death as a cell fate. Genes Dev. 20, 1–15 [DOI] [PubMed] [Google Scholar]
- 27. Weerasinghe P., Buja L. M. (2012) Oncosis: an important non-apoptotic mode of cell death. Exp. Mol. Pathol. 93, 302–308 [DOI] [PubMed] [Google Scholar]
- 28. Saito Y., Nishio K., Ogawa Y., Kimata J., Kinumi T., Yoshida Y., Noguchi N., Niki E. (2006) Turning point in apoptosis/necrosis induced by hydrogen peroxide. Free Radic. Res. 40, 619–630 [DOI] [PubMed] [Google Scholar]
- 29. Jänicke R. U., Ng P., Sprengart M. L., Porter A. G. (1998) Caspase-3 is required for α-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J. Biol. Chem. 273, 15540–15545 [DOI] [PubMed] [Google Scholar]
- 30. Orth K., Chinnaiyan A. M., Garg M., Froelich C. J., Dixit V. M. (1996) The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate lamin A. J. Biol. Chem. 271, 16443–16446 [PubMed] [Google Scholar]
- 31. Takahashi A., Alnemri E. S., Lazebnik Y. A., Fernandes-Alnemri T., Litwack G., Moir R. D., Goldman R. D., Poirier G. G., Kaufmann S. H., Earnshaw W. C. (1996) Cleavage of lamin A by Mch2α but not CPP32: multiple interleukin 1β-converting enzyme-related proteases with distinct substrate recognition properties are active in apoptosis. Proc. Natl. Acad. Sci. U.S.A. 93, 8395–8400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walsh J. G., Cullen S. P., Sheridan C., Lüthi A. U., Gerner C., Martin S. J. (2008) Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc. Natl. Acad. Sci. U.S.A. 105, 12815–12819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Radakovic-Fijan S., Rappersberger K., Tanew A., Hönigsmann H., Ortel B. (1999) Ultrastructural changes in PAM cells after photodynamic treatment with δ-aminolevulinic acid-induced porphyrins or photosan. J. Invest. Dermatol. 112, 264–270 [DOI] [PubMed] [Google Scholar]
- 34. Pham C. G., Bubici C., Zazzeroni F., Knabb J. R., Papa S., Kuntzen C., Franzoso G. (2007) Upregulation of Twist-1 by NF-κB blocks cytotoxicity induced by chemotherapeutic drugs. Mol. Cell. Biol. 27, 3920–3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martin L. J., Al-Abdulla N. A., Brambrink A. M., Kirsch J. R., Sieber F. E., Portera-Cailliau C. (1998) Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: a perspective on the contributions of apoptosis and necrosis. Brain Res. Bull. 46, 281–309 [DOI] [PubMed] [Google Scholar]
- 36. Bonfoco E., Krainc D., Ankarcrona M., Nicotera P., Lipton S. A. (1995) Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc. Natl. Acad. Sci. U.S.A. 92, 7162–7166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hampton M. B., Orrenius S. (1997) Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett. 414, 552–556 [DOI] [PubMed] [Google Scholar]
- 38. Dursun B., He Z., Somerset H., Oh D. J., Faubel S., Edelstein C. L. (2006) Caspases and calpain are independent mediators of cisplatin-induced endothelial cell necrosis. Am. J. Physiol. Renal Physiol. 291, F578–F587 [DOI] [PubMed] [Google Scholar]
- 39. Meurette O., Rebillard A., Huc L., Le Moigne G., Merino D., Micheau O., Lagadic-Gossmann D., Dimanche-Boitrel M. T. (2007) TRAIL induces receptor-interacting protein 1-dependent and caspase-dependent necrosis-like cell death under acidic extracellular conditions. Cancer Res. 67, 218–226 [DOI] [PubMed] [Google Scholar]
- 40. Fujita R., Ueda H. (2003) Protein kinase C-mediated necrosis-apoptosis switch of cortical neurons by conditioned medium factors secreted under the serum-free stress. Cell Death Differ. 10, 782–790 [DOI] [PubMed] [Google Scholar]
- 41. Ueda H., Fujita R., Yoshida A., Matsunaga H., Ueda M. (2007) Identification of prothymosin-α1, the necrosis-apoptosis switch molecule in cortical neuronal cultures. J. Cell Biol. 176, 853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brown J. M., Attardi L. D. (2005) The role of apoptosis in cancer development and treatment response. Nat. Rev. Cancer 5, 231–237 [DOI] [PubMed] [Google Scholar]
- 43. Ankarcrona M., Dypbukt J. M., Bonfoco E., Zhivotovsky B., Orrenius S., Lipton S. A., Nicotera P. (1995) Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 15, 961–973 [DOI] [PubMed] [Google Scholar]
- 44. Xiao A. Y., Wei L., Xia S., Rothman S., Yu S. P. (2002) Ionic mechanism of ouabain-induced concurrent apoptosis and necrosis in individual cultured cortical neurons. J. Neurosci. 22, 1350–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alnemri E. S., Livingston D. J., Nicholson D. W., Salvesen G., Thornberry N. A., Wong W. W., Yuan J. (1996) Human ICE/CED-3 protease nomenclature. Cell 87, 171. [DOI] [PubMed] [Google Scholar]
- 46. McIlwain D. R., Berger T., Mak T. W. (2013) Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 5, a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stridh H., Kimland M., Jones D. P., Orrenius S., Hampton M. B. (1998) Cytochrome c release and caspase activation in hydrogen peroxide- and tributyltin-induced apoptosis. FEBS Lett. 429, 351–355 [DOI] [PubMed] [Google Scholar]
- 48. Edelstein C. L., Shi Y., Schrier R. W. (1999) Role of caspases in hypoxia-induced necrosis of rat renal proximal tubules. J. Am. Soc. Nephrol. 10, 1940–1949 [DOI] [PubMed] [Google Scholar]
- 49. Debiton E., Madelmont J. C., Legault J., Barthomeuf C. (2003) Sanguinarine-induced apoptosis is associated with an early and severe cellular glutathione depletion. Cancer Chemother. Pharmacol. 51, 474–482 [DOI] [PubMed] [Google Scholar]
- 50. Niquet J., Seo D. W., Allen S. G., Wasterlain C. G. (2006) Hypoxia in presence of blockers of excitotoxicity induces a caspase-dependent neuronal necrosis. Neuroscience 141, 77–86 [DOI] [PubMed] [Google Scholar]
- 51. Lopez-Meraz M. L., Wasterlain C. G., Rocha L. L., Allen S., Niquet J. (2010) Vulnerability of postnatal hippocampal neurons to seizures varies regionally with their maturational stage. Neurobiol. Dis. 37, 394–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lopez-Meraz M. L., Niquet J., Wasterlain C. G. (2010) Distinct caspase pathways mediate necrosis and apoptosis in subpopulations of hippocampal neurons after status epilepticus. Epilepsia 51, Suppl. 3, 56–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yoon S., Park S. J., Han J. H., Kang J. H., Kim J. H., Lee J., Park S., Shin H. J., Kim K., Yun M., Chwae Y. J. (2014) Caspase-dependent cell death-associated release of nucleosome and damage-associated molecular patterns. Cell Death Disease 5, e1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Penaloza C., Lin L., Lockshin R. A., Zakeri Z. (2006) Cell death in development: shaping the embryo. Histochem. Cell Biol. 126, 149–158 [DOI] [PubMed] [Google Scholar]
- 55. Kroemer G., Galluzzi L., Brenner C. (2007) Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 87, 99–163 [DOI] [PubMed] [Google Scholar]
- 56. Wan K. F., Chan S. L., Sukumaran S. K., Lee M. C., Yu V. C. (2008) Chelerythrine induces apoptosis through a Bax/Bak-independent mitochondrial mechanism. J. Biol. Chem. 283, 8423–8433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Karch J., Kwong J. Q., Burr A. R., Sargent M. A., Elrod J. W., Peixoto P. M., Martinez-Caballero S., Osinska H., Cheng E. H., Robbins J., Kinnally K. W., Molkentin J. D. (2013) Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. eLife 2, e00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vogler M., Weber K., Dinsdale D., Schmitz I., Schulze-Osthoff K., Dyer M. J., Cohen G. M. (2009) Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 16, 1030–1039 [DOI] [PubMed] [Google Scholar]
- 59. Stubbs M., McSheehy P. M., Griffiths J. R., Bashford C. L. (2000) Causes and consequences of tumour acidity and implications for treatment. Mol. Med. Today 6, 15–19 [DOI] [PubMed] [Google Scholar]
- 60. Pelicano H., Carney D., Huang P. (2004) ROS stress in cancer cells and therapeutic implications. Drug Resist. Updat. 7, 97–110 [DOI] [PubMed] [Google Scholar]
- 61. Trachootham D., Alexandre J., Huang P. (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 8, 579–591 [DOI] [PubMed] [Google Scholar]