Abstract
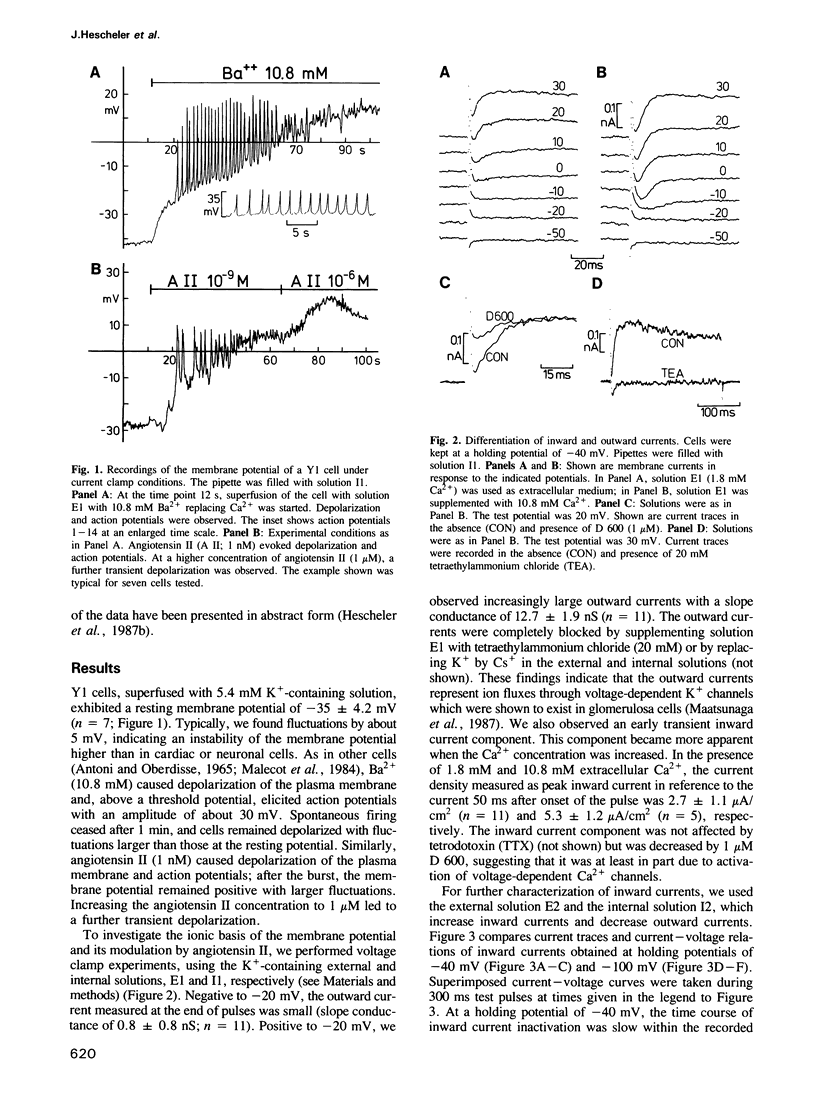
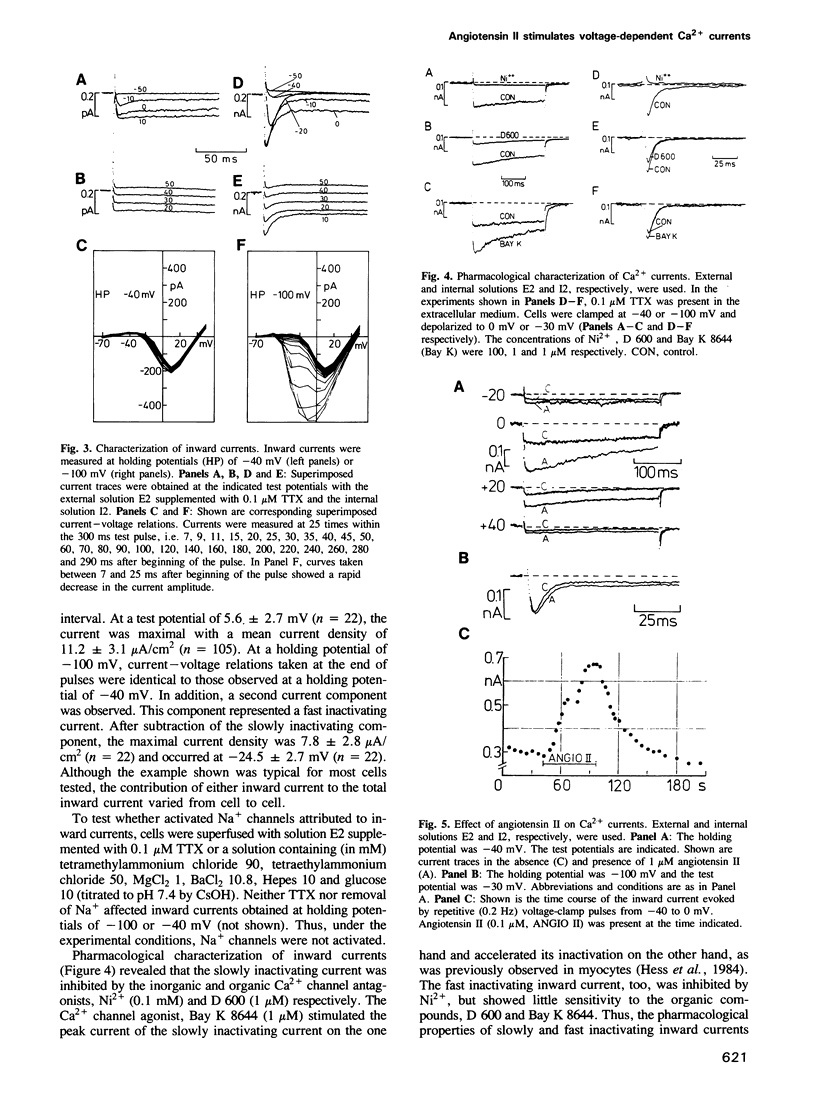
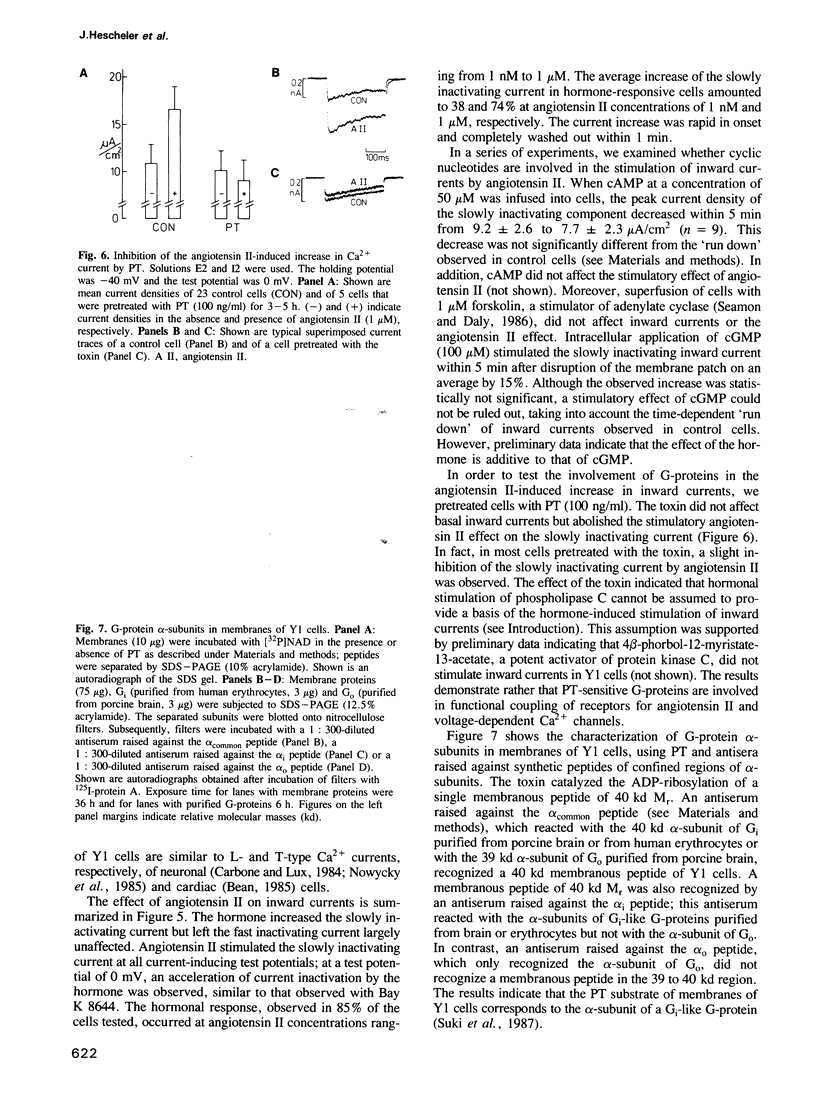
Biochemical studies suggest that stimulation of aldosterone secretion by angiotensin II involves activation of voltage-dependent Ca2+ channels. We used an adrenocortical cell line (Y1) to study the effect of angiotensin II on transmembranous currents. The hormone (1 nM to 1 microM) caused depolarization of the plasma membrane (from -35 to 10 mV) and elicited repetitive action potentials. Using the whole-cell clamp technique, we identified two types of voltage-dependent Ca2+ currents which differed with respect to their threshold potential and time course of inactivation. Angiotensin II (1 nM to 1 microM) stimulated a slowly inactivating Ca2+ current on average up to 1.7-fold whereas a fast inactivating Ca2+ current remained almost unaffected by the hormone. Ca2+ currents were not influenced by forskolin (1 microM) or intracellularly applied cAMP (50 microM). Pretreatment of cells with pertussis toxin abolished the hormonal stimulation of the slowly inactivating Ca2+ current but was without effect on control currents. The toxin ADP-ribosylated a single membranous peptide of 40 kd Mr. An antiserum raised against a synthetic peptide corresponding to a region common to all sequenced alpha-subunits of guanine nucleotide-binding proteins (G-proteins) and an antiserum raised against a peptide corresponding to a region of alpha-subunits of Gi-like G-proteins reacted with membranous 40 kd peptides, whereas an antiserum raised against a synthetic peptide corresponding to a region specific for the alpha-subunit of the G-protein, G0, failed to recognize a peptide in the 39 to 40 kd region.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera G., Catt K. J. Participation of voltage-dependent calcium channels in the regulation of adrenal glomerulosa function by angiotensin II and potassium. Endocrinology. 1986 Jan;118(1):112–118. doi: 10.1210/endo-118-1-112. [DOI] [PubMed] [Google Scholar]
- Antoni H., Oberdisse E. Elektrophysiologische Untersuchungen über die Barium-induzierte Schrittmacher-Aktivität im isolierten Säugetiermyokard. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965 Jun 15;284(3):259–272. [PubMed] [Google Scholar]
- Armstrong D., Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina J., Rosenthal W., Hildebrandt J. D., Birnbaumer L., Sekura R. D. Purification of Ns and Ni, the coupling proteins of hormone-sensitive adenylyl cyclases without intervention of activating regulatory ligands. Methods Enzymol. 1985;109:446–465. doi: 10.1016/0076-6879(85)09108-x. [DOI] [PubMed] [Google Scholar]
- Codina J., Yatani A., Grenet D., Brown A. M., Birnbaumer L. The alpha subunit of the GTP binding protein Gk opens atrial potassium channels. Science. 1987 Apr 24;236(4800):442–445. doi: 10.1126/science.2436299. [DOI] [PubMed] [Google Scholar]
- Enyedi P., Mucsi I., Hunyady L., Catt K. J., Spät A. The role of guanyl nucleotide binding proteins in the formation of inositol phosphates in adrenal glomerulosa cells. Biochem Biophys Res Commun. 1986 Nov 14;140(3):941–947. doi: 10.1016/0006-291x(86)90726-6. [DOI] [PubMed] [Google Scholar]
- Fakunding J. L., Catt K. J. Dependence of aldosterone stimulation in adrenal glomerulosa cells on calcium uptake: effects of lanthanum nd verapamil. Endocrinology. 1980 Nov;107(5):1345–1353. doi: 10.1210/endo-107-5-1345. [DOI] [PubMed] [Google Scholar]
- Fakunding J. L., Chow R., Catt K. J. The role of calcium in the stimulation of aldosterone production by adrenocorticotropin, angiotensin II, and potassium in isolated glomerulosa cells. Endocrinology. 1979 Aug;105(2):327–333. doi: 10.1210/endo-105-2-327. [DOI] [PubMed] [Google Scholar]
- Foster R., Lobo M. V., Rasmussen H., Marusic E. T. Calcium: its role in the mechanism of action of angiotensin II and potassium in aldosterone production. Endocrinology. 1981 Dec;109(6):2196–2201. doi: 10.1210/endo-109-6-2196. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Baukal A., Catt K. J. Angiotensin II receptors in bovine adrenal cortex. Modification of angiotensin II binding by guanyl nucleotides. J Biol Chem. 1974 Jan 25;249(2):664–666. [PubMed] [Google Scholar]
- Grahame-Smith D. G., Butcher R. W., Ney R. L., Sutherland E. W. Adenosine 3',5'-monophosphate as the intracellular mediator of the action of adrenocorticotropic hormone on the adrenal cortex. J Biol Chem. 1967 Dec 10;242(23):5535–5541. [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Aguilera G., Catt K. J. Selective enhancement of angiotensin II- and potassium-stimulated aldosterone secretion by the calcium channel agonist BAY K 8644. Endocrinology. 1986 Feb;118(2):869–874. doi: 10.1210/endo-118-2-869. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986 Aug;407(2):182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Hofmann F., Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 1986 Aug;407(2):123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Kreutter D., Rasmussen H. The temporal integration of the aldosterone secretory response to angiotensin occurs via two intracellular pathways. J Biol Chem. 1984 Dec 10;259(23):14448–14457. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Characteristics of angiotensin II-, K+- and ACTH-induced calcium influx in adrenal glomerulosa cells. Evidence that angiotensin II, K+, and ACTH may open a common calcium channel. J Biol Chem. 1985 Aug 5;260(16):9171–9176. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Effects of ANG II and K+ on Ca efflux and aldosterone production in adrenal glomerulosa cells. Am J Physiol. 1985 Jan;248(1 Pt 1):E36–E43. doi: 10.1152/ajpendo.1985.248.1.E36. [DOI] [PubMed] [Google Scholar]
- Kojima I., Shibata H., Ogata E. Pertussis toxin blocks angiotensin II-induced calcium influx but not inositol trisphosphate production in adrenal glomerulosa cell. FEBS Lett. 1986 Aug 18;204(2):347–351. doi: 10.1016/0014-5793(86)80841-9. [DOI] [PubMed] [Google Scholar]
- Kojima K., Kojima I., Rasmussen H. Dihydropyridine calcium agonist and antagonist effects on aldosterone secretion. Am J Physiol. 1984 Nov;247(5 Pt 1):E645–E650. doi: 10.1152/ajpendo.1984.247.5.E645. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis D. L., Weight F. F., Luini A. A guanine nucleotide-binding protein mediates the inhibition of voltage-dependent calcium current by somatostatin in a pituitary cell line. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9035–9039. doi: 10.1073/pnas.83.23.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luqman W. A., Matej L. A., Smith M. L. Comparison of prolactin levels in human semen and seminal plasma. J Endocrinol. 1979 Apr;81(1):131–133. doi: 10.1677/joe.0.0810131. [DOI] [PubMed] [Google Scholar]
- Malécot C., Coraboeuf E., Coulombe A. Automaticity of ventricular fibers induced by low concentrations of barium. Am J Physiol. 1984 Sep;247(3 Pt 2):H429–H439. doi: 10.1152/ajpheart.1984.247.3.H429. [DOI] [PubMed] [Google Scholar]
- Matsunaga H., Maruyama Y., Kojima I., Hoshi T. Transient Ca2+-channel current characterized by a low-threshold voltage in zona glomerulosa cells of rat adrenal cortex. Pflugers Arch. 1987 Apr;408(4):351–355. doi: 10.1007/BF00581128. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Kahn R. A., Manning D. R., Gilman A. G. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1986 Jan;83(2):265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal W., Binder T., Schultz G. NADP efficiently inhibits endogenous but not pertussis toxin-catalyzed covalent modification of membrane proteins incubated with NAD. FEBS Lett. 1987 Jan 26;211(2):137–143. doi: 10.1016/0014-5793(87)81424-2. [DOI] [PubMed] [Google Scholar]
- Rosenthal W., Koesling D., Rudolph U., Kleuss C., Pallast M., Yajima M., Schultz G. Identification and characterization of the 35-kDa beta subunit of guanine-nucleotide-binding proteins by an antiserum raised against transducin. Eur J Biochem. 1986 Jul 15;158(2):255–263. doi: 10.1111/j.1432-1033.1986.tb09745.x. [DOI] [PubMed] [Google Scholar]
- Sala G. B., Hayashi K., Catt K. J., Dufau M. L. Adrenocorticotropin action in isolated adrenal cells. The intermediate role of cyclic AMP in stimulation of corticosterone synthesis. J Biol Chem. 1979 May 25;254(10):3861–3865. [PubMed] [Google Scholar]
- Schimmer B. P. Cyclic nucleotides in hormonal regulation of adrenocortical function. Adv Cyclic Nucleotide Res. 1980;13:181–214. [PubMed] [Google Scholar]
- Schimmer B. P. Isolation of ACTH-resistant Y1 adrenal tumor cells. Methods Enzymol. 1985;109:350–356. doi: 10.1016/0076-6879(85)09099-1. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Dolphin A. C. Regulation of calcium currents by a GTP analogue: potentiation of (-)-baclofen-mediated inhibition. Neurosci Lett. 1986 Aug 15;69(1):59–64. doi: 10.1016/0304-3940(86)90414-3. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Suki W. N., Abramowitz J., Mattera R., Codina J., Birnbaumer L. The human genome encodes at least three non-allellic G proteins with alpha i-type subunits. FEBS Lett. 1987 Aug 10;220(1):187–192. doi: 10.1016/0014-5793(87)80900-6. [DOI] [PubMed] [Google Scholar]
- Ui M., Katada T., Murayama T., Kurose H., Yajima M., Tamura M., Nakamura T., Nogimori K. Islet-activating protein, pertussis toxin: a specific uncoupler of receptor-mediated inhibition of adenylate cyclase. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:145–151. [PubMed] [Google Scholar]
- Woodcock E. A., Johnston C. I. Inhibition of adenylate cyclase in rat adrenal glomerulosa cells by angiotensin II. Endocrinology. 1984 Jul;115(1):337–341. doi: 10.1210/endo-115-1-337. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]



