Background: IL-2 signaling occurs by cognate receptor phosphorylation, ultimately resulting in regulation of lymphocyte function.
Results: IL-2Rβ Thr(P)-450 positively regulates receptor complex stability and downstream STAT5 transcriptional activity.
Conclusion: IL-2Rβ Thr-450 is a novel ERK1/2-mediated phosphoregulatory site important for optimal IL-2 signal transduction.
Significance: IL-2Rβ threonine phosphorylation governs IL-2 signal transduction and may play a role in immune cell dysfunction.
Keywords: cell signaling, JAK, lymphocyte, protein kinase, protein phosphatase, signal transduction, STAT transcription factor, interleukin 2, receptor phosphorylation, serine/threonine
Abstract
T, B, and natural killer cells are required for normal immune response and are regulated by cytokines such as IL-2. These cell signals are propagated following receptor-ligand engagement, controlling recruitment and activation of effector proteins. The IL-2 receptor β subunit (IL-2Rβ) serves in this capacity and is known to be phosphorylated. Tyrosine phosphorylation of the β chain has been studied extensively. However, the identification and putative regulatory roles for serine and threonine phosphorylation sites have yet to be fully characterized. Using LC-MS/MS and phosphospecific antibodies, a novel IL-2/IL-15 inducible IL-2Rβ phosphorylation site (Thr-450) was identified. IL-2 phosphokinetic analysis revealed that phosphorylation of IL-2Rβ Thr-450 is rapid (2.5 min), transient (peaks at 15 min), and protracted compared with receptor tyrosine phosphorylation and occurs in multiple cell types, including primary human lymphocytes. Pharmacological and siRNA-mediated inhibition of various serine/threonine kinases revealed ERK1/2 as a positive regulator, whereas purified protein phosphatase 1 (PP1), dephosphorylated Thr-450 in vitro. Reconstitution assays demonstrated that Thr-450 is important for regulating IL-2R complex formation, recruitment of JAK3, and activation of AKT and ERK1/2 and a transcriptionally active STAT5. These results provide the first evidence of the identification and functional characterization for threonine phosphorylation of an interleukin receptor.
Introduction
IL-2 and its effector molecules are key for maintaining normal and efficient homeostasis of the immune system. Dysregulation can result in several immune disorders (1). It is an important regulator of several lymphoid cells, including B, natural killer, and various subsets of T cells (2–4), in which it promotes activation-induced cell death (5). Additionally, IL-2 promotes self-tolerance through the development and maintenance of regulatory cells essential for the negative regulation of the peripheral lymphoid compartment (6). These cellular responses are mediated through the IL-2 receptor (IL-2R)2 complex, composed of the affinity-conferring chain IL-2Rα (CD25) and the signaling subunits IL-2Rβ (CD122) and IL-2Rγ (CD132). Engagement of the receptor complex promotes recruitment and activation of several tyrosine kinases, including JAK1, JAK3, spleen tyrosine kinase, and lymphocyte-specific protein tyrosine kinase (6–8). Activated JAK1 and JAK3 phosphorylate tyrosine residues on the receptor, providing docking sites for the phosphotyrosine binding and Src homology 2 (SH2) domain-containing proteins SHC, and STAT3 and STAT5, respectively (9, 10). The β and γ subunits of the IL-2 receptor are also shared with the IL-15 receptor, which mediates signaling by common effector proteins (11). However, the differential expression and distribution of their distinct α subunits allows IL-2 and IL-15 to have unique cellular roles. For example, IL-2 serves to eliminate self-reactive T-cells, whereas IL-15 can support memory T cell survival (12).
The positive signal transduction mediated by IL-2 has been attributed to tyrosine phosphorylation of distinct residues within the IL-2Rβ and recruitment of, predominantly, SHC to Tyr-338 and STAT5 to Tyr-338, Tyr-392, and Tyr-510 (10, 13). Receptor-docked molecules become activated and initiate the JAK/STAT, PI3K, and MEK/ERK pathways, which regulate genes involved in cell differentiation, proliferation, and apoptosis (14–16). Surprisingly, activation of STAT5 has been observed in cells expressing IL-2Rβ with phosphodeletion mutations of its three key tyrosine residues (Tyr-338, Tyr-392, and Tyr-510) (17), suggesting that other receptor domains or phosphorylation sites may contribute to this response. Interestingly, IL-2 also induces serine and threonine phosphorylation of IL-2Rβ (18–20), although its effects on signal transduction have yet to be characterized. Recent evidence indicates that serine and threonine phosphorylation can have multiple effects on the IL-2 signaling cascade. Our group showed that activation of adenylate cyclase and PKA resulted in negative regulation of IL-2 signaling at multiple levels, characterized by heightened serine phosphorylation and reduced tyrosine phosphorylation of JAK3 (21). Moreover, IL-2-induced tyrosine and serine phosphorylation of STAT5 was reduced, along with the formation of a functional IL-2R complex. It is known that negative regulation of the JAK/STAT pathway is achieved through several mechanisms, including dephosphorylation of key residues by protein tyrosine phosphatases, sequestering phosphotyrosine docking sites by suppressor of cytokine signaling molecules (22), and inhibition of STAT5 binding to DNA by the protein inhibitor of activated STATs family (23). Our group and others have shown that serine/threonine phosphatases participate in the regulation of JAK/STAT activation. Inhibition of protein phosphatase type 2A (PP2A) diminishes STAT3, STAT6, STAT5, JAK3, and IL-2Rβ activation (18, 24–26). Serine phosphorylation of STAT5, JAK3, and IL-2Rβ was enhanced, whereas IL-2-induced tyrosine phosphorylation was attenuated (18).
To further elucidate the regulatory mechanisms of serine and threonine phosphorylation and impact on IL-2 signaling, this study investigated IL-2Rβ serine/threonine phosphorylation in response to IL-2 stimulation. Here we provide evidence of IL-2- or IL-15-induced IL-2Rβ phosphorylation at Thr-450 mediated by ERK1/2 and negatively regulated by PP1. In addition, phosphorylation of Thr-450 was found to play a role in stabilizing the IL-2R complex and to be required for full signaling of its downstream components, including STAT5B transcriptional activity. This study suggests that cytokine receptor residues such as IL-2Rβ Thr-450, and possibly others, serve to regulate signaling pathways in addition to their well studied tyrosine-phosphorylated residues.
Experimental Procedures
Cell Culture and Treatment
The human YT, Kit225, HuT 102 (27), HH (28), MT-2 (29), SUP-T1, and HEK293 cell lines were maintained in RPMI 1640 medium containing 10% fetal bovine serum (Atlanta Biologicals), 2 mm l-glutamine, and penicillin-streptomycin (50 mg/ml). Kit225 cells were supplemented with 100 nm human recombinant IL-2 (NCI Preclinical Repository). Human peripheral blood mononuclear cells from healthy donors (Research Blood Components) were purified by isocentrifugation (Ficoll-Hypaque) and activated with phytohemagglutinin (10 μg/ml) for 72 h as described previously (30). Quiescent human peripheral blood mononuclear cells or malignant hematopoietic cell lines were stimulated with human recombinant IL-2 (100 nm) or human recombinant IL-15 (100 nm) (NCI Preclinical Repository) at 37 °C for the indicated times. Kinase and phosphatase inhibition studies with wortmannin (Calbiochem), KT5720 (Sigma) and tofacitinib and trametinib (Selleck Chemicals) were performed for 1 h, whereas calyculin A (CA) (Invitrogen) treatment was performed for 15 min at 37 °C at the indicated concentrations.
Solubilization of Proteins, Immunoprecipitation, Western Blot, and Mass Spectrometry Analysis
Cells were pelleted, lysed, and subjected to immunoprecipitation and Western blot analysis as reported previously (31). For total cell lysate, protein concentration was determined by the bicinchoninic acid method (Pierce). Equal concentrations of protein per lane were separated by SDS-PAGE. Western blot assays were developed with horseradish peroxidase-conjugated goat anti-mouse IgG (heavy plus light chains) or goat anti-rabbit IgG (heavy plus light chains, KPL) and visualized using enhanced chemiluminescence and x-ray film. When reblotting, polyvinylidene difluoride membranes were incubated with stripping buffer (100 mm β-mercaptoethanol, 2% SDS, and 62.5 mm Tris-HCl (pH 6.7)) at 55 °C for 30 min, blocked, and then reprobed. Liquid chromatography-tandem mass spectrometry analysis was performed by the Taplin Biological Mass Spectrometry Facility (Harvard University) or the Biomolecule Analysis Core Facility of the Border Biomedical Research Center (The University of Texas at El Paso). The anti-phosphothreonine 450 (α-Thr(P)-450) IL-2Rβ rabbit polyclonal antibody was custom-generated by GenScript (Piscataway, NJ) using the immunogen LGPP(pT)PGVPDLVDFC (where pT indicates phosphothreonine) coupled to keyhole limpet hemocyanin. The anti-JAK3 antibody was used as described previously (32). A monoclonal mouse antibody made against the IL-2Rβ chain (561-IgG2) was a gift from Dr. Richard Robb (33). The anti-Tyr(P)-694 STAT5A and Tyr-699 STAT5B (Tyr(P)STAT5) monoclonal antibody, anti-STAT5 polyclonal antibody, anti-phospho-p44/42 MAPK (ERK1/2) Thr-202/Tyr-204 polyclonal antibody, anti-p44/42 MAPK (ERK1/2) polyclonal antibody, anti-AKT monoclonal antibody, and anti-phospho-AKT monoclonal antibody were purchased from Cell Signaling Technology and were used according to the protocol of the manufacturer, along with anti-phosphotyrosine monoclonal 4G10 (α-Tyr(P)) antibody (Upstate Biotechnology), anti-GAPDH monoclonal antibody (Fitzgerald), and anti-IL-2Rβ and anti-IL-2Rγ chain antibodies (Santa Cruz Biotechnology).
Plasmids, Site-directed Mutagenesis, and Transfection of HEK293 Cells
The pcDNA3.1/GS human IL-2Rβ and IL-2Rγ expression plasmids were purchased from Invitrogen. The pcDNA3.1 human JAK3 and STAT5B cDNAs (OriGene) were obtained as described previously (31, 34). Mutant forms of IL-2Rβ were prepared using the QuikChange site-directed mutagenesis kit (Stratagene) according to the instructions of the manufacturer. The primers used for the IL-2Rβ mutation were as follows: T450A (5′-TGGGGCCTCCCGCCCCAGGAGTC-3′) and T450E (5′-CCCTGGGGCCTCCCGAGCCAGGAGTCCCAGA-3′). All subclones and mutations were verified by DNA sequencing at the Genomic Analysis Core Facility of the Border Biomedical Research Center, The University of Texas at El Paso. Transient transfections of HEK293 cells were performed with Lipofectamine 2000 (Invitrogen) according to the instructions of the manufacturer.
In Vitro Dephosphorylation Assay
YT cells (20 × 106) were incubated without or with human recombinant IL-2 (100 nm) for 5 min or with 100 nm CA for 15 min prior to solubilization in lysis buffer (1% Triton X-100, 10 mm Tris-HCl (pH 8.0), and 50 mm NaCl). Cell lysates were incubated without or with 0.5 units of purified PP1 or PP2A (Millipore) at 37 °C for 60 min according to the instructions of the manufacturer. The reactions were stopped by addition of sample buffer containing 125 mm Tris-HCl (pH 6.8), 10% β-mercaptoethanol, 9.2% SDS, 0.04% bromphenol blue, and 20% glycerol and boiled for 5 min. Samples were resolved by SDS-PAGE, and phosphorylation of Thr-450 was measured by WB analysis.
siRNA-mediated Silencing of ERK1 and ERK2
ERK1 (SMARTpool, catalog no. M-003592-03-0010) and ERK2 (SMARTpool, catalog no. M-003555-04-0010) as well as control non-targeting (siGENOME, non-targeting siRNA pool #1, catalog no. D-001206-13-20) siRNAs were purchased from Dharmacon. Transfection of YT cells was carried out by electroporation using the Nucleofection system by Amaxa according to the instructions of the manufacturer. Briefly, YT cells (5 × 106) were suspended in 100 μl of transfection solution and transfected with 750 nm of ERK1 and 1 μm ERK2 or 1.750 μm control siRNA. Transfected cells were immediately diluted with prewarmed complete RPMI medium and cultured for 48 h. Cells were then quieted for 24 h in 1% FBS medium before incubation with or without IL-2 as indicated.
Luciferase and β-Galactosidase Assays
Subconfluent HEK293 cells in 10-cm dishes were transfected with the following plasmids: WT or mutant IL-2Rβ (6 μg), γc (6 μg), JAK3 (0.5 μg), STAT5B (3 μg), β-casein-luciferase reporter (3 μg), and pCMV-β-gal (1 μg). Cells were stimulated with or without 100 nm IL-2, transferred to 96-well plates (50,000 cells/well), and incubated for 48 h at 37 °C. Luciferase and β-gal activities were measured using ONE-Glo luciferase and Beta-Glo assay systems (Promega), respectively, according to the instructions of the manufacturer.
Results
Identification of a Novel IL-2-inducible Threonine Phosphorylation Site in Human IL-2Rβ
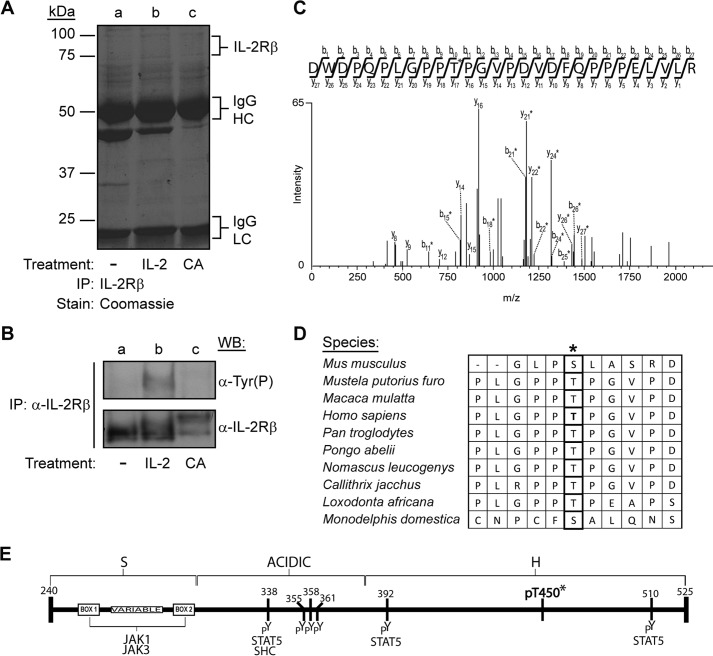
To identify unique and previously uncharacterized IL-2Rβ phosphoregulatory sites, the β subunit of the IL-2 receptor was analyzed by mass spectrometry. YT cells, which express ∼15,000 β receptors (35), were left untreated (Fig. 1A, lane a), stimulated with IL-2 for 10 min (Fig. 1A, lane b), or treated for 15 min with CA (Fig. 1A, lane c), a serine/threonine PP1 and PP2A inhibitor, to induce maximum phosphorylation of these residues. IL-2Rβ was immunoprecipitated, and the samples were separated by 10% SDS-PAGE and visualized by Coomassie Blue staining (Fig. 1A). An aliquot of the sample was analyzed by Western blot for tyrosine phosphorylation and total IL-2Rβ (Fig. 1B) to ensure IL-2-induced activation of the receptor. Indeed, IL-2 stimulation induced tyrosine phosphorylation of the IL-2Rβ (Fig. 1B, lane b, top panel). In addition, a reduction in protein migration was observed in the sample stimulated with IL-2 as well as in the CA-treated sample (Fig. 1B, lanes b and c, bottom panel), a phenomenon observed frequently in phosphorylated proteins (18). The bands corresponding to non-stimulated (75-kDa), IL-2-stimulated (80-kDa), and CA-treated (100-kDa) IL-2Rβ (Fig. 1A, lanes a–c) were excised, subjected to trypsin and Asp-N digestion, and then analyzed by LC-MS/MS. Spectrum analysis using the Sequest search algorithm revealed a combined protein coverage of 61%, 84%, and 99%, respectively, and the identification of several novel IL-2Rβ phosphorylated peptides. Of specific interest was the peptide DWDPQPLGPPTPGVPDVDFQPPPELVLR, which contains the phosphorylated IL-2Rβ Thr-450 residue (underlined). This phosphothreonine was identified in both IL-2-stimulated as well as CA-treated samples. The tandem mass spectrum for the peptide from IL-2-stimulated samples is shown in Fig. 1C. A comparable spectrum was obtained for the corresponding peptide in CA-treated YT cells, and phosphorylation of the site was confirmed in CA-treated, IL-2Rβ-transfected HEK293 cells (data not shown). To determine the extent of Thr-450 evolutionary conservation, the human IL-2Rβ protein sequence was aligned with IL-2 receptor β from several species (Fig. 1D). Thr-450 is flanked by prolines, and its primary amino acid sequence is well conserved among eight different species, mostly primates. The position of this novel threonine phosphorylation site, along with previously identified and reported IL-2Rβ tyrosine phosphorylation sites, are presented in Fig. 1E.
FIGURE 1.
Identification of Thr-450 as a novel proline-flanked phosphorylation site in human IL-2Rβ. YT cells were left untreated (−), stimulated with IL-2 for 10 min, or treated with CA (100 nm) for 15 min. Cell lysates were immunoprecipitated (IP) with α-IL-2Rβ and separated by SDS-PAGE. A and B, one set was Coomassie Blue-stained (A) (HC, heavy chain; LC, light chain), and the other (B) was Western-blotted with α-Tyr(P) or α-IL-2Rβ antibodies. C, tandem mass spectra of a monophosphorylated peptide showing site localization of Thr-450 from IL-2-stimulated cells are indicated by asterisks. D, evolutionarily conserved sequence alignment of the region surrounding Thr-450 (asterisk) from different organisms using the Clustal Omega program. E, schematic of the cytoplasmic domain architecture of human IL-2Rβ with known tyrosine and newly identified threonine (asterisk) phosphorylation sites. The numbers indicate amino acid residues of mature human IL-2Rβ. Putative binding sites for JAK1, JAK3, STAT5, and SHC are also indicated.
Generation of the Anti-Thr(P)-450 IL-2Rβ Phosphospecific Antibody
To verify that IL-2Rβ is phosphorylated at Thr-450 and to investigate the regulatory role of this phosphorylation site, a phosphospecific polyclonal antibody was generated. Dot blot analysis was performed with the immunizing phosphopeptide and the corresponding non-phosphorylated peptide (see “Experimental Procedures” for sequences) to determine whether the IL-2Rβ phosphospecific antibody cross-reacts with the non-phosphorylated form of the peptide. Decreasing amounts (1 μg, 0.1 μg, 10 ng, 1 ng, and 0.1 ng) of Thr(P)-450 or Thr-450 peptides were spotted on to PVDF membranes and blotted with α-Thr(P)-450 IL-2Rβ. The α-Thr(P)-450 IL-2Rβ antibody primarily recognized the phosphorylated peptide but not its non-phosphorylated counterpart, indicating strong and selective binding to the phosphorylated peptide (Fig. 2A, first panel, far left). In addition, dot-blotted membranes were probed using α-Thr(P)-450 IL-2Rβ preincubated with increasing amounts of non-phosphopeptide or phosphopeptide as indicated. The α-Thr(P)-450 IL-2Rβ phosphoantibody was specifically blocked by the phosphopeptide (Fig. 2A, fourth and fifth panels, right) but not the non-phosphopeptide (Fig. 2A, second and third panels, center), confirming antibody-peptide specificity.
FIGURE 2.
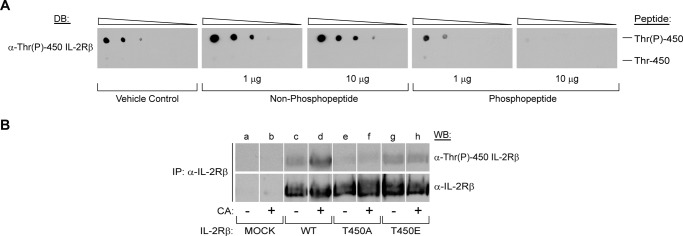
The IL-2Rβ Thr(P)-450 antibody preferentially recognizes phosphorylated peptides. A, a phosphospecific rabbit polyclonal IL-2Rβ antibody to Thr(P)-450 was generated and tested by dot blot (DB) analysis using decreasing concentrations (1 μg, 0.1 μg, 10 ng, 1 ng, and 0.1 ng) of IL-2Rβ Thr-450 (LGPPTPGVPDLVDFC) (top) and Thr-450 (LGPP(pT)PGVPDLVDFC) (bottom) peptides. For peptide competition analysis, the polyclonal Thr(P)-450 IL-2Rβ antibody was preblocked for 2 h at room temperature with vehicle control (first panel, far left), 1 or 10 μg of the non-phosphopeptide (second and third panels), or phosphopeptide (fourth and fifth panels) as indicated. B, HEK293 cells were transfected with plasmids encoding WT IL-2Rβ or the T450A or T450E mutants. 48 h post-transfection, cells were incubated in the absence (−) or presence (+) of CA (100 nm) for 60 min. IL-2Rβ was immunoprecipitated (IP), separated by SDS-PAGE, and Western-blotted as indicated. Representative data from three independent experiments are shown.
To further characterize this phosphospecific antibody, HEK293 cells were transfected with cDNA encoding WT IL-2Rβ, T450A, or T450E mutants. 48 h post-transfection, cells were treated with 100 nm CA for 1 h, and IL-2Rβ was immunoprecipitated, resolved by 10% SDS-PAGE, transferred to a PVDF membrane, and examined for Thr-450 phosphorylation by Western blotting. As shown in Fig. 2B, the α-Thr(P)-450 IL-2Rβ antibody recognized CA-treated WT IL-2Rβ (lane d) but not the T450A mutant (lane f). Recognition of the T450E mutant (Fig. 2B, lanes g and h) by the antibody was comparable with non-treated WT (Fig. 2B, lane c); however, CA-induced phosphorylation was not detectable in either Thr-450 mutants (Fig. 2B, lanes f and h). Reprobing these blots with α-IL-2Rβ antibody showed a similar expression of the protein. Taken together, the data confirm the specificity of this antibody toward the Thr-450-phosphorylated form of IL-2Rβ by Western blot analysis.
Phosphorylation of IL-2Rβ at Thr-450 Occurs in Response to IL-2 and IL-15 Stimulation of Human Lymphocytes
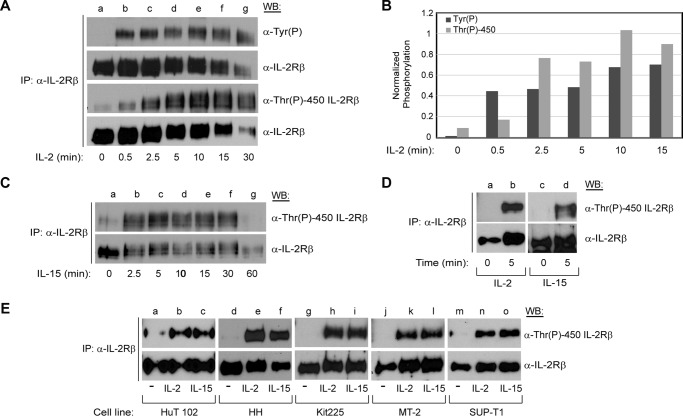
IL-2 and IL-15 share the β and γc subunits of their receptors and utilize common JAK/STAT signaling molecules, which results in shared functions, including T and B cell proliferation and the generation of cytotoxic T lymphocytes and natural killer cells. However, IL-2 and IL-15 also play distinct roles necessary for homeostasis of the immune response (12). To determine whether IL-2Rβ is phosphorylated at Thr-450 in response to physiological stimuli and to assess whether IL-2-induced phosphorylation of tyrosine and Thr-450 differ in kinetics, YT cells were subjected to a cytokine stimulation time course (0–30 min) with IL-2. Endogenous IL-2Rβ was immunoprecipitated and examined for phosphorylation by either α-Tyr(P) or α-Thr(P)-450 IL-2Rβ antibodies (Fig. 3A, lanes a–g). IL-2 stimulation resulted in phosphorylation of tyrosine and Thr-450 within 30 s (Fig. 3A, lane b). Although tyrosine phosphorylation reached maximal levels between 30 s and 2.5 min (Fig. 3A, lanes b and c), the Thr-450 phosphorylation signal was protracted and continued to increase until 10 min after IL-2 stimulation (Fig. 3A, lanes b–e). Dephosphorylation of tyrosine and Thr-450 began 30 min post-stimulation (Fig. 3A, lane g). It should be noted that internalization of the receptor begins 15–25 min after binding of IL-2 (36), hence the reduction in signal for total protein. The densitometry analysis of the representative blot is shown in Fig. 3B. To evaluate a putative pleiotropic role of Thr-450 phosphorylation, YT cells were stimulated with IL-15 and harvested at different time points (0–60 min), and then immunoprecipitated IL-2Rβ was examined for phosphorylation with α-Thr(P)-450 IL-2Rβ antibody. Similar to IL-2, IL-15 stimulation resulted in a phosphorylation signal of IL-2Rβ Thr-450 within 2.5 min that was sustained for 30 min (Fig. 3C, lanes b–f).
FIGURE 3.
Phosphorylation of IL-2Rβ Thr-450 displays rapid kinetics and is inducible by IL-2 and IL-15 in several lymphoid tumor cell lines and mitogen-activated primary lymphocytes. A, YT cells were grown to exhaustion and then stimulated with 100 nm IL-2 for the indicated times (0–30 min, lanes a–g), immunoprecipitated (IP) with α-IL-2Rβ, and Western-blotted with α-Tyr(P), α-IL-2Rβ, and α-Thr(P)-450 IL-2Rβ as indicated. B, a representative blot was used to quantitate Tyr(P) and Thr(P)-450 band intensities that were normalized to total IL-2Rβ using densitometric analysis and plotted for each time point. C, YT cells were stimulated with IL-15 for the indicated times (0–60 min, lanes a–g) and immunoprecipitated and Western-blotted as described in A. D, quiescent phytohemagglutinin-activated human PBMCs were stimulated with IL-2 (lane b) or IL-15 (lane d) for 5 min, and IL-2Rβ was immunoprecipitated, separated by SDS-PAGE, and Western-blotted as indicated. E, the indicated cell lines where grown to exhaustion and then stimulated with IL-2 (lanes b, e, h, k, and n) or IL-15 (lanes c, f, i, l, and o) for 5 min. IL-2Rβ was immunoprecipitated, separated by SDS-PAGE, and Western-blotted as indicated. Representative data from three independent experiments are shown.
To determine whether IL-2- and IL-15-induced phosphorylation of Thr-450 was confined to the natural killer-like cell line YT, other human lymphoid tumor cell lines were investigated, including human T cell lymphotrophic virus, type I-positive cutaneous T-cell lymphoblast HuT 102; cutaneous T cell lymphoma HH; T cell chronic lymphocytic leukemia Kit225; human T-cell lymphotrophic virus, type I-positive T cell leukemia MT-2; and T cell lymphoblastic leukemia SUP-T1. Cells were made quiescent by growing to exhaustion and then stimulated with IL-2 or IL-15 for 5 min. Endogenous IL-2Rβ was immunoprecipitated and resolved by SDS-PAGE for Western blot analysis. Both IL-2 (Fig. 3E, lanes b, e, h, k, and n) and IL-15 (Fig. 3E, lanes c, f, i, l, and o) stimulation induced phosphorylation of IL-2Rβ at Thr-450 within these human leukemia cell lines.
To test whether Thr-450 is phosphorylated in non-tumorigenic primary lymphocytes, phytohemagglutinin-activated primary human peripheral blood mononuclear cells (PBMCs) were made quiescent and then stimulated with IL-2 or IL-15 (Fig. 3D). Both cytokines induced IL-2Rβ Thr-450 phosphorylation within 5 min (Fig. 3D, lanes b and d). These data suggest that IL-2Rβ Thr-450 phosphorylation may be important for diverse biological functions mediated by IL-2 and IL-15. Therefore, the phosphorylation of IL-2Rβ Thr-450 occurred in multiple cell types, including primary human PBMCs, with the activation profiles indicating a general mechanism of IL-2Rβ activation.
Inhibition of JAK, MEK, and ERK Kinases Inhibits IL-2-mediated IL-2Rβ Thr-450 Phosphorylation
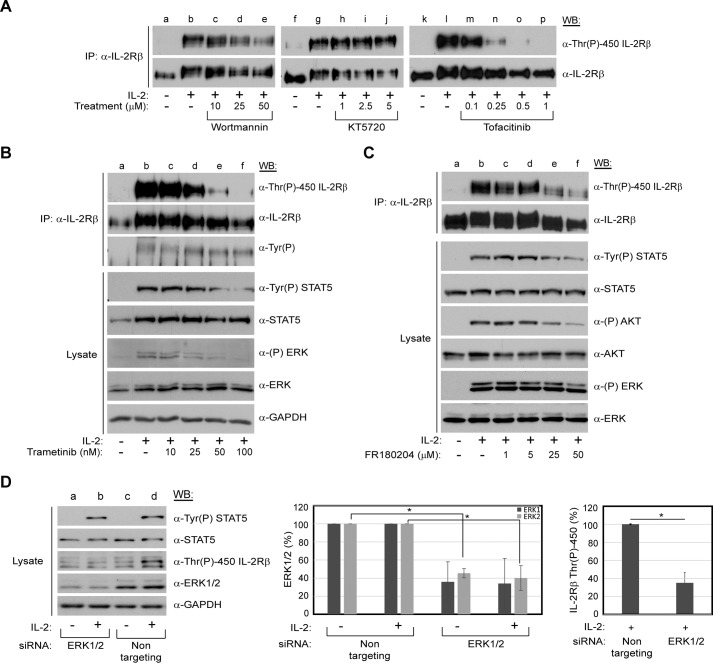
To identify the putative kinase(s) responsible for phosphorylation of IL-2Rβ Thr-450, YT cells were incubated with inhibitors of candidate serine/threonine kinases prior to IL-2 stimulation. The putative kinases were chosen on the basis of the pathways known to be activated by IL-2: JAK/mammalian target of rapamycin (mTOR), PI3K, and MEK/ERK. In addition, the intracellular domain of IL-2Rβ was analyzed using the PhosphoMotif Finder tool of the Human Protein Reference Database (37) for consensus serine/threonine kinase substrate motifs. Of interest were ERK1 and ERK2, which were reported to phosphorylate proline-flanked threonine residues and are activated by IL-2. Inhibition of JAK activation by tofacitinib resulted in abrogation of IL-2-induced Thr-450 phosphorylation in a dose-dependent manner (Fig. 4A, lanes m–p). In contrast, inhibition of PI3K by wortmannin (Fig. 4A, lanes c–e) or PKA with KT5720 (Fig. 4A, lanes h–j) had no visible effect even at doses much greater than their IC50 values (Fig. 4A). The mTOR inhibitor rapamycin was also tested and showed a similar ineffectiveness in blocking Thr-450 phosphorylation (data not shown). However, treatment with the highly specific MEK inhibitor trametinib resulted in a significant loss of IL-2-induced Thr-450 phosphorylation in a dose-dependent manner that corresponded with a loss of ERK1/2 and, unexpectedly, STAT5 activation (Fig. 4B, lanes c–f). Interestingly, IL-2-induced tyrosine phosphorylation of IL-2Rβ was unaffected by trametinib treatment, although the shift in electrophoretic mobility of the receptor reverted to its unstimulated form (Fig. 4B, lanes c–f). Additionally, blockade of ERK enzymatic activity with the inhibitor FR180204 resulted in a reduction of IL-2-induced Thr-450 phosphorylation (Fig. 4C, lanes c–f). It should be noted that FR180204 is an ATP-competitive inhibitor of ERK and does not affect its phosphorylation by MEK (38). Therefore, ERK is able to become phosphorylated. However, it is not able to enzymatically phosphorylate its targets. In addition, ERK phosphorylation is not autophosphorylation-dependent and, therefore, remains unaffected following drug treatment. Interestingly, activation of STAT5 and AKT was decreased notably. Taken together, these results suggest that IL-2Rβ Thr-450 phosphorylation is directly or indirectly dependent on IL-2-induced activation of the MEK/ERK pathway.
FIGURE 4.
Inhibition of JAK, MEK, and ERK kinases blocks IL-2 mediated IL-2Rβ Thr-450 phosphorylation. A–C, YT cells were grown to exhaustion, treated with increasing concentrations of inhibitors toward PI3K (wortmannin), PKA (KT5720), JAK (tofacitinib, A), MEK (trametinib, B), or ERK (FR180204, C) for 60 min and then stimulated with or without IL-2 (100 nm) for 5 min. Whole cell lysates or immunoprecipitated (IP) IL-2Rβ was separated by SDS-PAGE and Western-blotted as indicated. D, YT cells were nucleofected with ERK1 (750 nm) and ERK2-specific (1 μm) siRNA or non-targeting control siRNA (1.75 μm). 48 h post-electroporation, cells were starved for 24 h, harvested, and incubated with or without IL-2 (100 nm) for 5 min at 37 °C. Cell lysates were separated by SDS-PAGE and Western-blotted as indicated (left panel). Band intensities for ERK1/2 (center panel) and phospho-IL-2Rβ Thr-450 (right panel) were normalized to GAPDH using densitometric analysis and percent phosphorylation-plotted. Values represent the mean ± S.D. of three independent experiments. Statistical significance was determined using Student's t test. *, p < 0.05.
siRNA-mediated Knockdown of ERK1 and ERK2 Inhibits IL-2-induced IL-2Rβ Thr-450 Phosphorylation
To confirm the role of ERKs in phosphorylating Thr-450, siRNA-mediated knockdown of ERK1 and ERK2 was performed. ERK1 and ERK2 or non-targeting control siRNA were delivered into YT cells via electroporation. Cells were stimulated with IL-2 for 5 min at 72 h post-transfection. Protein phosphorylation and ERK1/2 protein levels were determined by Western blot analysis of total cell lysates. As demonstrated in Fig. 4D, ERK1/2-specific siRNA significantly reduced protein levels of ERK1 and ERK2, which correlated with a loss of IL-2-induced phosphorylation of IL-2Rβ Thr-450 (Fig. 4D, left panel, lane b). STAT5 phosphorylation served as a positive control for IL-2 cell stimulation (Fig. 4D, left panel, lanes b and d). Densitometric analysis indicated that ERK1/2 expression from three separate experiments was reduced ∼60% compared with the non-targeting siRNA control (Fig. 4D, center panel), which correlated with a statistically significant 60% reduction of IL-2Rβ Thr-450 phosphorylation signal (Fig. 4D, right panel). These findings demonstrate that ERK1 and ERK2 have a direct effect on IL-2-induced phosphorylation of IL-2Rβ Thr-450.
PP1 but Not PP2A Negatively Regulates Phosphorylation of IL-2Rβ Thr-450
We have demonstrated previously that inhibition of PP1 and PP2A with CA induces an electrophoretic mobility shift in IL-2Rβ characteristic of phosphorylated proteins (18). In this study, mass spectrometry analysis of IL-2Rβ revealed that Thr-450 is phosphorylated following CA treatment (data not shown). Interestingly, ERK1/2 can become activated by CA-induced inhibition of PP1 and PP2A (39). To delineate the kinetics of PP1 and PP2A inhibition on Thr-450 phosphorylation, YT cells were treated with 100 nm CA for 0–60 min. IL-2Rβ was immunoprecipitated from soluble cell lysates, separated by SDS-PAGE, and subjected to Western blot analysis with α-Thr(P)-450 IL-2Rβ antibody (Fig. 5A). Consistent with the mass spectrometry data, CA treatment induced phosphorylation of IL-2Rβ Thr-450 with rapid kinetics. Phosphorylation of Thr-450 was detectable as early as 5 min, and the signal was sustained until 15 min and returned to basal levels after 60 min (Fig. 5A, lanes c, e, and g). The membrane was stripped and reprobed for total IL-2Rβ to ensure equal gel loading. To further differentiate the role of PP1 from PP2A in the regulation of Thr-450 phosphorylation, an in vitro phosphatase assay was performed. YT cells were left untreated, stimulated with IL-2, or treated with 100 nm CA for 15 min. Soluble cell lysates were subjected to in vitro dephosphorylation using purified PP1 or PP2A enzymes, separated by SDS-PAGE, and analyzed for IL-2Rβ Thr-450 phosphorylation by Western blot. As shown in Fig. 5B, dephosphorylation using purified PP1 reversed the IL-2- and CA-induced phosphorylation of Thr-450 (lanes e and f), whereas samples incubated with purified PP2A (lanes g–i) remained similar to the control (lanes a–c). Interestingly, the electrophoretic mobility shift caused by CA was not reversed by treatment with either purified PP1 (Fig. 5B, lanes e and f) or PP2A (Fig. 5B, lanes h and i). Taken together, these data indicate that inhibition of PP1 and PP2A by CA induces phosphorylation of IL-2Rβ Thr-450 in vivo and that PP1 directly dephosphorylates it in vitro.
FIGURE 5.
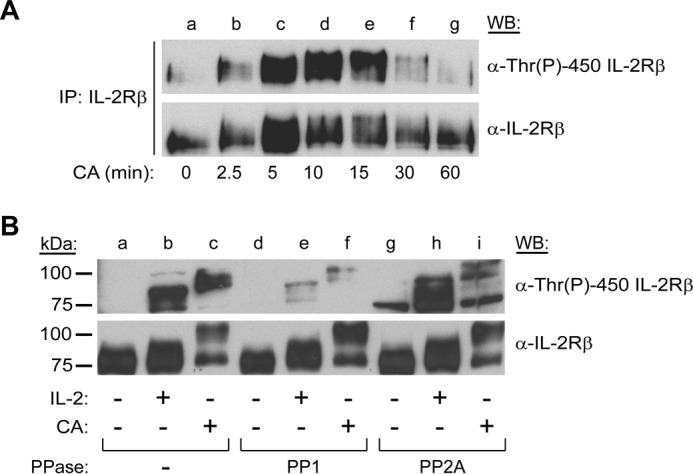
PP1 regulates IL-2Rβ Thr-450 phosphorylation. A, YT cells were left untreated or incubated with CA (100 nm) for 0–60 min (lanes a–g), IL-2Rβ was immunoprecipitated and Western-blotted (WB) using α-Thr(P)-450 IL-2Rβ. The membrane was stripped and reblotted for total IL-2Rβ as indicated. B, YT cells were incubated with or without IL-2 (100 nm) for 5 min (lanes b, e, and h) or CA (100 nm) for 15 min (lanes c, f, and i). Whole cell lysates were subjected to dephosphorylation using purified PP1 (lanes d–f) or PP2A (lanes g–i) for 60 min at 37 °C or left untreated (lanes a–c) before separation by SDS-PAGE and Western blot analysis as indicated. Representative data from three independent experiments are shown.
Phosphorylation of Thr-450 Is Required for Optimal IL-2 Signaling and Receptor Complex Formation
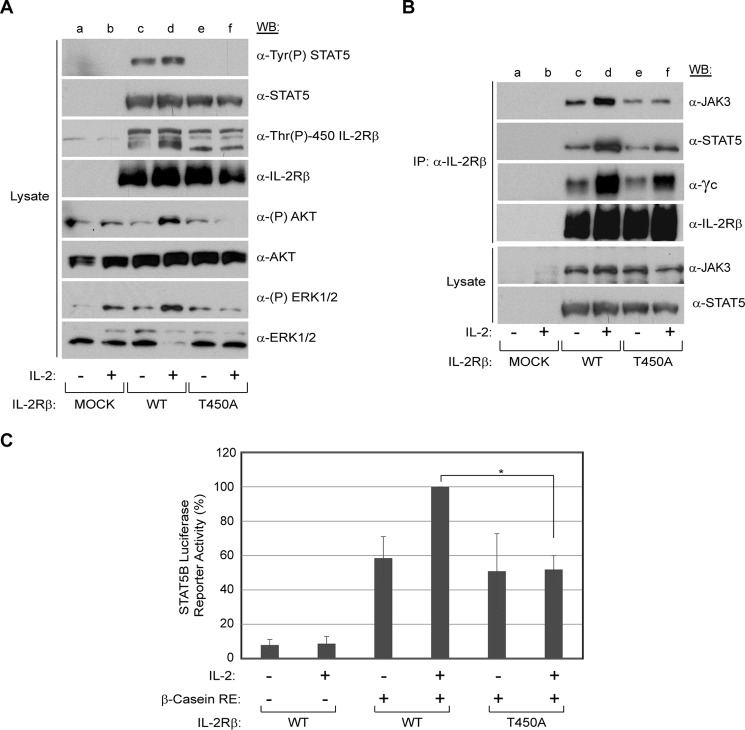
IL-2 promotes the formation of a heterotrimeric receptor complex (high affinity, Kd 10−11 m) consisting of two essential signaling subunits, IL-2Rβ, the γc (intermediate affinity receptor, Kd 10−9 m), and the affinity-conferring subunit IL-2Rα (low affinity, Kd 10−8 m) (40, 41). Failure in the assembly of IL-2Rβ and γc upon stimulation of IL-2 results in the blockade of downstream signaling components (42, 43). Previous work from our group showed that CA-induced serine and threonine phosphorylation disrupted IL-2-induced IL-2R complex formation and activation of downstream targets (18). To investigate the functional role of Thr-450 phosphorylation on IL-2 signaling, a HEK293 reconstitution system was employed using amino acid substitution (T450A) within IL-2Rβ. Plasmids encoding γc, JAK3, and STAT5B were cotransfected into HEK293 cells with either the WT or T450A forms of IL-2Rβ. 48 h post-transfection, cells were made quiescent and then left untreated or stimulated with IL-2 for 10 min. Whole cell lysates were analyzed for activation of key signaling proteins of the JAK/STAT, PI3K, and MEK/ERK pathways, which are important for lymphocyte differentiation, proliferation, and survival (44). As shown in Fig. 6A, STAT5 was observed to be constitutively activated in cells transfected with γc, JAK3, STAT5, and WT IL-2Rβ (lane c). However, such phosphorylation appears to be modestly induced by IL-2 stimulation (Fig. 6A, lane d). Importantly, tyrosine phosphorylation of STAT5 was lost in cells transfected with the phospho-deletion mutant (T450A) (Fig. 6A, lanes e and f). Activation of the PI3K pathway was assessed in terms of phosphorylation of its downstream target AKT. IL-2-induced phosphorylation of AKT was observed in cells transfected with WT (Fig. 6A, lane d) but not in cells expressing the T450A mutant (Fig. 6A, lane f). Similarly, IL-2-induced phosphorylation of ERK1/2 was detected in cells transfected with the WT receptor (Fig. 6A, lane d) but not with the phospho-deletion mutant (Fig. 6A, lane f). To determine the role of IL-2-induced IL-2Rβ Thr-450 phosphorylation in the assembly of the IL-2R complex, the IL-2Rβ subunit was immunoprecipitated from soluble lysates and probed for the association of γc, JAK3, and STAT5B by Western blot analysis (Fig. 6B). IL-2 stimulation resulted in coimmunoprecipitation of JAK3, STAT5B, and γc with WT IL-2Rβ (Fig. 6B, lane d). Importantly, this association was reduced considerably when Thr-450 on IL-2Rβ was mutated to alanine (Fig. 6B, lane f). These results suggest that phosphorylation of Thr-450 is important for the assembly of the IL-2Rβ complex in addition to regulating the phosphorylation of the downstream signaling molecules STAT5B, AKT, and ERK1/2.
FIGURE 6.
IL-2Rβ Thr-450 phosphorylation is a positive regulator for activation of downstream signaling molecules, receptor complex formation, and STAT5B transcriptional activity in a reconstituted HEK293 cell system. HEK293 cells were transfected with γc, JAK3, STAT5B, and IL-2Rβ (WT or T450A) and incubated for 48 h in complete medium, followed by 48 h of incubation in 1% FBS medium. Transfected cells were stimulated without (−) or with IL-2 (+) for 10 min. A, whole cell lysates were separated by SDS-PAGE and Western-blotted as indicated. B, IL-2Rβ was immunoprecipitated (IP), separated by SDS-PAGE, and analyzed by WB for coimmunoprecipitation of JAK3, STAT5, and γc as indicated. Input controls for immunoprecipitated JAK3 and STAT5 are indicated. Representative data from three independent experiments are shown. C, HEK293 cells were transfected with IL-2Rβ (WT or T450A), γc, JAK3, STAT5B, β-casein-luciferase, and pCMV-β-galactosidase. 48 h post-transfection, cells were incubated with or without IL-2 for an additional 48 h, and luciferase activity was measured and normalized to β-gal activity. Each treatment was performed in triplicate. Results are presented as the mean ± S.D. of three independent experiments. Statistical significance was determined using Student's t test. *, p < 0.05.
Phosphorylation of Thr-450 Is Important for Maximum IL-2-induced STAT5B Transcriptional Activity
The STAT5A and STAT5B transcription factors become activated in response to several growth factors and cytokines, including IL-2, which induces tyrosine phosphorylation of the receptor and provides docking sites for STATs to bind through their SH2 domains (45). Receptor-bound STATs become activated through tyrosine and serine phosphorylation, form dimers, and translocate to the nucleus to activate genes related to the differentiation and proliferation of cells (46). To determine whether phosphorylation of IL-2Rβ Thr-450 is important for STAT5B transcriptional activity in vivo, luciferase reporter assays were performed using the HEK293 reconstitution system (Fig. 6C). Plasmids encoding γc, JAK3, and STAT5B were cotransfected into HEK293 cells with either the WT or T450A forms of IL-2Rβ along with a β-casein-firefly luciferase reporter and a β-gal reporter construct. 48 h post-transfection, cells were made quiescent and then left untreated or stimulated with IL-2 for 48 h. Luciferase activity was first normalized to β-gal activity and then to IL-2-stimulated WT IL-2Rβ samples. Upon IL-2 stimulation, the luciferase reporter activity of STAT5B was reduced ∼50% when signaling through the IL-2Rβ T450A mutant compared with the WT (Fig. 6C). Additionally, a reduced proliferative capacity in response to IL-2 was observed in cells expressing the IL-2Rβ T450A phospho-deletion mutant compared with the WT receptor (data not shown). These data suggests that IL-2Rβ Thr-450 phosphorylation is important for effective transduction of the L-2 signal and STAT5B transcriptional activity.
Discussion
IL-2 receptor engagement activates the JAK/STAT, PI3K, and MEK/ERK signaling pathways to promote cellular differentiation, proliferation, and survival. IL-2Rβ plays a critical role in the activation of downstream effector molecules. Indeed, mice with targeted deletion of the receptor display an irregular immunoglobulin profile, dysregulated T cell activation and B cell differentiation, and absence of natural killer or T regulatory cells, which results in severe autoimmunity and death (47). In humans, dysregulation or polymorphisms of IL-2Rβ have been associated with several autoimmune diseases as well as severe combined immunodeficiency disorders (48–54). Although regulation of the signal transduction cascade has yet to be fully elucidated, IL-2 immunotherapy has been used for years to treat renal cell carcinoma and metastatic melanoma, whereas IL-15 appears to elicit promising results in ongoing clinical trials (55, 56). Therefore, understanding the regulation and function of IL-2Rβ is critically important.
This study identifies a novel phosphorylation site at Thr-450 on IL-2Rβ and demonstrates it to be an important regulator of IL-2 signal transduction. This newly discovered phosphosite was observed to be rapidly and transiently phosphorylated in response to IL-2 and IL-15 in several malignant hematopoietic cell lines as well as non-tumorigenic primary lymphocytes (Fig. 3). Inhibition of ERK1/2 resulted in loss of IL-2 induced Thr-450 phosphorylation (Fig. 4), whereas purified PP1 reversed IL-2- and CA-induced phosphorylation of this residue in vitro (Fig. 5). Examination of the WT receptor versus a T450A mutant revealed Thr-450 phosphorylation to be important for IL-2-induced activation of STAT5, AKT, and ERK1/2 as well as STAT5B transcriptional activity and IL-2R complex formation (Fig. 6). Taken together, these findings support a fundamentally important role of Thr-450 phosphorylation in regulating IL-2 signal transduction. It is tempting to speculate that this previously unrecognized positive regulatory site could be manipulated to create novel therapeutic strategies for various immune disorders.
Previously identified phosphoregulatory sites in IL-2Rβ that are fundamental for the propagation of the IL-2 signals include Tyr-338, Tyr-394, and Tyr-510, to which SHC and/or STAT5 bind (6). Importantly, IL-2 also induces threonine phosphorylation of the receptor. Previously, we have shown induction of IL-2Rβ threonine phosphorylation in response to IL-2 stimulation and CA treatment via phosphoamino acid analysis (18), whereas other groups identified Thr-79, Thr-256, Thr-507, and Thr-522 as phosphosites through proteomic discovery mode mass spectrometry (20, 57, 58), although no site-specific characterization was performed. This work indicates that phosphorylation of IL-2Rβ Thr-450 provides an additional positive regulatory mechanism that modulates IL-2 signaling. Residue Thr-450 is confined to a PPTP sequence, a known ERK1 and ERK2 targeting motif (59). It was rapidly phosphorylated following IL-2 stimulation or CA treatment, and its conservation among higher organisms (Fig. 1D) suggests that this could be a result of a recent evolutionary gain-of-function mutation, possibly to support a more complex immune system. The role of threonine phosphorylation in cytokine receptors is not well established. Two γc family members are reported to be threonine-phosphorylated, IL-4R and IL-9R (57), although no functional characterization has been described. Interestingly, Thr-756 within the IL-4R is located within the PPT motif recognized by ERK1/2, and phosphorylation of Thr-520 within IL-9R is important for promoting receptor interaction with the adaptor protein 14-3-3ζ (60).
The transient nature of phosphorylation makes it ideal for the regulation of signal transduction. It can activate proteins and subsequently alter their cellular localization and substrate binding affinity to regulate processes including differentiation, proliferation, and the cell cycle. It is also rapidly reversible to terminate the signal. In this study, IL-2 and IL-15 induced the rapid and transient phosphorylation of IL-2Rβ Thr-450 in several lymphoid tumor cell lines as well as in normal human primary lymphocytes (Fig. 3). This strongly suggests a general mechanism of IL-2Rβ activation important for the diverse biological functions of IL-2 and IL-15. We are currently investigating whether constitutive phosphorylation of IL-2Rβ Thr-450 is present in primary lymphoid tumors, potentially providing new insights into the driving factors of hematological malignancies.
Many serine/threonine kinases are known to regulate IL-2 signal transduction (61, 62), although not directly at the receptor level. To elucidate the regulators of IL-2Rβ Thr-450 phosphorylation, pharmacological inhibition of serine/threonine kinases was performed. IL-2 induced Thr-450 phosphorylation was reduced by blocking MEK or ERK activation or inhibiting ERK1/2 expression by siRNA (Fig. 4). Interestingly, tyrosine phosphorylation of the receptor was independent of Thr-450 phosphorylation because blockade of the latter did not affect the former (Fig. 4B). Surprisingly, reduced STAT5 and AKT phosphorylation correlated with ERK1/2 and Thr-450 phosphorylation inhibition (Fig. 4, B–D). Taken together, these results suggest that Thr-450 phosphorylation could play a positive regulatory role on IL-2 signal transduction in lymphocytes.
PP1 is a ubiquitous phosphatase that regulates numerous cellular processes, including cell cycle progression, transcription of genes, and protein synthesis. The catalytic subunit of PP1 relies on ∼200 regulatory proteins for specificity and forms a holoenzyme with target-specific properties (63, 64). In this study, PP1 was able to dephosphorylate IL-2Rβ Thr-450 in vitro (Fig. 5). Importantly, IL-2 has been shown to transiently decrease PP1 activity in antigen-specific human T cells (65). Therefore, it is tempting to envision a feedback loop mechanism in which IL-2 leads to inhibition of PP1 and ERK1/2 activation dependent upon IL-2Rβ Thr-450 phosphorylation.
Thr-450 is located in the H region of the IL-2Rβ, which contains the known STAT5 docking sites Tyr-392 and Tyr-510 (6) (14). Deleting the region between these tyrosine residues results in down-regulation of IL-2Rα expression (66, 67), suggesting a positive regulatory role for this receptor domain. In agreement with these studies, phosphorylation of Thr-450 was found to be important for activation of the downstream signaling molecules STAT5B, AKT, and ERK1/2 (Fig. 6A). Impaired activation of such proteins coincides with a decrease in STAT5B transcriptional activity, as demonstrated by luciferase reporter assays (Fig. 6C). Additionally, coimmunoprecipitation studies revealed that phosphorylation of Thr-450 was necessary for optimal IL-2-induced IL-2R complex formation because binding of γc, JAK3, and STAT5B to the IL-2Rβ T450A mutant was greatly reduced (Fig. 6B). The canonical model for IL-2 signaling suggests that IL-2 binds IL-2Rβ and γc, resulting in the recruitment and activation of JAK1 and JAK3 and phosphorylation of the receptor within highly conserved and defined tyrosine residues. Phosphorylated tyrosines on IL-2Rβ serve as docking sites for SHC and STAT5, initiating the MEK/ERK and PI3K pathways. An additional feedforward mechanisms may include IL-2 activation of the MEK/ERK pathway, resulting in phosphorylation of Thr-450 to augment the signaling cascade. On the basis of the phosphorylation kinetics shown here, IL-2 first induces phosphorylation of IL-2Rβ on tyrosine residues, followed by a protracted phosphorylation at residue Thr-450 (Fig. 3A). Conceivably, Thr-450 phosphorylation results in conformational and electrostatic changes that stabilize the interaction between the components of the receptor, allowing for a maximum IL-2 signal to be transmitted. Inability of the receptor to form a stable complex likely results in attenuation of the three signaling pathways (JAK/STAT, MEK/ERK, and PI3K), as demonstrated by the loss of STAT5, ERK1/2, and AKT phosphorylation (Fig. 6) and proliferation (68, 69).
In conclusion, this work demonstrated that the IL-2Rβ chain harbors an IL-2/IL-15-inducible phosphorylation site that has been mapped to Thr-450. It is transiently phosphorylated with kinetics distal to receptor tyrosine phosphorylation. The current evidence suggests that IL-2Rβ Thr-450 is phosphorylated by ERK1/2 and dephosphorylated by PP1. More importantly, it appears to stabilize the receptor complex and downstream effectors, including STAT5 and AKT. Whether this site represents a therapeutic target for controlling IL-2/IL-15 signaling for ablating certain disorders such as graft versus host disease or lymphomas dependent on these pathways remains to be determined.
Author Contributions
R. A. K. conceived the study and revised the manuscript. R. A. K., J. A. R., and B. E. R. designed the experiments and analyzed the data. B. E. R. performed the experiments and prepared the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank the staff of the Border Biomedical Research Center Core Laboratories, including the Bioinformatics Computing Core Facility, Biomolecule Analysis Core Facility; the Cytometry, Screening, and Imaging Core Facility; the Genomic Analysis Core Facility; and the Statistical Consulting Laboratory for services and facilities provided. We also thank Amy J. Arrieta for technical assistance.
This work was supported, in whole or in part, by National Institute on Minority Health and Health Disparities/National Institutes of Health Grant 2G12MD007592. This work was also supported by grants from the Lizanell and Colbert Coldwell Foundation (to R. A. K.), the Edward N. and Margaret G. Marsh Foundation, and the Woman's Auxiliary Fellowship (to B. E. R.). The authors declare that they have no conflicts of interest with the contents of this article.
- IL-2R
- IL-2 receptor
- SH2
- Src homology 2
- CA
- calyculin A
- WB
- Western blot
- mTOR
- mammalian target of rapamycin
- PBMCs
- peripheral blood mononuclear cells.
References
- 1. Atkinson T. P. (2012) Immune deficiency and autoimmunity. Curr. Opin. Rheumatol. 24, 515–521 [DOI] [PubMed] [Google Scholar]
- 2. Olejniczak K., Kasprzak A. (2008) Biological properties of interleukin 2 and its role in pathogenesis of selected diseases: a review. Med. Sci. Monit. 14, RA179–189 [PubMed] [Google Scholar]
- 3. Lowenthal J. W., Zubler R. H., Nabholz M., MacDonald H. R. (1985) Similarities between interleukin-2 receptor number and affinity on activated B and T lymphocytes. Nature 315, 669–672 [DOI] [PubMed] [Google Scholar]
- 4. Trinchieri G., Matsumoto-Kobayashi M., Clark S. C., Seehra J., London L., Perussia B. (1984) Response of resting human peripheral blood natural killer cells to interleukin 2. J. Exp. Med. 160, 1147–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Refaeli Y., Van Parijs L., London C. A., Tschopp J., Abbas A. K. (1998) Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity 8, 615–623 [DOI] [PubMed] [Google Scholar]
- 6. Nelson B. H., Willerford D. M. (1998) Biology of the interleukin-2 receptor. Adv. Immunol. 70, 1–81 [DOI] [PubMed] [Google Scholar]
- 7. Kirken R. A., Rui H., Malabarba M. G., Farrar W. L. (1994) Identification of interleukin-2 receptor-associated tyrosine kinase p116 as novel leukocyte-specific Janus kinase. J. Biol. Chem. 269, 19136–19141 [PubMed] [Google Scholar]
- 8. Tanaka N., Asao H., Ohbo K., Ishii N., Takeshita T., Nakamura M., Sasaki H., Sugamura K. (1994) Physical association of JAK1 and JAK2 tyrosine kinases with the interleukin 2 receptor β and γ chains. Proc. Natl. Acad. Sci. U.S.A. 91, 7271–7275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buitenhuis M., Coffer P. J., Koenderman L. (2004) Signal transducer and activator of transcription 5 (STAT5). Int. J. Biochem. Cell Biol. 36, 2120–2124 [DOI] [PubMed] [Google Scholar]
- 10. Delespine-Carmagnat M., Bouvier G., Allée G., Fagard R., Bertoglio J. (1999) Biochemical analysis of interleukin-2 receptor β chain phosphorylation by p56(lck). FEBS Lett. 447, 241–246 [DOI] [PubMed] [Google Scholar]
- 11. Osinalde N., Sanchez-Quiles V., Akimov V., Guerra B., Blagoev B., Kratchmarova I. (2015) Simultaneous dissection and comparison of IL-2 and IL-15 signaling pathways by global quantitative phosphoproteomics. Proteomics 15, 520–531 [DOI] [PubMed] [Google Scholar]
- 12. Waldmann T. A. (2006) The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6, 595–601 [DOI] [PubMed] [Google Scholar]
- 13. Friedmann M. C., Migone T. S., Russell S. M., Leonard W. J. (1996) Different interleukin 2 receptor β-chain tyrosines couple to at least two signaling pathways and synergistically mediate interleukin 2-induced proliferation. Proc. Natl. Acad. Sci. U.S.A. 93, 2077–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaffen S. L. (2001) Signaling domains of the interleukin 2 receptor. Cytokine 14, 63–77 [DOI] [PubMed] [Google Scholar]
- 15. Molina J. R., Adjei A. A. (2006) The Ras/Raf/MAPK pathway. J. Thorac. Oncol. 1, 7–9 [PubMed] [Google Scholar]
- 16. Morgan T. M., Koreckij T. D., Corey E. (2009) Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr. Cancer Drug Targets 9, 237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu A., Zhu L., Altman N. H., Malek T. R. (2009) A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity 30, 204–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ross J. A., Cheng H., Nagy Z. S., Frost J. A., Kirken R. A. (2010) Protein phosphatase 2A regulates interleukin-2 receptor complex formation and JAK3/STAT5 activation. J. Biol. Chem. 285, 3582–3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asao H., Takeshita T., Nakamura M., Nagata K., Sugamura K. (1990) Interleukin 2 (IL-2)-induced tyrosine phosphorylation of IL-2 receptor p75. J. Exp. Med. 171, 637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Osinalde N., Moss H., Arrizabalaga O., Omaetxebarria M. J., Blagoev B., Zubiaga A. M., Fullaondo A., Arizmendi J. M., Kratchmarova I. (2011) Interleukin-2 signaling pathway analysis by quantitative phosphoproteomics. J. Proteomics 75, 177–191 [DOI] [PubMed] [Google Scholar]
- 21. Rodriguez G., Ross J. A., Nagy Z. S., Kirken R. A. (2013) Forskolin-inducible cAMP pathway negatively regulates T-cell proliferation by uncoupling the interleukin-2 receptor complex. J. Biol. Chem. 288, 7137–7146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shuai K., Liu B. (2003) Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 3, 900–911 [DOI] [PubMed] [Google Scholar]
- 23. Morales J. K., Falanga Y. T., Depcrynski A., Fernando J., Ryan J. J. (2010) Mast cell homeostasis and the JAK-STAT pathway. Genes Immun. 11, 599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woetmann A., Nielsen M., Christensen S. T., Brockdorff J., Kaltoft K., Engel A. M., Skov S., Brender C., Geisler C., Svejgaard A., Rygaard J., Leick V., Odum N. (1999) Inhibition of protein phosphatase 2A induces serine/threonine phosphorylation, subcellular redistribution, and functional inhibition of STAT3. Proc. Natl. Acad. Sci. U.S.A. 96, 10620–10625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woetmann A., Brockdorff J., Lovato P., Nielsen M., Leick V., Rieneck K., Svejgaard A., Geisler C., Ødum N. (2003) Protein phosphatase 2A (PP2A) regulates interleukin-4-mediated STAT6 signaling. J. Biol. Chem. 278, 2787–2791 [DOI] [PubMed] [Google Scholar]
- 26. Wang Y., Malabarba M. G., Nagy Z. S., Kirken R. A. (2004) Interleukin 4 regulates phosphorylation of serine 756 in the transactivation domain of Stat6: roles for multiple phosphorylation sites and Stat6 function. J. Biol. Chem. 279, 25196–25203 [DOI] [PubMed] [Google Scholar]
- 27. Bunn P. A., Jr., Foss F. M. (1996) T-cell lymphoma cell lines (HUT102 and HUT78) established at the National Cancer Institute: history and importance to understanding the biology, clinical features, and therapy of cutaneous T-cell lymphomas (CTCL) and adult T-cell leukemia-lymphomas (ATLL). J. Cell Biochem. Suppl. 24, 12–23 [DOI] [PubMed] [Google Scholar]
- 28. Starkebaum G., Loughran T. P., Jr., Waters C. A., Ruscetti F. W. (1991) Establishment of an IL-2 independent, human T-cell line possessing only the p70 IL-2 receptor. Int. J. Cancer 49, 246–253 [DOI] [PubMed] [Google Scholar]
- 29. Miyoshi I., Kubonishi I., Yoshimoto S., Shiraishi Y. (1981) A T-cell line derived from normal human cord leukocytes by co-culturing with human leukemic T-cells. Gann 72, 978–981 [PubMed] [Google Scholar]
- 30. Ross J. A., Nagy Z. S., Kirken R. A. (2008) The PHB1/2 phosphocomplex is required for mitochondrial homeostasis and survival of human T cells. J. Biol. Chem. 283, 4699–4713 [DOI] [PubMed] [Google Scholar]
- 31. Cheng H., Ross J. A., Frost J. A., Kirken R. A. (2008) Phosphorylation of human Jak3 at tyrosines 904 and 939 positively regulates its activity. Mol. Cell Biol. 28, 2271–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malabarba M. G., Rui H., Deutsch H. H., Chung J., Kalthoff F. S., Farrar W. L., Kirken R. A. (1996) Interleukin-13 is a potent activator of JAK3 and STAT6 in cells expressing interleukin-2 receptor-γ and interleukin-4 receptor-α. Biochem. J. 319, 865–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Voss S. D., Sondel P. M., Robb R. J. (1992) Characterization of the interleukin 2 receptors (IL-2R) expressed on human natural killer cells activated in vivo by IL-2: association of the p64 IL-2R γ chain with the IL-2R β chain in functional intermediate-affinity IL-2R. J. Exp. Med. 176, 531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mitra A., Ross J. A., Rodriguez G., Nagy Z. S., Wilson H. L., Kirken R. A. (2012) Signal transducer and activator of transcription 5b (Stat5b) serine 193 is a novel cytokine-induced phospho-regulatory site that is constitutively activated in primary hematopoietic malignancies. J. Biol. Chem. 287, 16596–16608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Robb R. J., Rusk C. M., Yodoi J., Greene W. C. (1987) Interleukin 2 binding molecule distinct from the Tac protein: analysis of its role in formation of high-affinity receptors. Proc. Natl. Acad. Sci. U.S.A. 84, 2002–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duprez V., Ferrer M., Dautry-Varsat A. (1992) High-affinity interleukin 2 receptor α and β chains are internalized and remain associated inside the cells after interleukin 2 endocytosis. J. Biol. Chem. 267, 18639–18643 [PubMed] [Google Scholar]
- 37. Amanchy R., Periaswamy B., Mathivanan S., Reddy R., Tattikota S. G., Pandey A. (2007) A curated compendium of phosphorylation motifs. Nat. Biotechnol. 25, 285–286 [DOI] [PubMed] [Google Scholar]
- 38. Ohori M., Kinoshita T., Okubo M., Sato K., Yamazaki A., Arakawa H., Nishimura S., Inamura N., Nakajima H., Neya M., Miyake H., Fujii T. (2005) Identification of a selective ERK inhibitor and structural determination of the inhibitor-ERK2 complex. Biochem. Biophys. Res. Commun. 336, 357–363 [DOI] [PubMed] [Google Scholar]
- 39. Nifoussi S. K., Ratcliffe N. R., Ornstein D. L., Kasof G., Strack S., Craig R. W. (2014) Inhibition of protein phosphatase 2A (PP2A) prevents Mcl-1 protein dephosphorylation at the Thr-163/Ser-159 phosphodegron, dramatically reducing expression in Mcl-1-amplified lymphoma cells. J. Biol. Chem. 289, 21950–21959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang X., Rickert M., Garcia K. C. (2005) Structure of the quaternary complex of interleukin-2 with its α, β, and γc receptors. Science 310, 1159–1163 [DOI] [PubMed] [Google Scholar]
- 41. Takeshita T., Asao H., Ohtani K., Ishii N., Kumaki S., Tanaka N., Munakata H., Nakamura M., Sugamura K. (1992) Cloning of the γ chain of the human IL-2 receptor. Science 257, 379–382 [DOI] [PubMed] [Google Scholar]
- 42. Kirken R. A., Malabarba M. G., Xu J., DaSilva L., Erwin R. A., Liu X., Hennighausen L., Rui H., Farrar W. L. (1997) Two discrete regions of interleukin-2 (IL2) receptor β independently mediate IL2 activation of a PD98059/rapamycin/wortmannin-insensitive Stat5a/b serine kinase. J. Biol. Chem. 272, 15459–15465 [DOI] [PubMed] [Google Scholar]
- 43. Kirken R. A., Rui H., Malabarba M. G., Howard O. M., Kawamura M., O'Shea J. J., Farrar W. L. (1995) Activation of JAK3, but not JAK1, is critical for IL-2-induced proliferation and STAT5 recruitment by a COOH-terminal region of the IL-2 receptor β-chain. Cytokine 7, 689–700 [DOI] [PubMed] [Google Scholar]
- 44. Fung M. M., Rohwer F., McGuire K. L. (2003) IL-2 activation of a PI3K-dependent STAT3 serine phosphorylation pathway in primary human T cells. Cell Signal. 15, 625–636 [DOI] [PubMed] [Google Scholar]
- 45. Leonard W. J. (1996) STATs and cytokine specificity. Nat. Med. 2, 968–969 [DOI] [PubMed] [Google Scholar]
- 46. Lin J. X., Leonard W. J. (2000) The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene 19, 2566–2576 [DOI] [PubMed] [Google Scholar]
- 47. Kim H. P., Imbert J., Leonard W. J. (2006) Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 17, 349–366 [DOI] [PubMed] [Google Scholar]
- 48. Amu S., Strömberg K., Bokarewa M., Tarkowski A., Brisslert M. (2007) CD25-expressing B-lymphocytes in rheumatic diseases. Scand. J. Immunol. 65, 182–191 [DOI] [PubMed] [Google Scholar]
- 49. Barton A., Thomson W., Ke X., Eyre S., Hinks A., Bowes J., Plant D., Gibbons L. J., Wellcome Trust Case Control Consortium, YEAR Consortium, BIRAC Consortium, Wilson A. G., Bax D. E., Morgan A. W., Emery P., Steer S., Hocking L., Reid D. M., Wordsworth P., Harrison P., Worthington J. (2008) Rheumatoid arthritis susceptibility loci at chromosomes 10p15, 12q13 and 22q13. Nat. Genet. 40, 1156–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Espino-Paisán L., De La Calle H., Fernández-Arquero M., Figueredo M. A., De La Concha E. G., Urcelay E., Santiago J. L. (2011) Study of polymorphisms in 4q27, 10p15, and 22q13 regions in autoantibodies stratified type 1 diabetes patients. Autoimmunity 44, 624–630 [DOI] [PubMed] [Google Scholar]
- 51. Gregersen P. K., Olsson L. M. (2009) Recent advances in the genetics of autoimmune disease. Annu. Rev. Immunol. 27, 363–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bucheton B., Argiro L., Chevillard C., Marquet S., Kheir M. M., Mergani A., El-Safi S. H., Dessein A. J. (2007) Identification of a novel G245R polymorphism in the IL-2 receptor β membrane proximal domain associated with human visceral leishmaniasis. Genes Immun. 8, 79–83 [DOI] [PubMed] [Google Scholar]
- 53. Pál Z., Antal P., Millinghoffer A., Hullám G., Pálóczi K., Tóth S., Gabius H. J., Molnár M. J., Falus A., Buzás E. I. (2010) A novel galectin-1 and interleukin 2 receptor β haplotype is associated with autoimmune myasthenia gravis. J. Neuroimmunol. 229, 107–111 [DOI] [PubMed] [Google Scholar]
- 54. Gilmour K. C., Fujii H., Cranston T., Davies E. G., Kinnon C., Gaspar H. B. (2001) Defective expression of the interleukin-2/interleukin-15 receptor β subunit leads to a natural killer cell-deficient form of severe combined immunodeficiency. Blood 98, 877–879 [DOI] [PubMed] [Google Scholar]
- 55. Rosenberg S. A., Yang J. C., Topalian S. L., Schwartzentruber D. J., Weber J. S., Parkinson D. R., Seipp C. A., Einhorn J. H., White D. E. (1994) Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 271, 907–913 [PubMed] [Google Scholar]
- 56. Conlon K. C., Lugli E., Welles H. C., Rosenberg S. A., Fojo A. T., Morris J. C., Fleisher T. A., Dubois S. P., Perera L. P., Stewart D. M., Goldman C. K., Bryant B. R., Decker J. M., Chen J., Worthy T. A., Figg W. D., Sr., Peer C. J., Sneller M. C., Lane H. C., Yovandich J. L., Creekmore S. P., Roederer M., Waldmann T. A. (2015) Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 33, 74–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hornbeck P. V., Kornhauser J. M., Tkachev S., Zhang B., Skrzypek E., Murray B., Latham V., Sullivan M. (2012) PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 40, D261–D270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Depontieu F. R., Qian J., Zarling A. L., McMiller T. L., Salay T. M., Norris A., English A. M., Shabanowitz J., Engelhard V. H., Hunt D. F., Topalian S. L. (2009) Identification of tumor-associated, MHC class II-restricted phosphopeptides as targets for immunotherapy. Proc. Natl. Acad. Sci. U.S.A. 106, 12073–12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang X., Gabuzda D. (1998) Mitogen-activated protein kinase phosphorylates and regulates the HIV-1 Vif protein. J. Biol. Chem. 273, 29879–29887 [DOI] [PubMed] [Google Scholar]
- 60. Sliva D., Gu M., Zhu Y. X., Chen J., Tsai S., Du X., Yang Y. C. (2000) 14-3-3zeta interacts with the alpha-chain of human interleukin 9 receptor. Biochem. J. 345, 741–747 [PMC free article] [PubMed] [Google Scholar]
- 61. Mazurkiewicz-Munoz A. M., Argetsinger L. S., Kouadio J. L., Stensballe A., Jensen O. N., Cline J. M., Carter-Su C. (2006) Phosphorylation of JAK2 at serine 523: a negative regulator of JAK2 that is stimulated by growth hormone and epidermal growth factor. Mol. Cell Biol. 26, 4052–4062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McCubrey J. A., May W. S., Duronio V., Mufson A. (2000) Serine/threonine phosphorylation in cytokine signal transduction. Leukemia 14, 9–21 [DOI] [PubMed] [Google Scholar]
- 63. Peti W., Nairn A. C., Page R. (2013) Structural basis for protein phosphatase 1 regulation and specificity. FEBS J. 280, 596–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Heroes E., Lesage B., Görnemann J., Beullens M., Van Meervelt L., Bollen M. (2013) The PP1 binding code: a molecular-lego strategy that governs specificity. FEBS J. 280, 584–595 [DOI] [PubMed] [Google Scholar]
- 65. Brockdorff J., Nielsen M., Dobson P., Geisler C., Röpke C., Svejgaard A., Odum N. (1997) Interleukin 2 induces a transient downregulation of protein phosphatase 1 and 2A activity in human T cells. Tissue Antigens 49, 228–235 [DOI] [PubMed] [Google Scholar]
- 66. Fujii H., Ogasawara K., Otsuka H., Suzuki M., Yamamura K., Yokochi T., Miyazaki T., Suzuki H., Mak T. W., Taki S., Taniguchi T. (1998) Functional dissection of the cytoplasmic subregions of the IL-2 receptor βac chain in primary lymphocyte populations. EMBO J. 17, 6551–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Imbert V., Rezzonico R., Reichenbach P., Nabholz M. (2002) Induction of interleukin-2 receptor α (IL-2Rα) expression by interleukin-2: important role of the interleukin-2 receptor β chain region between the two Stat5 docking sites. Eur. Cytokine Netw. 13, 331–339 [PubMed] [Google Scholar]
- 68. Lockyer H. M., Tran E., Nelson B. H. (2007) STAT5 is essential for Akt/p70S6 kinase activity during IL-2-induced lymphocyte proliferation. J. Immunol. 179, 5301–5308 [DOI] [PubMed] [Google Scholar]
- 69. Nyga R., Pecquet C., Harir N., Gu H., Dhennin-Duthille I., Régnier A., Gouilleux-Gruart V., Lassoued K., Gouilleux F. (2005) Activated STAT5 proteins induce activation of the PI 3-kinase/Akt and Ras/MAPK pathways via the Gab2 scaffolding adapter. Biochem. J. 390, 359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]