Background: Pituitary adenylate cyclase-activating peptide (PACAP) is a neurotrophic peptide involved in the development and function of the nervous system.
Results: PACAP targets RCAN1, a Down syndrome-related gene, via activation of the PKA-CREB pathway.
Conclusion: Proper expression of RCAN1 is necessary for neuronal differentiation.
Significance: Identification of RCAN1 as a PACAP target gene could contribute to the understanding of mechanisms in neurodevelopment.
Keywords: cAMP response element-binding protein (CREB), differentiation, neuron, neuropeptide, PKA, DSCR1, PACAP, RCAN1
Abstract
Pituitary adenylate cyclase-activating peptide (PACAP) is a neurotrophic peptide involved in a wide range of nervous functions, including development, differentiation, and survival, and various aspects of learning and memory. Here we report that PACAP induces the expression of regulator of calcineurin 1 (RCAN1, also known as DSCR1), which is abnormally expressed in the brains of Down syndrome patients. Increased RCAN1 expression is accompanied by activation of the PKA-cAMP response element-binding protein pathways. EMSA and ChIP analyses demonstrate the presence of a functional cAMP response element in the RCAN1 promoter. Moreover, we show that PACAP-dependent neuronal differentiation is significantly disturbed by improper RCAN1 expression. Our data provide the first evidence of RCAN1, a Down syndrome-related gene, as a novel target for control of the neurotrophic function of PACAP.
Introduction
Regulator of calcineurin 1 (RCAN1) has been identified as a protein product of the Down syndrome candidate region 1 (DSCR1) gene on human chromosome 21, the trisomy of which is associated with the phenotypic characteristics of Down syndrome (DS)2 (1). The RCAN1 gene consists of seven exons that are alternatively spliced and undergoes differential promoter usage to produce different isoforms (2, 3). Among the isoforms, RCAN1.4, encoded by exon 4, and RCAN1.1, encoded by exon 1, are the major isoforms that are differentially expressed in many tissues and cells, such as the CNS, skeletal muscle, and heart (1, 3). The expression of RCAN1 isoforms is differentially induced by various intracellular signaling pathways (4–6). The RCAN1.4 protein ranges from 25–29 kDa in size and is induced by diverse stimuli, including calcium, depolarization, and oxidative stress (2). After ischemic insult, up-regulation of RCAN1.4 in the peri-infarct cortex has also been reported (7). RCAN1.1, a 36- to 41-kDa protein, is up-regulated by glucocorticoid and dexamethasone in human leukemic cells via the glucocorticoid response element (8, 9). Chronic RCAN1 overexpression is associated with DS and Alzheimer disease, and RCAN1 deficiency has been reported in Huntington disease (2, 4, 10, 11). Several model systems that mimic the improper dosage of RCAN1 exhibit aberrations that resemble disease pathology (12–14). Mouse RCAN1 overexpression causes DS-like hippocampal deficits that alter learning and memory (13). Disturbance of brain function by a loss-of-function Nebula mutant, the Drosophila homolog, has been suggesting consistently that RCAN1 expression is important for nervous system development and function (14, 15). RCAN1 was named according to its capacity to regulate calcineurin, a Ca2+/calmodulin-dependent serine/threonine phosphatase, by inhibiting or activating its phosphatase activity (4).
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a neuropeptide that was originally isolated from ovine hypothalamic extracts on the basis of its capacity to stimulate cAMP synthesis in rat anterior pituitary cells (16). The sequence of PACAP has been remarkably conserved throughout evolution, suggesting that this peptide is involved in the regulation of important biological functions (17). In support of this role, the Drosophila homolog of mammalian PACAP has been implicated as essential for associative learning and memory processes (18). PACAP is expressed throughout the central and peripheral nervous systems and regulates neuronal survival, neurotransmitter phenotype, axonal growth, and growth cone attraction (19, 20). PACAP mediates its functions via binding to G protein-coupled receptor family members in both the developing and mature nervous systems (17). In the developing CNS, PACAP decreases the population of mitotic cells and promotes neuroblast differentiation (21). In the mature brain, PACAP has been reported to act as a neurohormone, a neuromodulator, and a neurotrophic factor and to increase cell survival following various neuronal injures (22, 23). Although the effect of PACAP through its G protein-coupled PAC1 receptor and the associated downstream signaling pathways have long been studied, little is known concerning the proteins that control the neurotrophic effects of PACAP.
In this report, we examined the possible effect of PACAP on the expression of the DS-related gene RCAN1. We found that PACAP induced RCAN1.4 expression through cAMP response element-binding protein (CREB) activation. We characterized the RCAN1 gene promoters and identified a functional cAMP response element (CRE). Furthermore, we found that constitutive alterations of RCAN1 expression prevented PACAP-dependent neuronal differentiation. Our data reveal a new regulatory target that controls the neurotrophic actions of PACAP via CREB activation.
Experimental Procedures
Materials and Expression Vectors
Anti-phospho-CREB (Ser-133), anti-CREB, anti-phospho-ERK, and anti-ERK antibodies were purchased from Cell Signaling Technology. Anti-Synapsin-1 antibody was purchased from Millipore. Anti-RCAN1 antibody was purchased from Sigma-Aldrich. Normal rabbit IgG and anti-GAPDH antibodies were purchased from Santa Cruz Biotechnology. PACAP38, actinomycin D, BAPTA-AM (1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)), and EGTA were purchased from Sigma-Aldrich. H-89 was purchased from Cayman. PD98059 and U0126 were purchased from Calbiochem. The expression vectors for pCG-CREB and pCG-CREB (S133A) were gifts from K. Saeki. The expression vector for A-CREB was a gift from D. D. Ginty. The RCAN1.4 gene was isolated from rat brain and was cloned into pcDNA. The series of pRS-RCAN1 shRNA expression vectors was purchased from Origene (Rockville, MD).
Mice
Ts65Dn mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained as described previously (24). The genotypes of the neonates were determined at birth using PCR followed by restriction endonuclease digestion as described previously (25). Briefly, DNA amplification was performed on genomic DNA extracted from tails of neonatal mice using the following primers: forward, 5′-AAATAGTAGCATCTCATGAGTG-3′; reverse, 5′-CATAGTGCATCTTAGACAAGC-3′. The amplified PCR fragments were digested with SacI (New England Biolabs) and visualized on a 2.0% agarose gel containing GelStar stain (Lonza Bioscience, Rockland, ME), resulting in three bands of 246, 175, and 71 bp in Ts65Dn mice and only two bands of 175 and 71 bp in littermate euploid controls. Ts65Dn neonates (P0-P1) and their littermate controls were then used for isolation of cortical neurons.
Primary Cultures of Cortical Neurons
Primary cortical neuron cultures were prepared from postnatal (P0-P1) ICR mice. Cortical hemispheres were dissected under sterile conditions, and the cells were dissociated with minimum Eagle's medium containing 0.2% trypsin. Cells were then plated onto poly-d-lysine (25 μg/ml)-coated 6-well plates for 2 h. The cultures were maintained in Neurobasal medium (Invitrogen) supplemented with 2% B27 (Invitrogen), glutamax (Invitrogen), and 1% penicillin-streptomycin.
Generation of Stable Cell Lines
PC12 cells were maintained in DMEM supplemented with 5% FBS, 10% horse serum, and 1% penicillin-streptomycin. Cells were plated in 60-mm dishes and transfected with 1 μg of each expression vector using the Lipofectamine 2000 method (Invitrogen). To obtain stable cell lines, transfected cells were selected in 250 μg/ml of G418-containing (for pcDNA-control and pcDNA-RCAN1.4) or puromycin-containing growth medium (for pRS-control and pRS-shRCAN1). The expression levels were immunoblotted with anti-RCAN1 antibody.
Cloning of the RCAN1.4 Promoter
A 1002-bp fragment of the 5′ flanking region of exon 4 of the RCAN1 gene (Gene ID no. ENSRNOG00000001979) was isolated from rat PC12 cells by genomic PCR, and the PCR products were inserted into the promoterless pGL3-basic luciferase vector (Promega, pGL3-RCAN1.4-luc). The 5′ deletion mutants of the RCAN1.4 promoter were produced from pGL3-RCAN1.4-luc using a PCR-based method. The putative transcription factor-binding sites in the 5′ flanking region of the RCAN1 gene were analyzed using TFSEARCH promoter analysis software.
EMSA
EMSA was performed according to the instructions of the manufacturer (Pierce Biotechnology). 4 μg of nuclear extract was incubated with biotin-labeled wild-type (5′-ACTAGGGTGTTGACGTCACCTCTTTCCA-3′) and mutant (5′-ACTAGGGTGTTGTTCTCACCTCTTTCCA-3′) CRE synthetic oligonucleotides. The reaction products were separated on a 6% non-denaturing polyacrylamide gel and transferred to a nylon membrane. After the oligos were fixed on the membrane by baking for 1 h at 80 °C, the membrane was incubated with a streptavidin-HRP mixture for 15 min. The membrane was then developed with a substrate solution and exposed on x-ray film.
ChIP Assays
ChIP analysis was performed using a ChIP assay kit (Millipore Corp.). Cells were cross-linked in 1% formaldehyde solution for 10 min at 37 °C. After washing with PBS, cells were resuspended in lysis buffer (10 mm Tris-Cl (pH 8.0), 140 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% SDS, 0.1% deoxycholate, 1 mm Na3VO4, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 10 mm NaF, and 0.2 mm phenylmethylsulfonyl fluoride). Cell lysates were sonicated eight times for 5 s on ice. Precleared supernatant was incubated with either anti-phospho-CREB or normal rabbit IgG antibodies overnight. Immunoprecipitated DNA was recovered by phenol/chloroform/isoamyl alcohol extraction and amplified by PCR with primers corresponding to the RCAN1 promoter region containing the CRE. The primer pair sequences were 5′-CTTCGAGGCTCAGCAAACCTTAG-3′ (forward) and 5′-CAGACAGGGACAAAGTGTAAGTT-3′ (reverse).
Quantitative Real-time PCR
Total RNA was converted into cDNA using Moloney Murine Leukemia Virus reverse transcriptase (Promega). Real-time PCR was performed using SYBR Green real-time PCR master mix (TOYOBO) according to the instructions of the manufacturer. The primer sequences were as follows: β-actin, 5′-AGAGGGAAATCGTGCGTGAC-3′ (forward) and 5′-CGATAGTGATGACCTGACCGT-3′ (reverse); RCAN1.1, 5′-CATCGCCTGTCACCTGGAC-3′ (forward) and 5′-GACAGGGGGTTGCTGAAGTT-3′ (reverse); and RCAN1.4, 5′-CTGTGTGGCAAACGGTGATG-3′ (forward) and 5′-GACAGGGGGTTGCTGAAGTT-3′ (reverse). All samples were run in triplicate and were normalized to β-actin. PCR amplification data were analyzed and quantified using CFX ManagerTM software version 3.0 (Bio-Rad).
Western Blot Analysis
The cells were lysed, and equal amounts of protein were separated by 10% SDS-PAGE. Proteins were then transferred to nitrocellulose membranes. The membranes were washed with Tris-buffered saline with 0.05% Tween 20, and the signals were then visualized using ECL reagent.
Quantitative Analysis of Neurite Outgrowth
Four days after treatment with PACAP, images of PC12 cells were acquired randomly on a computer-assisted inverted microscope. Differentiation was evaluated by counting the number of neurite-bearing cells and calculating the percentage out of the total number of cells in a field. Cells with at least one neurite with a length greater than the diameter of the cell body were counted as neurite-bearing cells.
Reporter Gene Assays
PC12 cells were transfected with the indicated expression vectors using the Lipofectamine 2000 method (Invitrogen). Luciferase activity was measured with a luciferase assay system (Promega) and normalized for transfection efficiency using a β-galactosidase-expressing vector (pCMV5.lacZ) and the Galacto-Star system (PerkinElmer Life Sciences).
Statistics
Densitometric scans of Western blot analyses were quantified using ImageJ software. Values are expressed as the mean ± S.D. of three independent experiments. Statistical significance between two groups was performed using Student's t test. Differences between two means with p < 0.05 were considered to be significant.
Results
PACAP Induces RCAN1.4 Protein Expression
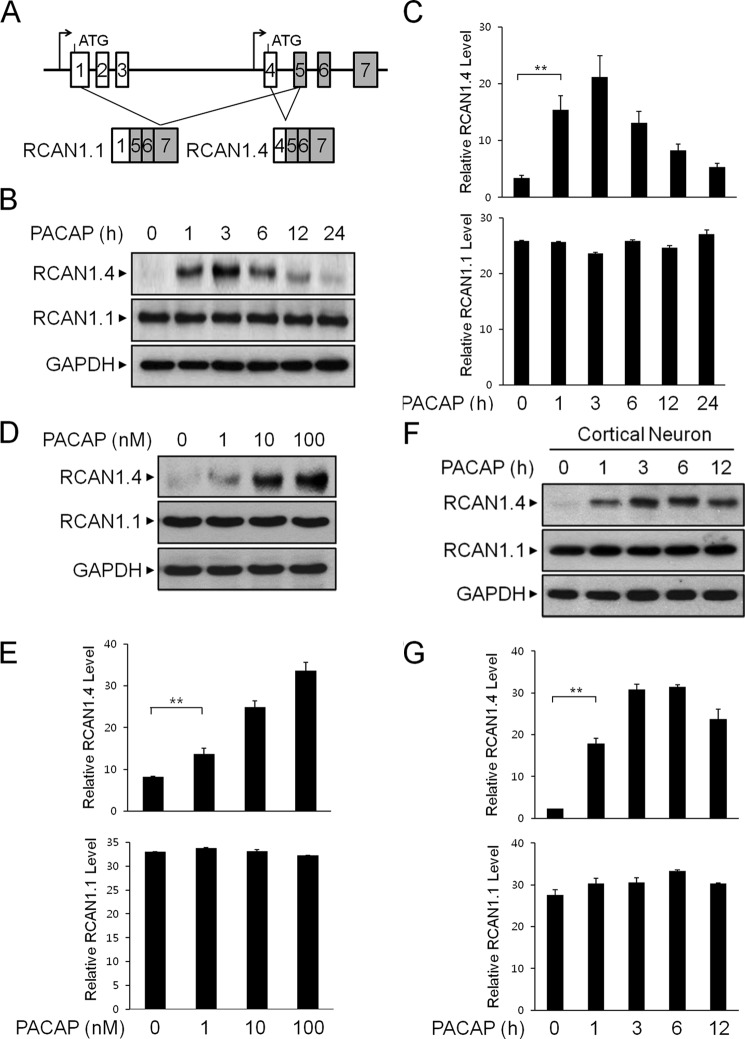
The RCAN1 gene has been shown to undergo differential promoter usage to produce different isoforms (Fig. 1A) (3). To investigate whether the neurotrophic peptide PACAP could affect the steady-state level of RCAN1, neuronal PC12 cells were treated with PACAP, and the changes in RCAN1 expression were examined at various time points up to 24 h. By immunoblot analysis, we observed that the application of 10 nm PACAP induced a time-dependent increase in RCAN1.4 protein expression (Fig. 1, B and C). The increase of RCAN1.4 protein expression in response to PACAP reached a maximum level 3 h after treatment and decreased thereafter (Fig. 1, B and C). However, RCAN1.1 levels remained constant throughout PACAP treatment. The PACAP-mediated increase of RCAN1.4 protein expression was observed with concentrations of PACAP of up to 100 nm (Fig. 1, D and E). The increase in RCAN1.4 expression in response to PACAP was observed consistently in primary cortical neurons, confirming the effect of PACAP on RCAN1.4 protein induction in neurons (Fig. 1, F and G). Collectively, these results indicate that PACAP can effectively induce RCAN1.4 protein expression.
FIGURE 1.
RCAN1.4 protein expression is induced by PACAP. A, use of different promoters to produce RCAN1 isoforms. B and C, PC12 cells were treated with PACAP (10 nm) for the indicated times. The cell extracts were immunoblotted with anti-RCAN1 and anti-GAPDH antibodies (B) and then quantified (C). D and E, PC12 cells were treated with the indicated concentrations for 3 h. The cell extracts were immunoblotted with anti-RCAN1 and anti-GAPDH antibodies (D) and then quantified (E). F and G, mouse primary cortical neurons were treated with PACAP (10 nm) for the indicated times. The cell extracts were immunoblotted with anti-RCAN1 and anti-GAPDH antibodies (F) and then quantified (G). Data are mean ± S.D. of three independent experiments. **, p < 0.01.
PACAP Induces RCAN1.4 Transcription
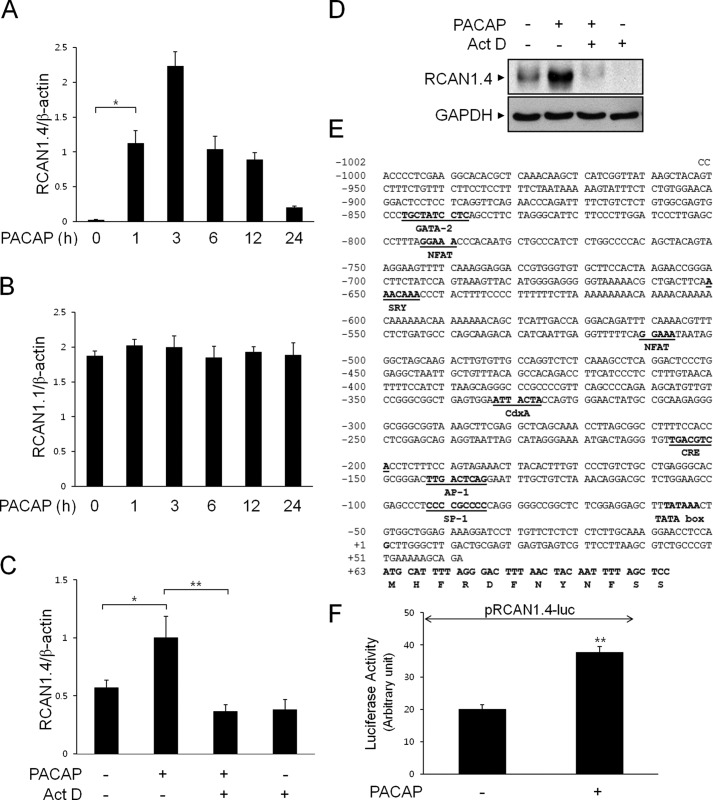
We next analyzed the expression of RCAN1 transcripts in response to PACAP using quantitative real-time PCR (Fig. 2, A and B). Consistently, we found that PACAP treatment caused a time-dependent accumulation of RCAN1.4 but not RCAN1.1 transcripts (Fig. 2, A and B). To examine the mechanisms for increased RCAN1.4 expression in response to PACAP, we first examined whether RCAN1 mRNA induction was dependent on transcription. For this experiment, PC12 cells were preincubated with actinomycin D for 10 min to prevent de novo RNA synthesis prior to PACAP treatment, and RCAN1.4 mRNA levels were assessed by real-time PCR (Fig. 2C). As shown in Fig. 2C, RCAN1.4 mRNA induction in response to PACAP was attenuated significantly by pretreatment with actinomycin D, suggesting that PACAP-mediated RCAN1.4 induction is due to increased gene transcription. The involvement of RCAN1.4 transcription in response to PACAP was further supported by inhibition of RCAN1.4 protein expression with actinomycin D (Fig. 2D).
FIGURE 2.
RCAN1.4 transcription is induced by PACAP. A and B, PC12 cells were treated with PACAP (10 nm) for the indicated times, and RCAN1.4 (A) and RCAN1.1 (B) mRNA levels were measured by quantitative real-time PCR. *, p < 0.05. C and D, cells were pretreated with or without actinomycin D (Act D, 0.5 μg/ml) for 30 min, followed by treatment with PACAP (10 nm) for 3 h. mRNA levels of RCAN1 were measured by quantitative real-time PCR (C), and the cell extracts were immunoblotted with anti-RCAN1 and anti-GAPDH antibodies (D). E, characterization of the rat RCAN1.4 gene promoter. F, cells were transfected with the RCAN1.4-luciferase reporter construct (pRCAN1.4-luc). After 24 h, cells were treated with or without PACAP (10 nm) for 20 h and analyzed for luciferase activity. The results were normalized to β-galactosidase activity and are presented as mean ± S.D. of three independent experiments. **, p < 0.01.
To investigate PACAP-induced RCAN1.4 transcription in PC12 cells, we isolated a 1002-bp fragment of the 5′ flanking region of exon 4 of the rat RCAN1 gene from genomic DNA by PCR (Fig. 2E). The PCR products were then inserted into the promoterless luciferase reporter plasmid pGL3-basic. The resulting RCAN1.4-luc plasmid was transiently transfected into PC12 cells, and reporter activity was measured (Fig. 2F). As shown in Fig. 2F, PACAP induced increased RCAN1.4 promoter activity compared with untreated cells. These results demonstrate that transcriptional activation is related to RCAN1.4 up-regulation in response to PACAP.
PACAP Induces RCAN1.4 Expression via PKA-dependent Transcriptional Activation
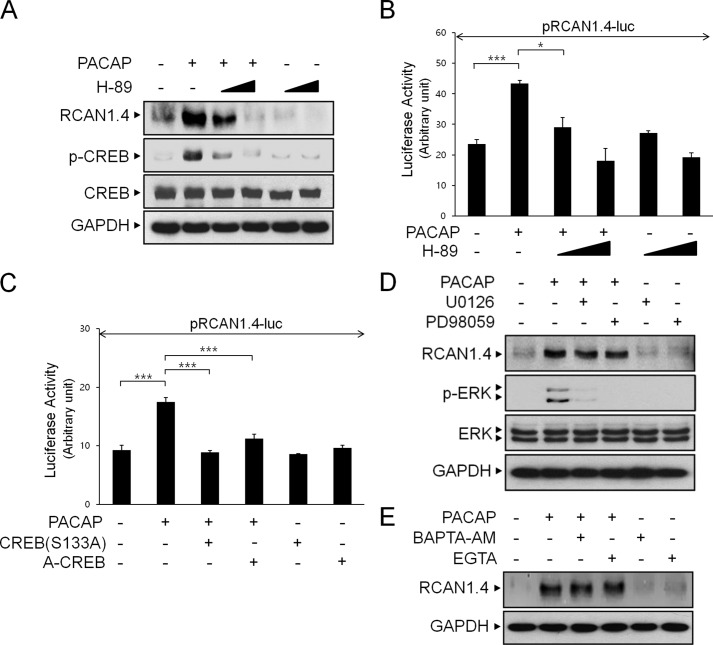
Previous studies have identified a variety of signaling cascades triggered by PACAP (26). Because PKA is one of the major intracellular effectors activated by PACAP, we set out to determine whether PKA is required for the PACAP-induced increase in RCAN1.4 expression. As shown in Fig. 3A, pharmacological inhibition of PKA with a well known inhibitor, H-89, prevented PACAP-induced RCAN1.4 protein expression in a dose-dependent manner. We then examined whether PKA mediated the stimulatory effect of PACAP on RCAN1.4 promoter activity. We consistently observed that PKA inhibition significantly prevented PACAP-induced RCAN1.4 transcription (Fig. 3B).
FIGURE 3.
PACAP-induced RCAN1.4 expression is dependent on the PKA-CREB signaling pathway. A, PC12 cells were pretreated with or without H-89 (10 or 50 μm) for 30 min, followed by treatment with PACAP (10 nm) for 3 h. The cell extracts were immunoblotted with anti-RCAN1, anti-phospho-CREB, anti-CREB, and anti-GAPDH antibodies. B and C, cells were transfected with the RCAN1.4-luciferase reporter construct (pRCAN1.4-luc) alone or together with the indicated expression vector. After 24 h, cells were treated with PACAP in the presence or absence of H-89 (10 or 50 μm) for 20 h. The results were normalized to β-galactosidase activity and are presented as mean ± S.D. of three independent experiments. *, p < 0.05; ***, p < 0.001. D and E, cells were pretreated with or without U0126 (10 μm), PD98059 (100 μm), BAPTA-AM (20 μm), and EGTA (500 μm) for 30 min, followed by treatment with PACAP (10 nm) for 3 h. The cell extracts were immunoblotted with anti-RCAN1, anti-phospho-ERK, anti-ERK, and anti-GAPDH antibodies.
To further determine whether PACAP requires PKA signaling to increase RCAN1.4 promoter activity, we examined the effect of CREB, one of the downstream targets of PKA. For this experiment, we expressed dominant-negative mutant CREB, such as S133A-CREB and A-CREB, which are known to prevent the transcriptional activation of CREB, prior to PACAP treatment in PC12 cells (27) (Fig. 3C). As shown in Fig. 3C, S133A-CREB and A-CREB expression significantly prevented increased RCAN1.4 reporter activity in response to PACAP. Taken together, these results indicate that the PKA-CREB pathway mediates the stimulatory effect of PACAP on RCAN1.4 expression.
The MEK-ERK pathways have been shown to be activated by PACAP (28, 29). We next determined whether the MEK-ERK signaling pathways were involved in the PACAP-induced RCAN1 expression. As shown in Fig. 3D, although both U0126 and PD98059 efficiently blocked PACAP-induced ERK phosphorylation, the increased RCAN1 expression level in response to PACAP was not altered, indicating that the MEK-ERK pathways are not involved in PACAP-dependent RCAN1 induction.
PACAP stimulates the calcium current by inducing both Ca2+ release from intracellular stores and Ca2+ influx from extracellular fluid in a variety of cells (30). We next determined whether the increased calcium level in response to PACAP is crucial for RCAN1 induction. For this experiment, cells were preincubated with BAPTA-AM and EGTA, Ca2+ chelators (Fig. 3E). As shown in Fig. 3E, neither of these reagents had any effect on PACAP-dependent RCAN1 induction, indicating that increased calcium is not involved in RCAN1 induction.
CREB Binds to the RCAN1 Promoter in Vivo
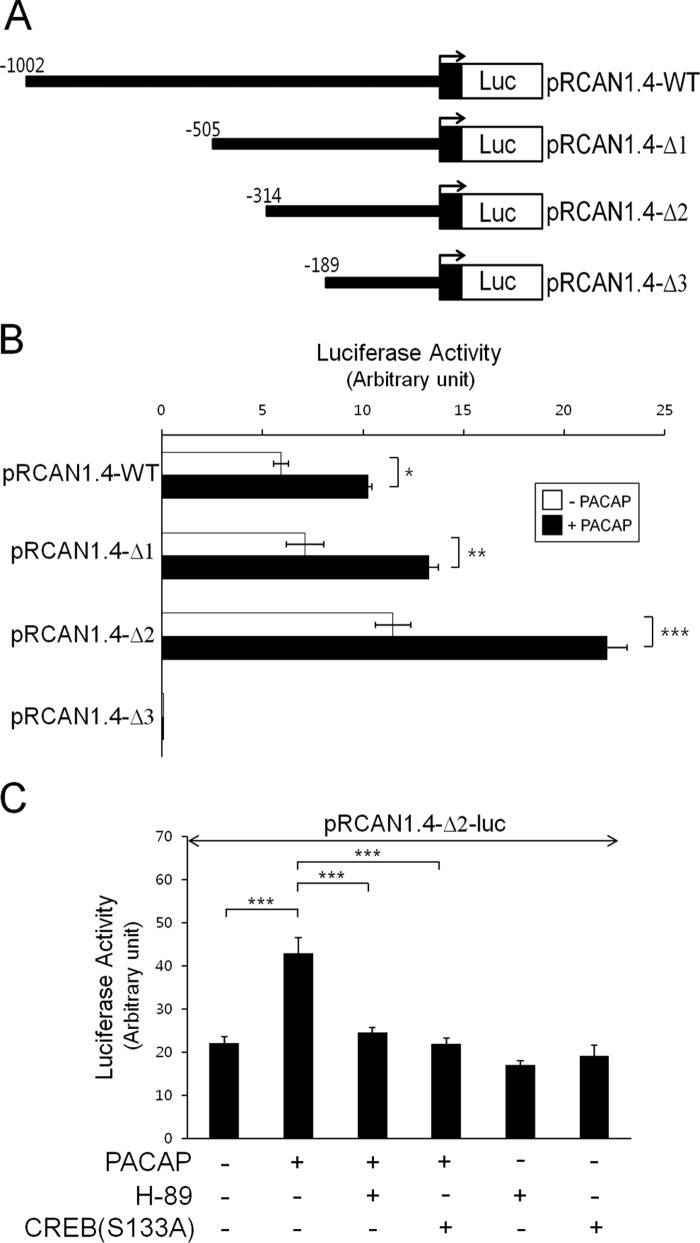
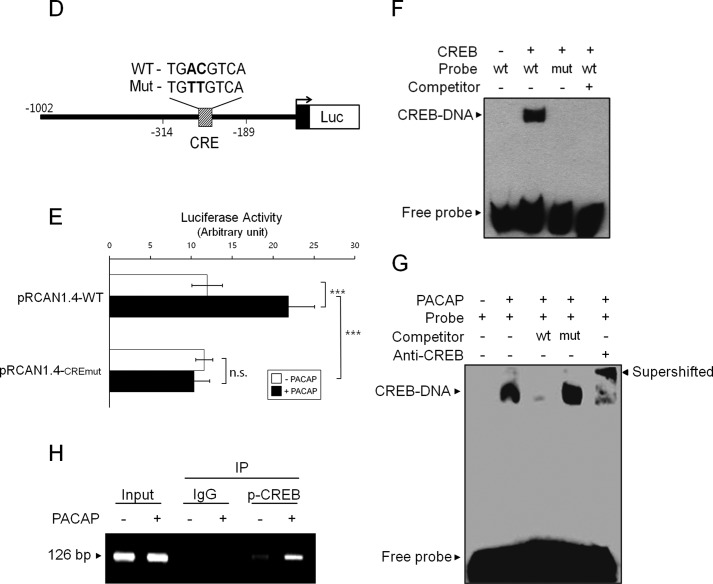
To identify the PACAP-responsive region of the RCAN1 promoter, we constructed a series of 5′-truncated luciferase reporter plasmids containing different upstream deletions of the RCAN1 promoter (Fig. 4A). The pRCAN1.4-Δ1, pRCAN1.4-Δ2, and pRCAN1.4-Δ3 inserts are the fragments of the RCAN1 promoter from −505, −314, and −189 bp to +1 bp, respectively (Fig. 4A). Reporter activity measured from the transient transfections with these plasmids into PC12 cells revealed that the promoter region between −314 to +1 bp (pRCAN1.4-Δ2) had a higher basal activity compared with other constructs and that PACAP augmented its promoter activity (Fig. 4B). The deletion of an additional 125 bp from −314 had no inductive effect on promoter activity in response to PACAP (pRCAN1.4-Δ3) (Fig. 4B). Therefore, it appears that the regulatory elements between nucleotides −314 and −189 of the RCAN1.4 promoter are responsible for the PACAP-dependent induction of its activity.
FIGURE 4.
PACAP induces CREB binding to the RCAN1 promoter. A, the 5′-deletion RCAN1.4-luciferase (Luc) reporter constructs. B, C, and E, PC12 cells were transfected with the indicated expression vectors. After 24 h, cells were treated with or without PACAP (10 nm) in the presence or absence of H-89 (10 μm) for 20 h and analyzed for luciferase activity. The results were normalized to β-galactosidase activity and are presented as mean ± S.D. of three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant. D, the CRE mutant RCAN1.4-luciferase reporter constructs. F, DNA-binding activity was analyzed by EMSA using nuclear extracts prepared from PC12 cells transfected with either an empty vector or a CREB expression vector. G, EMSA was performed using nuclear extracts from PC12 cells treated with or without PACAP (10 nm). The binding complex was incubated in the presence or absence of anti-CREB antibody. H, ChIP was performed with either normal IgG or anti-phospho-CREB (Ser-133) antibodies. PC12 cells were treated with or without PACAP (10 nm) for 30 min prior to the ChIP assay. The immunoprecipitated (IP) DNA was amplified by PCR using primers covering CRE in the RCAN1.4 promoter.
To verify the presence of a PACAP-responsive element between nucleotides −314 and −189 of the RCAN1 promoter, we examined whether PACAP-regulated reporter activity of pRCAN1.4-Δ2 occurred via activation of the PKA-CREB pathway. As shown in Fig. 4C, the PACAP-responsive reporter activity of this region was blocked significantly by the PKA inhibitor H-89. The presence of a PACAP-regulated element in this reporter fragment was further confirmed by the suppression of PACAP-dependent reporter activity with overexpression of the inactive mutant S133A-CREB (Fig. 4C).
To define the promoter elements responsible for mediating the effects of PACAP on RCAN1 expression, a computer-based search of this 125-bp region for transcription factor binding sites was performed using TFSEARCH, and a putative CRE was identified. To investigate the role of this putative CRE in PACAP-mediated transactivation of the RCAN1 promoter, we constructed a CRE mutant reporter plasmid by site-directed mutagenesis (Fig. 4D). We found that mutation of the CRE site led to the complete inhibition of PACAP-induced RCAN1 promoter activity (Fig. 4E). These results indicate that the CRE site located between −314 and −189 bp is responsible for PACAP-induced RCAN1 promoter activity (Fig. 4E).
To determine whether CREB is able to bind to this CRE on the RCAN1 promoter, we performed EMSA using a labeled oligonucleotide probe corresponding to the RCAN1 sequence from −314 to −189 bp. As shown in Fig. 4F, we observed a DNA-protein complex in the nuclear extracts obtained from CREB-transfected PC12 cells, and this complex was inhibited by the addition of a 10-fold molar excess of unlabeled self-competitor (Fig. 4F). However, we could not detect any complex using the mutant CRE oligonucleotide probe from the same nuclear extracts, raising the possibility that CREB is able to bind to this RCAN1 promoter region.
We next determined the binding of CREB in response to PACAP in this region (Fig. 4G). A labeled wild-type probe containing the CRE region in the RCAN1 promoter was incubated with nuclear extracts derived from PC12 cells treated with or without PACAP. As shown in Fig. 4G, a DNA-protein complex was observed in PACAP-treated nuclear extracts and was inhibited by the addition of unlabeled wild-type CRE oligonucleotides but not by mutant CRE (Fig. 4G). Moreover, the complex was supershifted by the addition of anti-CREB antibodies, indicating that PACAP mediated CREB binding in this CRE region (Fig. 4G).
To verify whether the enhanced expression of RCAN1 mRNA in response to PACAP was correlated with physiological binding of CREB to the RCAN1 promoter in vivo, we performed a ChIP assay (Fig. 4H). The chromatins immunoprecipitated with anti-phospho-CREB antibodies were amplified by PCR using primers covering the putative CRE site in the RCAN1 promoter. As shown in Fig. 4H, the amount of phospho-CREB bound to the CRE was enhanced significantly in cells treated with PACAP compared with control cells. These findings indicate that PACAP activates RCAN1 gene expression by binding to the CRE of the RCAN1 promoter in vivo.
Altered RCAN1 Expression Prevents PACAP-induced Neuritogenesis
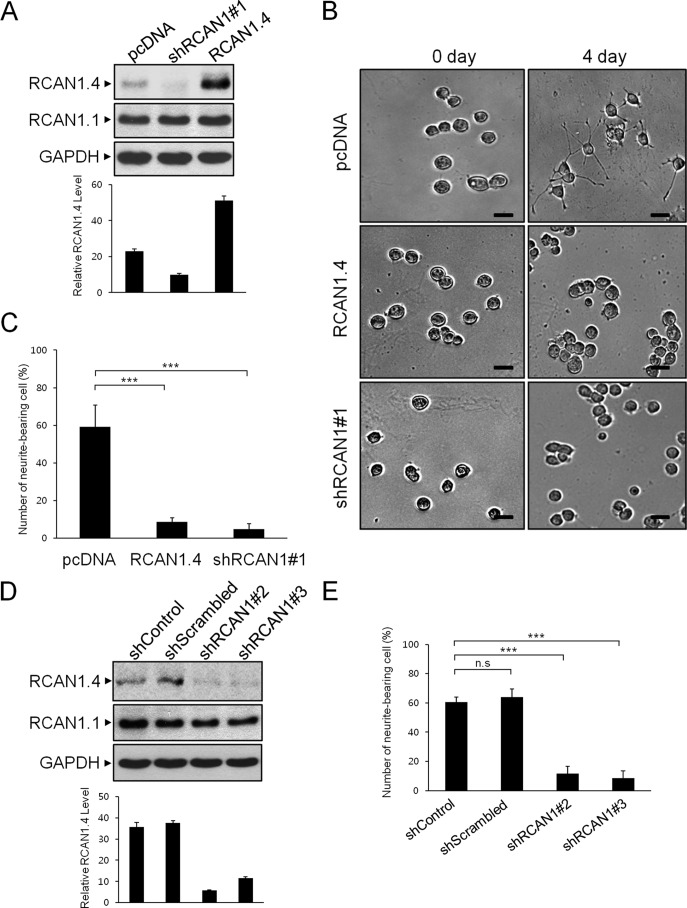
To investigate the functional role of enhanced RCAN1 expression by PACAP, we generated PC12 cells with either stably knocked down endogenous RCAN1 expression or stably overexpressed RCAN1. Protein expression in PC12 cells with stably knocked down or overexpressed RCAN1 was analyzed by Western blot (Fig. 5A). Because PACAP is known to induce PC12 cell differentiation, we investigated whether altered RCAN1 expression could affect the neurotrophic activities of PACAP. As shown in Fig. 5B, treatment with PACAP for 4 days resulted in neurite formation, a hallmark of neuronal differentiation, in mock-transfected control PC12 cells. In contrast, PACAP-dependent neurite extensions were inhibited significantly in cells with both stably knocked down and stably overexpressed RCAN1. Quantification of RCAN1 knockdown and RCAN1-overexpressed cells revealed a significant reduction in the number of neurite-bearing cells compared with control cells following PACAP stimulation (Fig. 5C). The perturbation of PACAP-dependent neurite outgrowth was observed consistently in cells expressing two other kinds of shRNA vectors targeting RCAN1.4, precluding its off-target effects (Fig. 5, D and E). These results indicate that altered RCAN1 expression prevents PACAP-dependent neurotrophic actions in PC12 cells.
FIGURE 5.
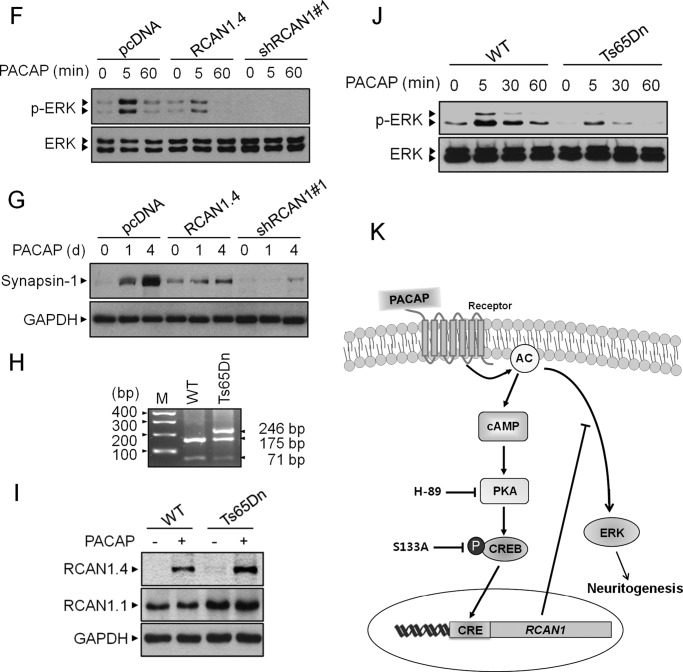
RCAN1 modulates PACAP-induced neuronal differentiation. A, PC12 cells were transfected with an empty (pcDNA), RCAN1 knockdown (shRCAN1#1), or RCAN1 overexpression (RCAN1.4) vector, and transfected cells were selected either in G418- or puromycin-containing growth medium. Stable clones were identified by immunoblotting using an anti-RCAN1 antibody. B, phase-contrast images of PC12 cells treated with PACAP (10 nm) on days 0 and 4. Scale bars = 30 μm. C, differentiation was evaluated by calculating the percentage of neurite-bearing cells versus the total number of cells in a field. Cells with at least one neurite with a length greater than the diameter of the cell body were counted as neurite-bearing cells. The results are the mean ± S.D. of three independent experiments. D, PC12 cells were transfected with the indicated shRNA expression vectors, and the protein extracts were immunoblotted with anti-RCAN1 antibody. E, differentiation was evaluated as in C. ***, p < 0.001; n.s., not significant. F and G, stable cells were treated with PACAP (10 nm) for the indicated times, and the cell extracts were immunoblotted with anti-phospho ERK, anti-ERK, anti-Synapsin-1, and GAPDH antibodies. H, genotyping results of a WT and a Ts65Dn trisomy mouse. M, marker. I and J, the cortical neurons of a wild-type and a TS65Dn mouse were treated with PACAP (10 nm), and the cell extracts were immunoblotted with the indicated antibodies. K, schematic summarizing the participation of RCAN1.4 in PACAP-mediated neuronal differentiation.
Numerous pieces of evidence suggested that the activation of ERK)/MAPK mediates the neurotrophic effects of PACAP in pheochromocytoma 12 (PC12) cells (31, 32). We next examined the effect of PACAP on of ERK1/2 phosphorylation levels in these cells. As shown in Fig. 5F, treatment with PACAP induced ERK1/2 phosphorylation after 5 min of stimulation in mock-transfected control cells. However, the effect of PACAP on inducing ERK activation was inhibited significantly in cells with both stably knocked down and stably overexpressed RCAN1 (Fig. 5F).
To further analyze the effects of RCAN1 expression on neuronal differentiation, we examined the expression of Synapsin-1, a target for synapse formation during neuronal differentiation (Fig. 5G). Western blot analysis indicated that Synapsin-1 levels were increased in response to PACAP in mock-transfected control cells (Fig. 5G). PACAP-induced Synapsin-1 expression was decreased consistently and significantly in cells with both stably knocked down and stably overexpressed RCAN1.
To develop the relevance of RCAN1 expression in DS pathogenesis, we used the mouse trisomy 21 model, Ts65Dn (24). Consistent with the previous report (25), genotyping results showed three bands of 246, 175, and 71 bp in Ts65Dn and two bands of 175 and 71 bp in a littermate euploid control mouse (Fig. 5H). In the neonatal primary cortical neuron cultures from these mice, we observed that the level of RCAN1.4 expression in response to PACAP was enhanced in Ts65Dn compared with that of the euploid control (Fig. 5I). Furthermore, PACAP-dependent ERK activation was inhibited in Ts65Dn mouse cortical neurons (Fig. 5J). Taken together, these results suggest that constitutive alteration of RCAN1 expression disrupts PACAP-dependent neurotrophic effects, thereby raising the physiological relevance of this study in DS pathogenesis (Fig. 5K).
Discussion
RCAN1 (or DSCR1) is widely expressed in the CNS during early embryonic development and is later restricted to regions with differentiating neurons (33). Nebula, the Drosophila homologue of RCAN1, is up-regulated in the head during development (2). The induction of RCAN1 expression by various signaling pathways regulates many cellular functions. For example, transient RCAN1 induction can protect against acute stress conditions, including oxidative stress (34, 35). Additionally, RCAN1 induction through calcium signaling has been implicated in cardiac valve formation and skeletal muscle hypertrophy (2, 36). However, chronic RCAN1 overexpression is associated with DS and Alzheimer disease, and RCAN1 deficiency has been reported in Huntington disease (2, 4, 10, 11). Moreover, several model systems that mimic improper RCAN1 expression showed aberrations resembling disease pathology (12–14). Furthermore, overexpression of mouse RCAN1 caused DS-like hippocampal deficits that alter learning and memory (13). Consistently, disturbance of brain function through Nebula loss-of-function and overexpression mutants has been reported in Drosophila (14, 15). Prolonged RCAN1 overexpression can cause severe metabolic disturbances via mitochondrial dysfunction and autophagy (37, 38). Altered RCAN1 expression regulated dendritic spine morphology and synaptic activity by binding to the fragile X mental retardation protein, leading to intellectual disability (39). Accordingly, our studies focusing on RCAN1 knockdown and overexpression showed significant impairments in PACAP-dependent neuronal differentiation and demonstrated the physiological relevance of this study in DS pathogenesis. Therefore, the proper regulation of RCAN1 expression might be involved in normal brain functions such as neurogenesis and neurodevelopment.
The effect of PACAP on the induction of RCAN1 expression occurred through the canonical PKA-dependent intracellular signaling pathway. Indeed, we identified a functional CREB-binding site in the RCAN1 promoter. In the brain, CREB is a key factor that regulates neural functions in differentiation, learning, memory, and synaptic plasticity by altering the expression of a variety of target genes through the interaction with CRE within their promoters (40, 41, 42–44). These genes include a neurotransmitter synthesis enzyme, neurotransmitters such as NGF and BDNF, and the neurotransmitter receptor subunit GluR1 (40, 45–47). Together with these findings, CREB-mediated RCAN1 transcription is likely critical to maintaining normal brain function.
Whether the effect of RCAN1 on neuronal differentiation in response to PACAP is direct or indirect remains unclear. RCAN1 encodes a repeated SP motif, a putative DNA binding domain, and a proline-rich region with the characteristics of a SH3 domain ligand (1, 12, 48). These features suggest that RCAN1 could be involved in transcriptional regulation and signal transduction in neuronal differentiation through its physical association with diverse partners. In support of this notion, several important neuronal differentiation regulators, including GSK-3, PKA, and MAPK, have been reported to bind and phosphorylate RCAN1 in vitro and in vivo (12, 48, 49).
Because RCAN1 was first identified as an enzymatic regulator of the serine/threonine phosphatase calcineurin, RCAN1 may indirectly influence a wide range of calcineurin-regulated phosphorylations. Calcineurin has been implicated in diverse nervous system signaling processes by altering certain substrate phosphorylation states. For example, the affinity of MAP2 and tau for microtubules, which is important for neurite outgrowth and axonal transport, increased following calcineurin activation (50). Calcineurin also regulates neurotransmitter release and vesicle recycling through the dephosphorylation of synapsin I and dynamin I along with amphiphysin I and II (51–53). In murine and human ES cells, proper regulation of calcineurin activity is required for neural differentiation (54). In addition to its direct effects on phosphorylation, calcineurin also regulated axon terminal remodeling by regulating nuclear factor of activated T cells-dependent gene transcription at the initial stage of synapse formation (55). Therefore, RCAN1 would be responsible for the proper regulation of calcineurin signaling pathways, which is necessary for differentiation. Accordingly, Cabin1, another calcineurin regulator, is expressed in neurons undergoing axon growth and regulated synaptogenesis (56). Therefore, further characterization of the mechanisms by which RCAN1 controls neuronal differentiation may help to elucidate DS-related pathogeneses such as brain development, mental retardation, and Alzheimer disease. Our observations showing RCAN1 as a PACAP-responsive gene are relevant for the development of in vivo therapeutic targets for the maintenance of neural functions.
DS is the most prevalent developmental disorder caused by the triplication of human chromosome 21 (HSA21). DS is characterized by a combination of several clinical abnormalities such as mental retardation, defects in neuronal differentiation, learning disabilities, cardiovascular defects, hypotonia, and dementia. The gene dosage effect in HSA21 is believed to play a significant role in determining the phenotypic features of DS. Therefore, the proper regulation of RCAN1 expression might be involved in normal brain functions such as neurogenesis and neurodevelopment.
Author Contributions
E. H. L. performed most of the experiments. S. S. K. designed and cloned all the constructs. S. L. and K. H. B. characterized and maintained TS65Dn mice. S. R. S. designed the study and wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by Ministry of Education, Science, and Technology Grants 2009-0065231 and 2010-0023815. This work was also a result of study on the Leaders Industry-University Cooperation Project supported by the Ministry of Education. The authors declare that they have no conflicts of interest with the contents of this article.
- DS
- Down syndrome
- PACAP
- pituitary adenylate cyclase-activating peptide
- CREB
- cAMP response element-binding protein
- CRE
- cAMP response element
- P
- postnatal day.
References
- 1. Fuentes J. J., Pritchard M. A., Planas A. M., Bosch A., Ferrer I., Estivill X. (1995) A new human gene from the Down syndrome critical region encodes a proline-rich protein highly expressed in fetal brain and heart. Hum. Mol. Genet. 4, 1935–1944 [DOI] [PubMed] [Google Scholar]
- 2. Harris C. D., Ermak G., Davies K. J. (2005) Multiple roles of the DSCR1 (Adapt78 or RCAN1) gene and its protein product calcipressin 1 (or RCAN1) in disease. Cell Mol. Life Sci. 62, 2477–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fuentes J. J., Pritchard M. A., Estivill X. (1997) Genomic organization, alternative splicing, and expression patterns of the DSCR1 (Down syndrome candidate region 1) gene. Genomics 44, 358–361 [DOI] [PubMed] [Google Scholar]
- 4. Fuentes J. J., Genescà L., Kingsbury T. J., Cunningham K. W., Pérez-Riba M., Estivill X., de la Luna S. (2000) DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum. Mol. Genet. 9, 1681–1690 [DOI] [PubMed] [Google Scholar]
- 5. Crawford D. R., Leahy K. P., Abramova N., Lan L., Wang Y., Davies K. J. (1997) Hamster adapt78 mRNA is a Down syndrome critical region homologue that is inducible by oxidative stress. Arch. Biochem. Biophys. 342, 6–12 [DOI] [PubMed] [Google Scholar]
- 6. U M., Shen L., Oshida T., Miyauchi J., Yamada M., Miyashita T. (2004) Identification of novel direct transcriptional targets of glucocorticoid receptor. Leukemia 18, 1850–1856 [DOI] [PubMed] [Google Scholar]
- 7. Cho K. O., Kim Y. S., Cho Y. J., Kim S. Y. (2008) Upregulation of DSCR1 (RCAN1 or Adapt78) in the peri-infarct cortex after experimental stroke. Exp. Neurol. 212, 85–92 [DOI] [PubMed] [Google Scholar]
- 8. Hirakawa Y., Nary L. J., Medh R. D. (2009) Glucocorticoid evoked upregulation of RCAN1–1 in human leukemic CEM cells susceptible to apoptosis. J. Mol. Signal. 4, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagao K., Iwai Y., Miyashita T. (2012) RCAN1 is an important mediator of glucocorticoid-induced apoptosis in human leukemic cells. PLoS ONE 7, e49926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ermak G., Morgan T. E., Davies K. J. (2001) Chronic overexpression of the calcineurin inhibitory gene DSCR1 (Adapt78) is associated with Alzheimer's disease. J. Biol. Chem. 276, 38787–38794 [DOI] [PubMed] [Google Scholar]
- 11. Ermak G., Hench K. J., Chang K. T., Sachdev S., Davies K. J. (2009) Regulator of calcineurin (RCAN1–1L) is deficient in Huntington disease and protective against mutant huntingtin toxicity in vitro. J. Biol. Chem. 284, 11845–11853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rothermel B., Vega R. B., Yang J., Wu H., Bassel-Duby R., Williams R. S. (2000) A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J. Biol. Chem. 275, 8719–8725 [DOI] [PubMed] [Google Scholar]
- 13. Martin K. R., Corlett A., Dubach D., Mustafa T., Coleman H. A., Parkington H. C., Merson T. D., Bourne J. A., Porta S., Arbonés M. L., Finkelstein D. I., Pritchard M. A. (2012) Over-expression of RCAN1 causes Down syndrome-like hippocampal deficits that alter learning and memory. Hum. Mol. Genet. 21, 3025–3041 [DOI] [PubMed] [Google Scholar]
- 14. Chang K. T., Min K. T. (2009) Upregulation of three Drosophila homologs of human chromosome 21 genes alters synaptic function: implications for Down syndrome. Proc. Natl. Acad. Sci. U.S.A. 106, 17117–17122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang K. T., Shi Y. J., Min K. T. (2003) The Drosophila homolog of Down's syndrome critical region 1 gene regulates learning: implications for mental retardation. Proc. Natl. Acad. Sci. U.S.A. 100, 15794–15799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miyata A., Arimura A., Dahl R. R., Minamino N., Uehara A., Jiang L., Culler M. D., Coy D. H. (1989) Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 164, 567–574 [DOI] [PubMed] [Google Scholar]
- 17. Vaudry D., Gonzalez B. J., Basille M., Yon L., Fournier A., Vaudry H. (2000) Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol. Rev. 52, 269–324 [PubMed] [Google Scholar]
- 18. Feany M. B., Quinn W. G. (1995) A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science 268, 869–873 [DOI] [PubMed] [Google Scholar]
- 19. Gonzalez B. J., Basille M., Vaudry D., Fournier A., Vaudry H. (1997) Pituitary adenylate cyclase-activating polypeptide promotes cell survival and neurite outgrowth in rat cerebellar neuroblasts. Neuroscience 78, 419–430 [DOI] [PubMed] [Google Scholar]
- 20. Villalba M., Bockaert J., Journot L. (1997) Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J. Neurosci. 17, 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu N., DiCicco-Bloom E. (1997) Pituitary adenylate cyclase-activating polypeptide is an autocrine inhibitor of mitosis in cultured cortical precursor cells. Proc. Natl. Acad. Sci. U.S.A. 94, 3357–3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dickson L., Finlayson K. (2009) VPAC and PAC receptors: from ligands to function. Pharmacol. Ther. 121, 294–316 [DOI] [PubMed] [Google Scholar]
- 23. Fahrenkrug J. (2006) PACAP: a multifacetted neuropeptide. Chronobiol. Int. 23, 53–61 [DOI] [PubMed] [Google Scholar]
- 24. Roper R. J., VanHorn J. F., Cain C. C., Reeves R. H. (2009) A neural crest deficit in Down syndrome mice is associated with deficient mitotic response to Sonic hedgehog. Mech. Dev. 126, 212–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lorenzi H., Duvall N., Cherry S. M., Reeves R. H., Roper R. J. (2010) PCR prescreen for genotyping the Ts65Dn mouse model of Down syndrome. BioTechniques 48, 35–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vaudry D., Falluel-Morel A., Bourgault S., Basille M., Burel D., Wurtz O., Fournier A., Chow B. K., Hashimoto H., Galas L., Vaudry H. (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 61, 283–357 [DOI] [PubMed] [Google Scholar]
- 27. Ahn S., Olive M., Aggarwal S., Krylov D., Ginty D. D., Vinson C. (1998) A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol. Cell. Biol. 18, 967–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sakai Y., Hashimoto H., Shintani N., Katoh H., Negishi M., Kawaguchi C., Kasai A., Baba A. (2004) PACAP activates Rac1 and synergizes with NGF to activate ERK1/2, thereby inducing neurite outgrowth in PC12 cells. Brain Res. Mol. Brain Res. 123, 18–26 [DOI] [PubMed] [Google Scholar]
- 29. Moroo I., Tatsuno I., Uchida D., Tanaka T., Saito J., Saito Y., Hirai A. (1998) Pituitary adenylate cyclase activating polypeptide (PACAP) stimulates mitogen-activated protein kinase (MAPK) in cultured rat astrocytes. Brain Res. 795, 191–196 [DOI] [PubMed] [Google Scholar]
- 30. Tatsuno I., Arimura A. (1994) Pituitary adenylate cyclase-activating polypeptide (PACAP) mobilizes intracellular free calcium in cultured rat type-2, but not type-1, astrocytes. Brain Res. 662, 1–10 [DOI] [PubMed] [Google Scholar]
- 31. Hernandez A., Kimball B., Romanchuk G., Mulholland M. W. (1995) Pituitary adenylate cyclase-activating peptide stimulates neurite growth in PC12 cells. Peptides 16, 927–932 [DOI] [PubMed] [Google Scholar]
- 32. Ravni A., Bourgault S., Lebon A., Chan P., Galas L., Fournier A., Vaudry H., Gonzalez B., Eiden L. E., Vaudry D. (2006) The neurotrophic effects of PACAP in PC12 cells: control by multiple transduction pathways. J. Neurochem. 98, 321–329 [DOI] [PubMed] [Google Scholar]
- 33. Casas C., Martínez S., Pritchard M. A., Fuentes J. J., Nadal M., Guimerà J., Arbonés M., Flórez J., Soriano E., Estivill X., Alcántara S. (2001) Dscr1, a novel endogenous inhibitor of calcineurin signaling, is expressed in the primitive ventricle of the heart and during neurogenesis. Mech. Dev. 101, 289–292 [DOI] [PubMed] [Google Scholar]
- 34. Leahy K. P., Crawford D. R. (2000) adapt78 protects cells against stress damage and suppresses cell growth. Arch. Biochem. Biophys. 379, 221–228 [DOI] [PubMed] [Google Scholar]
- 35. Ermak G., Harris C. D., Davies K. J. (2002) The DSCR1 (Adapt78) isoform 1 protein calcipressin 1 inhibits calcineurin and protects against acute calcium-mediated stress damage, including transient oxidative stress. FASEB J. 16, 814–824 [DOI] [PubMed] [Google Scholar]
- 36. Davies K. J., Harris C. D., Ermak G. (2001) The essential role of calcium in induction of the DSCR1 (ADAPT78) gene. Biofactors 15, 91–93 [DOI] [PubMed] [Google Scholar]
- 37. Ermak G., Sojitra S., Yin F., Cadenas E., Cuervo A. M., Davies K. J. (2012) Chronic expression of RCAN1–1L protein induces mitochondrial autophagy and metabolic shift from oxidative phosphorylation to glycolysis in neuronal cells. J. Biol. Chem. 287, 14088–14098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ermak G., Davies K. J. (2013) Chronic high levels of the RCAN1-1 protein may promote neurodegeneration and Alzheimer disease. Free Radic. Biol. Med. 62, 47–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang W., Zhu J. Z., Chang K. T., Min K. T. (2012) DSCR1 interacts with FMRP and is required for spine morphogenesis and local protein synthesis. EMBO J. 31, 3655–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lonze B. E., Ginty D. D. (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623 [DOI] [PubMed] [Google Scholar]
- 41. Pittenger C., Huang Y. Y., Paletzki R. F., Bourtchouladze R., Scanlin H., Vronskaya S., Kandel E. R. (2002) Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron 34, 447–462 [DOI] [PubMed] [Google Scholar]
- 42. Sheng M., Greenberg M. E. (1990) The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 4, 477–485 [DOI] [PubMed] [Google Scholar]
- 43. Tao X., Finkbeiner S., Arnold D. B., Shaywitz A. J., Greenberg M. E. (1998) Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20, 709–726 [DOI] [PubMed] [Google Scholar]
- 44. Ji L., Mochon E., Arcinas M., Boxer L. M. (1996) CREB proteins function as positive regulators of the translocated bcl-2 allele in t(14;18) lymphomas. J. Biol. Chem. 271, 22687–22691 [DOI] [PubMed] [Google Scholar]
- 45. Kim K. S., Lee M. K., Carroll J., Joh T. H. (1993) Both the basal and inducible transcription of the tyrosine hydroxylase gene are dependent upon a cAMP response element. J. Biol. Chem. 268, 15689–15695 [PubMed] [Google Scholar]
- 46. Finkbeiner S., Tavazoie S. F., Maloratsky A., Jacobs K. M., Harris K. M., Greenberg M. E. (1997) CREB: a major mediator of neuronal neurotrophin responses. Neuron 19, 1031–1047 [DOI] [PubMed] [Google Scholar]
- 47. Sakai N., Thome J., Newton S. S., Chen J., Kelz M. B., Steffen C., Nestler E. J., Duman R. S. (2002) Inducible and brain region-specific CREB transgenic mice. Mol. Pharmacol. 61, 1453–1464 [DOI] [PubMed] [Google Scholar]
- 48. Genescà L., Aubareda A., Fuentes J. J., Estivill X., De La Luna S., Pérez-Riba M. (2003) Phosphorylation of calcipressin 1 increases its ability to inhibit calcineurin and decreases calcipressin half-life. Biochem. J. 374, 567–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim S. S., Oh Y., Chung K. C., Seo S. R. (2012) Protein kinase A phosphorylates Down syndrome critical region 1 (RCAN1). Biochem. Biophys. Res. Commun. 418, 657–661 [DOI] [PubMed] [Google Scholar]
- 50. Mandelkow E. M., Biernat J., Drewes G., Gustke N., Trinczek B., Mandelkow E. (1995) Tau domains, phosphorylation, and interactions with microtubules. Neurobiol. Aging 16, 355–362; discussion 362–353 [DOI] [PubMed] [Google Scholar]
- 51. King M. M., Huang C. Y., Chock P. B., Nairn A. C., Hemmings H. C., Jr., Chan K. F., Greengard P. (1984) Mammalian brain phosphoproteins as substrates for calcineurin. J. Biol. Chem. 259, 8080–8083 [PubMed] [Google Scholar]
- 52. Jovanovic J. N., Benfenati F., Siow Y. L., Sihra T. S., Sanghera J. S., Pelech S. L., Greengard P., Czernik A. J. (1996) Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc. Natl. Acad. Sci. U.S.A. 93, 3679–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nichols R. A., Suplick G. R., Brown J. M. (1994) Calcineurin-mediated protein dephosphorylation in brain nerve terminals regulates the release of glutamate. J. Biol. Chem. 269, 23817–23823 [PubMed] [Google Scholar]
- 54. Cho A., Tang Y., Davila J., Deng S., Chen L., Miller E., Wernig M., Graef I. A. (2014) Calcineurin signaling regulates neural induction through antagonizing the BMP pathway. Neuron 82, 109–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yoshida T., Mishina M. (2005) Distinct roles of calcineurin-nuclear factor of activated T-cells and protein kinase A-cAMP response element-binding protein signaling in presynaptic differentiation. J. Neurosci. 25, 3067–3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hammond D. R., Udvadia A. J. (2010) Cabin1 expression suggests roles in neuronal development. Dev. Dyn. 239, 2443–2451 [DOI] [PubMed] [Google Scholar]