Background: PAK1 is phosphorylated and activated by the protein kinase CK2 in response to EGF.
Results: Upon EGF stimulation, CKIP-1 recruits CK2 to PAK1 in membrane ruffles in a PI3K-dependent manner.
Conclusion: PI3K activates PAK1 at the plasma membrane by promoting CK2 phosphorylation of PAK1 via CKIP-1.
Significance: This is the first evidence of spatial regulation of PAK1 activity.
Keywords: prostate cancer, protein kinase, protein phosphorylation, signal transduction, signaling
Abstract
Upon growth factor stimulation, PAK1 is recruited to the plasma membrane and activated by a mechanism that requires its phosphorylation at Ser-223 by the protein kinase CK2. However, the upstream signaling molecules that regulate this phosphorylation event are not clearly defined. Here, we demonstrate a major role of the CK2α-interacting protein CKIP-1 in activation of PAK1. CK2α, CKIP-1, and PAK1 are translocated to membrane ruffles in response to the epidermal growth factor (EGF), where CKIP-1 mediates the interaction between CK2α and PAK1 in a PI3K-dependent manner. Consistently, PAK1 mediates phosphorylation and modulation of the activity of p41-Arc, one of its plasma membrane substrate, in a fashion that requires PI3K and CKIP-1. Moreover, CKIP-1 knockdown or PI3K inhibition suppresses PAK1-mediated cell migration and invasion, demonstrating the physiological significance of the PI3K-CKIP-1-CK2-PAK1 signaling pathway. Taken together, these findings identify a novel mechanism for the activation of PAK1 at the plasma membrane, which is critical for cell migration and invasion.
Introduction
p21-activated kinase 1 (PAK1),2 a major downstream effector of the Rho-family GTPases Cdc42 and Rac1, plays a critical role in the regulation of cell morphology and motility (1). In its inactive state, PAK1 forms a trans-inhibited dimer, where the N-terminal autoinhibitory domain (AID) on one PAK1 molecule binds to and blocks the C-terminal catalytic domain of its counterpart (2, 3). This autoinhibition is relieved through binding of GTPases to the PBD (p21-binding domain)/CRIB (Cdc42/Rac-interacting) domain that partially overlaps with AID, leading to autophosphorylation at specific sites, including Thr-423 within the activation loop, and consequent activation of PAK1 (4). PAK1 is recruited to the plasma membrane via the SH3-containing proteins Nck and Grb2 upon stimulation by growth factors, leading to efficient activation of PAK1 (5–9).
CK2 (casein kinase 2) is a serine/threonine kinase that regulates a broad range of biological processes, including cell growth and survival, gene expression, and actin cytoskeleton organization (10, 11). CK2 is a tetramer composed of two α catalytic subunits and two β regulatory subunits, but the precise mechanism of its regulation is not fully understood. Emerging evidence shows that, in cancer cells, CK2 is frequently elevated and translocated into the nucleus to regulate gene expression (12, 13) and also targeted to the plasma membrane to regulate actin cytoskeleton organization by acting on proteins such as WASP (14), CRN2 (15), myosin-9 (16), and WAVE2 (17). Therefore, the regulated translocation of CK2 to different cellular compartments may be critical for its functional regulation (11).
The pleckstrin homology (PH) domain-containing protein CKIP-1, identified as a CK2α-interacting protein (18), recruits CK2α to the plasma membrane (19). Accumulating evidence indicates that CKIP-1 is involved in various cellular processes including cell proliferation, differentiation, morphology, and migration in various human cell lines including human osteosarcoma U2-OS and macrophage (20–22). CKIP-1 binds to multiple proteins (e.g. CK2α, CPα, Akt, C-Jun ATM, IFP/Nmi, and Smurf1) via its PH domain, implicating its role as a scaffold protein in various signaling pathways (23). Of note, CKIP-1 appears to associate with the plasma membrane through binding to phosphoinositides and to regulate muscle differentiation in mouse myoblast C2C12 cells (24) as well as myoblast fusion in mouse and zebrafish, in PI3K-dependent fashion (25). These observations have raised the possibility that CKIP-1 may serve as a key controller of cellular processes regulated by PI3K.
We have recently demonstrated that CK2 catalyzes phosphorylation of PAK1 at Ser-223, which is critical for the activation of the kinase (26). Here, we show evidence that CKIP-1 appears to recruit CK2α to PAK1 in response to EGF in a PI3K-dependent manner. Therefore, inhibition of either CKIP-1 or PI3K activity blocks PAK1-mediated actin cytoskeleton dynamics and cell migration. These results demonstrate the role of CKIP-1 in EGF-induced activation of PAK1, providing a novel regulatory mechanism for PAK1 signaling.
Experimental Procedures
Cell Cultures
The human prostate cancer PC3 cells were maintained in RPMI 1640 containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin in a humidified incubator (37 °C and 5% CO2). The benign prostate epithelial RWPE-1 cells were cultured in keratinocyte serum-free medium (K-SFM) containing 5 ng/ml of epidermal growth factor (EGF) and 50 μg/ml of bovine pituitary extract (BPE). CKIP-1 knockdown PC3 and RWPE-1 cells were grown in suitable medium containing 2 μg/ml of puromycin.
Lentiviral Infection
Standard lentiviral transduction was performed as described previously (27). In brief, lentiviral transduction was used for CKIP-1 knockdown and GFP-PAK1WT overexpression in prostate cancer cells (PC3 and RWPE-1). Lentiviral short hairpin RNAs (shRNAs) specific for CKIP-1 were purchased from Sigma-Aldrich (sh1, TRCN00001-65913; sh2, TRCN0000165914). For viral production, 293T cells were cotransfected with pLKO.1-shNT- or pLKO.1-CKIP-1-specific shRNAs, and packaging and envelope vectors psPAX2 and pCMV-VSV-G using the LipofectamineTM LTX transfection reagent (Invitrogen). Transfection was performed as described previously (27).
Western Blot Analysis
Whole cell extracts were prepared in RIPA buffer (50 mm Tris, pH 7.4, 15 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) with protease inhibitors (28). For immunoprecipitation, cells were lysed in IP lysis buffer (25 mm HEPES pH 7.4, 150 mm NaCl, 1 mm EDTA, 0.5% Triton X-100) and mixed with protein G-Sepharose beads (GE Healthcare) prebound to mouse normal IgG and anti-GFP (Santa Cruz Biotechnology) for 4 h with rotation at 4 °C. Pull-down beads were washed four times with IP wash buffer (25 mm HEPES pH 7.4, 1 m NaCl, 1 mm EDTA, 0.5% Triton X-100). Plasma membrane fractions were prepared using Mem-PERTM Eukaryotic Membrane Protein Extraction Reagent Kit (Thermo Scientific) following the manufacturer's protocols. Protein concentration was quantified using Bradford to calculate the ratio of each sample volume. The protein samples were resolved on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. Antibodies used in this study include anti-phospho-PAK1S223 (26), anti-phospho-PAK1S144, anti-PAK1, anti-Na/K-ATPase (Cell Signaling), anti-CK2α, anti-GFP (Santa Cruz Biotechnology), anti-α-tubulin (Neo Markers), anti-phospho-p41-ArcT21 (ECM Biosciences), and anti-CKIP-1 (Sigma-Aldrich) antibodies.
Immunofluorescence Staining
Cells were grown on glass coverslips and transfected with either control (NT) or CKIP-1-specific shRNA constructs. After 24 h, cells were washed twice in cold PBS before fixation with 4% PFA for 10 min at room temperature. After fixation, the cells were washed three times with PBS for 5 min each and incubated with the blocking buffer containing 5% BSA and 5% normal goat serum. Coverslips were incubated with primary antibodies overnight at 4 °C or with rhodamine-labeled phalloidin in PBS for 30 min at room temperature. After washing three times with PBS, cells were treated with secondary antibodies for 1 h. DNA was stained with 0.1 μg/ml DAPI (4′, 6-diamidino-2-phenylindole) in PBS for 1 min. Cells were mounted in a mounting medium (KPL) and imaged on an Olympus IX71 inverted microscope (Olympus Imaging America Inc).
Cell Migration and Invasion Assays
For the wound-healing migration assay, PC3 cells were seeded at 70% confluence into 6-well culture dishes and, 24 h later, infected with CKIP-1-specific or control shRNA lentivirus. When the cells grew to confluence, scratch wounds were made on the monolayers using sterile 200 μl pipette tips. After 24 h of incubation, the wounded monolayers were washed gently twice with 1× PBS to remove the detached cells and replenished with fresh medium. The cells were fixed with 4% paraformaldehyde for 30 min and then photographed. Wound areas were measured using ImageJ software. Cell invasion assay was performed using the cell invasion kit (Transwell Boyden's chamber with TranswellTM Permeable Support Inserts Coated with Cultrex® BME (basement membrane extract, Corning Costar) according to the manufacturer's instruction. Briefly, serum-starved PC3 and RWPE-1 (2 × 105 cells/well) cells were added to the upper chamber, whereas suitable media containing 10% FBS was added to the lower chamber and cultured in an incubator for 24 h. Nonmigrating cells were gently removed from the upper surface of the filter with a cotton swab; migrating cells which were attached to the lower surface of the filter were fixed and stained with hematoxylin for counting.
Soft Agar Colony Forming Assay
Anchorage-independent growth was determined by examining cell growth in soft agar. RWPE-1 cells (3 × 104) were seeded in 0.3% agar on top of a base layer of 0.6% agar containing K-SFM (keratinocyte serum-free medium) and 1 μg/ml of puromycin. The cells were placed in a 37 °C, 5% CO2 incubator. After 2 weeks, colonies were stained with crystal violet (0.05%) and counted. Colonies were counted under a microscope (Nikon Eclipse Ti; Melville, NY). All assays were performed in triplicate.
Results
PAK1, CK2α, and CKIP-1 Are Recruited to Membrane Ruffles in Response to EGF
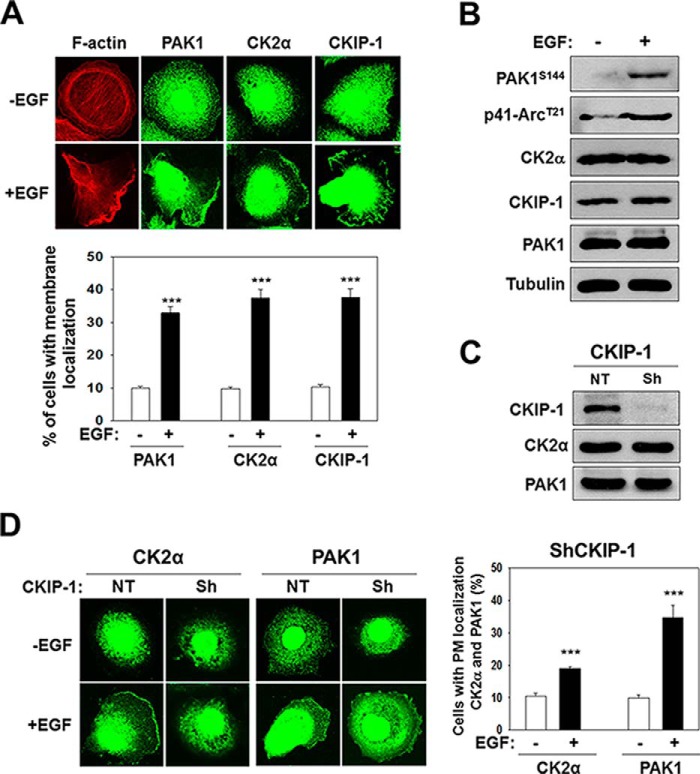
PAK1 regulates cell polarity and migration in response to growth factors by modulating the actin cytoskeleton (29–31). Immunofluorescence microscopy indicates that EGF induces the formation of membrane ruffles at the leading edge of polarized PC3 prostate cancer cells (Fig. 1A, F-actin) by promoting the recruitment of PAK1, CK2α, and CKIP-1 to membrane ruffles (Fig. 1A). Increased PAK1 activity is associated with auto-phosphorylation of specific sites including Ser-144 (32). Activated PAK1 phosphorylates Thr-21 of p41-Arc, a subunit of the human Arp2/3 complex, at the plasma membrane (33). Thus, PAK1 activity can be assessed by the phosphorylation state of Ser-144 of PAK1 (PAK1S144) and T21 of p41-Arc (p41-ArcT21). EGF treatment does not affect the expression of CK2α, CKIP-1, or PAK1, but does increase phosphorylation of PAK1S144 and p41-ArcT21 (Fig. 1B). Given that CKIP-1 interacts with CK2α (19), we addressed the role of CKIP-1 in CK2 activation of PAK1. CKIP-1 does not regulate the expression of CK2α or PAK1 (Fig. 1C); instead, it mediates the EGF-induced recruitment of CK2α to membrane ruffles (Fig. 1D). It is of note, however, that PAK1 localization to the plasma membrane is not regulated by CKIP-1 (Fig. 1D). Thus, CKIP-1 seems to play a key role in EGF-induced PAK1 activation by recruiting CK2α to the plasma membrane.
FIGURE 1.
PAK1, CK2α, and CKIP-1 are recruited to membrane ruffles in response to EGF. PC3 cells were serum-starved for 24 h and then incubated in the absence (−) or the presence (+) of EGF (100 ng/ml) for 1 h. A, fluorescence microscopy images of PC3 cells stained with rhodamine-labeled phalloidin (red) or an antibody to PAK1, CK2α, or CKIP-1 (green) (top) and quantification of PM localization of PAK1, CK2α, and CKIP-1 (bottom). B, whole-cell lysates of PC3 cells were analyzed by Western blotting using antibodies to the indicated proteins. C, total lysates of PC3 cells with (Sh) or without (NT) CKIP-1 knockdown were analyzed by Western blotting using anti-CK2α or anti-PAK1 antibody. D, fluorescence microscopy images of CKIP-1 knockdown PC3 cells stained with anti-CK2α or anti-PAK1 antibody (left) and quantification of PM localization of PAK1 and CK2α (right). The quantitative evaluation of the immuno-staining patterns was performed on at least 1000 cells per sample, in at least three independent experiments, and the results were expressed as the percentages of cells with PM localization of the respective proteins (A and D). ***, p < 0.001.
CKIP-1 Mediates EGF-induced PAK1 Interaction with CK2α
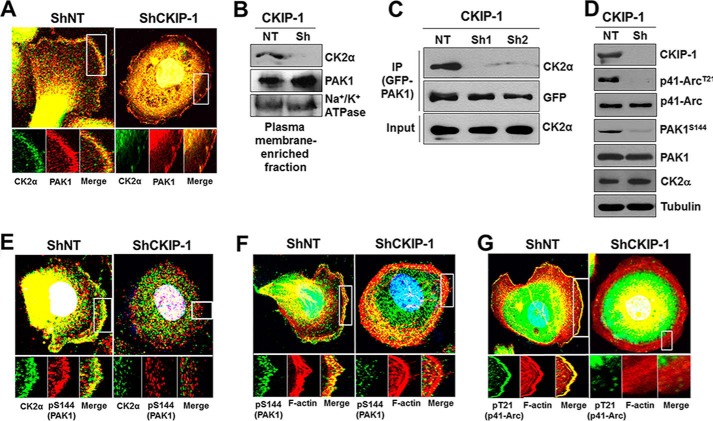
To gain more insights into the role of CKIP-1 in EGF-induced PAK1 activation, we assessed the effect of CKIP-1 knockdown on the interaction between CK2α and PAK1. Upon EGF treatment in PC3 cells, PAK1 and CK2α are colocalized and interact with each other at the plasma membrane (Fig. 2, A and C). However, this interaction is greatly reduced by CKIP-1 knockdown (ShCKIP-1 in Fig. 2, A and C). This is perhaps because CKIP-1 silencing results in decreased localization of CK2α to the plasma membrane (Fig. 2B), which renders PAK1 inactive (PAK1S144) and unable to phosphorylate its substrate p41-Arc at Thr-21 (p41-ArcT21) (Fig. 2D). Consequently, CKIP-1 silencing decreases the colocalization of CK2α with an active form of PAK1 (phospho-PAK1S144) (Fig. 2E) and that of F-actin with either phospho-PAK1S144 (Fig. 2F) or phospho-p41-ArcT21 (Fig. 2G) to the cell periphery. Collectively, these results indicate that CKIP-1 functions to promote CK2-dependent phosphorylation and activation of PAK1 by recruiting CK2α to PAK1 to the plasma membrane.
FIGURE 2.
CKIP-1 recruits CK2α to PAK1 to membrane ruffles in response to EGF. PC3 cells with (Sh) or without (NT) CKIP-1 knockdown were serum-starved for 24 h and then treated with EGF (100 ng/ml) for 1 h. A, PC3 cells were costained with anti-CK2α (green) and anti-PAK1 (red) antibodies. B, plasma membrane-enriched fraction of PC3 cells was analyzed by Western blotting using antibodies to CKIP-1, CK2α, or PAK1. The plasma membrane Na+/K+-ATPase was used as a loading control. C, GFP-PAK1 expressed in PC3 cells (a ShNT and two Sh-CKIP-1s) was immunoprecipitated using anti-GFP antibody (IP), and blots were probed with antibodies to the indicated proteins. D, whole-cell lysates of PC3 cells with (Sh) or without (NT) CKIP-1 knockdown were blotted with antibodies to the indicated proteins. E, colocalization of CK2α (green) with an activated form of PAK1, PAK1S144 (pS144, red) in PC3 cells. F and G, PC3 cells were costained with rhodamine-labeled phalloidin (red) and either anti-phospho-PAK1S144 antibody (green) (F) or anti-phospho-p41-ArcT21 antibody (green) (G). DNA was stained with DAPI (blue) in A, E, F, and G.
Expression of a Constitutively Active PAK1 (PAK1S233E) Bypasses the Requirement for CKIP-1 in PAK1-induced cell Polarity and Migration
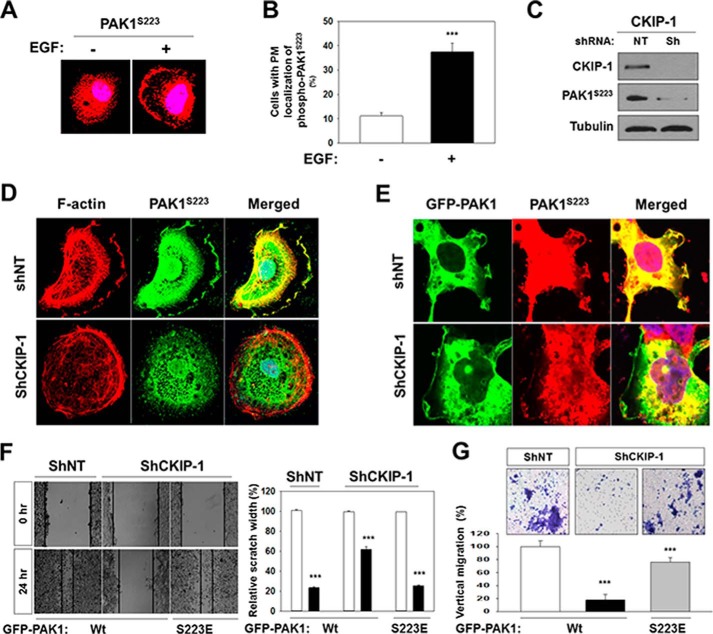
Our recent work shows that CK2 phosphorylates Ser-223 of PAK1, and that a phosphomimetic mutation at Ser-223 (Ser to Glu) generates a constitutively active PAK1 (26). We find that, upon EGF treatment, PAK1 is recruited to the plasma membrane (Fig. 1A) and phosphorylated at Ser-223 (Fig. 3, A and B). To understand further CKIP-1 regulation of PAK1, we infected CKIP-1 knockdown PC3 cells with a lentivirus expressing GFP-PAK1 and examined the activity and localization of PAK1 using anti-phospho-specific PAK1 (S223) antibody. Western blot analysis shows that CKIP-1 silencing decreases S223 phosphorylation of PAK1 (Fig. 3C). In addition, fluorescence microscopy analysis shows that CKIP-1 knockdown results in decreased phosphorylation at Ser-223 (Fig. 3D, middle) and, consequently, in loss of cell polarity (Fig. 3D, CKIP-1), and that the polarization defect of CKIP-1 knockdown cells (Fig. 2, shCKIP-1) is partially rescued by overexpression of GFP-PAK1 (Fig. 3E, shCKIP-1).
FIGURE 3.
Expression of a constitutively active PAK1 (PAK1S233E) bypasses the requirement for CKIP-1 in EGF-induced cell polarity and migration. PC3 cells were serum-starved for 24 h and then stimulated by EGF (100 ng/ml) for 1 h. A, PC3 cells were stained with anti-phospho-PAK1S144 antibody (red). B, quantification of cells stained with anti-phospho-PAK1S223 antibody. Cells with PM localization of phospho-PAK1S144 were quantified as described above (Fig. 1). ***, p < 0.001. C, Western blot analysis of Ser-223 phosphorylation of PAK1. Whole cell lysates of CKIP-1 knockdown (CKIP-1) and control (NT) PC3 cells expressing GFP-PAK1 were blotted with antibodies to the indicated proteins. D, PC3 cells stimulated by EGF were stained with anti-phospho-PAK1S223 antibody (green) and rhodamine-labeled phalloidin (red). E, immunofluorescence analysis of the localization of GFP-PAK1 and phospho-PAK1S223. PC3 cells expressing GFP-PAK1 were stained with anti-phospho-PAK1S223 antibody (middle). DNA was stained with DAPI (blue) in D and E. F, wound healing assay of PC3 cells expressing GFP-PAK1 or GFP-PAK1S223E (left). The results were expressed as the percentages of the remaining area as determined by normalizing the area of wound after 24 h (black bars) to the initial wound area at 0 h (white bars, set to 100%) (left). Each bar represents the mean ± S.E. of five fields counted (***, p < 0.001) (right). G, invasion assay of PC3 cells expressing GFP-PAK1Wt or GFP-PAK1S223E. Cell invasiveness was assessed using a Transwell Boyden chamber assay (top). The invasion rate was determined by counting the number of cells having migrated through the membrane into the lower chamber, and the results were expressed as a percentage relative to control cells (shNT cells expressing GFP-PAK1, set to 100%). Each bar represents the mean ± S.E. of five fields counted (***, p < 0.001) (bottom).
To examine the physiological significance of CKIP-1 regulation of PAK1 activity, we investigated whether PAK1-induced cell migration and invasion are affected by CKIP-1 knockdown. Wound healing assays show that CKIP-1 silencing inhibits migration of PC3 cells expressing wild type GFP-PAK1 by 70%, but this inhibitory effect is not observed in PC3 cells expressing GFP-PAK1S223E (Fig. 3F). Similarly, cell invasion assays using Transwell Boyden's chamber system indicate that invasion of PC3 cells expressing GFP-PAK1 is inhibited 70–80% by CKIP-1 knockdown, and that, however, CKIP-1 inhibition does not significantly reduce the invasiveness of PC3 cells expressing GFP-PAK1S223E (Fig. 3G). These results indicate that CKIP-1 knockdown suppresses PAK1-mediated cell migration and invasion.
The CKIP-1-mediated Interaction between CK2α and PAK1 Is Regulated by PI3K
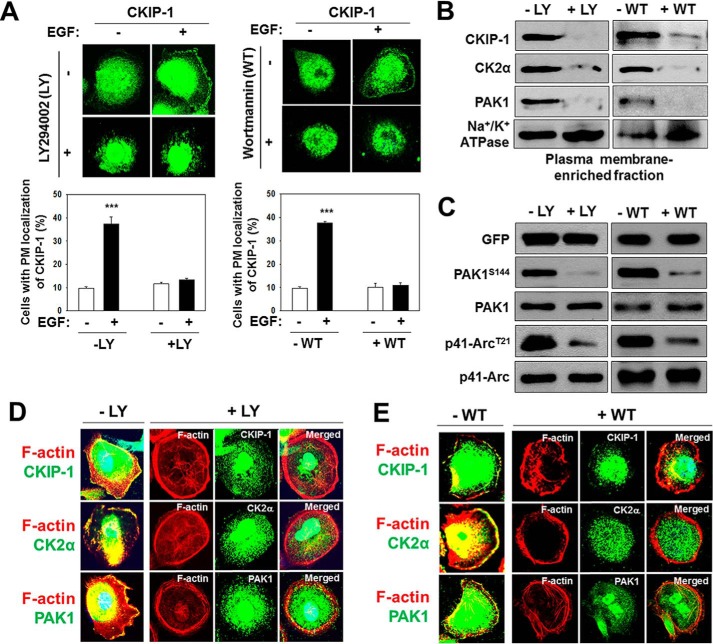
CKIP-1, as a pleckstrin homology domain-containing protein, is recruited by phosphatidylinositol 3-phosphate (PI3P) to the plasma membrane in a PI3K (phosphatidylinositol 3-kinase)-dependent manner (24). This notion is further supported by the finding that the inhibition of PI3K signaling reduces plasma membrane localization of CKIP-1 (34). An early study showed that PAK1 regulates the invasiveness of breast cancer cells via PI3K signaling (35). These observations suggest that PI3K regulates PAK1 activity through CKIP-1. To address this idea, we treated PC3 cells with the PI3K inhibitors LY294002 and wortmannin and examined the subcellular localization of CKIP-1, CK2α, and PAK1. Both LY294002 and wortmannin inhibit the EGF-induced membrane localization of CKIP-1 (Fig. 4A), CK2α and PAK1 (Fig. 4, D and E), and this may be associated with a decrease in the formation of membrane ruffles (Fig. 4, A, D, and E). In agreement, Western blot analysis demonstrates low levels of CKIP-1 and CK2α at the plasma membrane (Fig. 4B) and decreased PAK1 activity, as measured by the phosphorylation of PAK1S144 and p41-ArcT21 (Fig. 4C), in PC3 cells treated with LY294002 or wortmannin.
FIGURE 4.
PI3K regulates the localization of CKIP-1 to the plasma membrane. PC3 cells were serum-starved 24 h, stimulated by EGF (100 ng/ml) for 1 h, and then treated with LY294002 (25 μm) for 4 h or wortmannin (WT, 100 nm) for 1 h. A, fluorescence microscopy images of PC3 cells stained with anti-CKIP-1 antibody (top) and quantification of PM localization of CKIP-1 as described in Fig. 1 (bottom). B, plasma membrane-enriched fraction of PC3 cells was analyzed by Western blotting using antibodies to CKIP-1, CK2α, and PAK1. Na+/K+-ATPase was used as loading control. D, PAK1 activity in PC3 cells treated with (+) or without (−) LY294002 or wortmannin. C, Western blot analysis of the phosphorylation of PAK1S144 and p41-ArcT21. Whole-cell lysates were blotted with antibodies to the indicated proteins. D and E, PC3 cells were costained with rhodamine-labeled Phalloidin (red) and either anti-CKIP-1, CK2α, or PAK1 antibody (green). DNA was stained with DAPI in D and E.
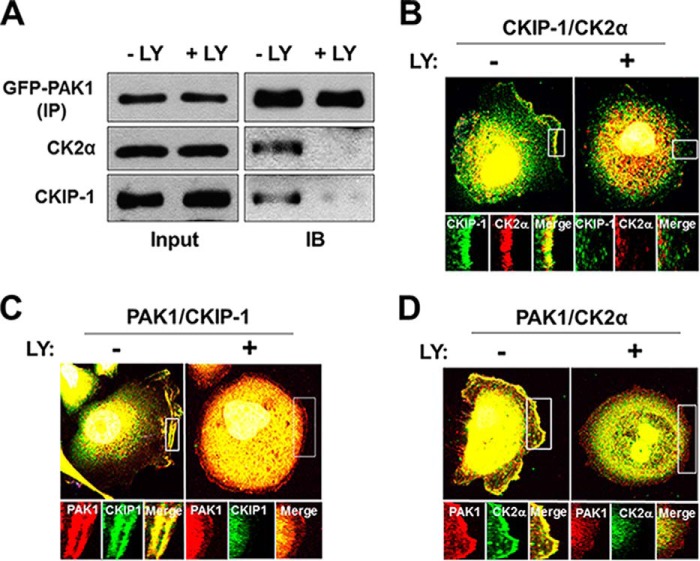
Consistently, the PI3K inhibitors LY294002 (Fig. 5) and wortmannin (data not shown) disrupt the interaction of PAK1 with CK2α and CKIP-1 (Fig. 5A) and inhibit the colocalization of CKIP-1 with either CK2α (Fig. 5B) or PAK1 (Fig. 5C) and that of PAK1 with CK2α (Fig. 5D). Thus, PI3K may regulate the CKIP-1-mediated interaction between CK2α and PAK1 by controlling the plasma membrane localization of CKIP-1.
FIGURE 5.
PI3K regulates the interaction of PAK1 with CK2α and CKIP-1. PC3 cells were incubated and treated with EGF and LY294002 as described above (Fig. 4). A, Western blot analysis of the interaction of PAK1 with CK2α and CKIP-1. GFP-PAK1 expressed in PC3 cells (−LY and +LY) was immunoprecipitated using anti-GFP antibody (IP) and blotted with anti-GFP, anti-CK2α, or anti-CKIP-1 antibody (IB). B, PC3 cells were costained with anti-CKIP-1 (green) and anti-CK2α (red) antibodies. C and D, PC3 cells were costained with anti-PAK1 antibody (red) and either anti-CKIP-1 antibody (green) (C) or anti-CK2α antibody (green) (D). DNA was stained with DAPI in B, C, and D.
Expression of a Constitutively Active PAK1 (PAK1S233E) Bypasses the Requirement for PI3K in the EGF-induced Activation of PAK1
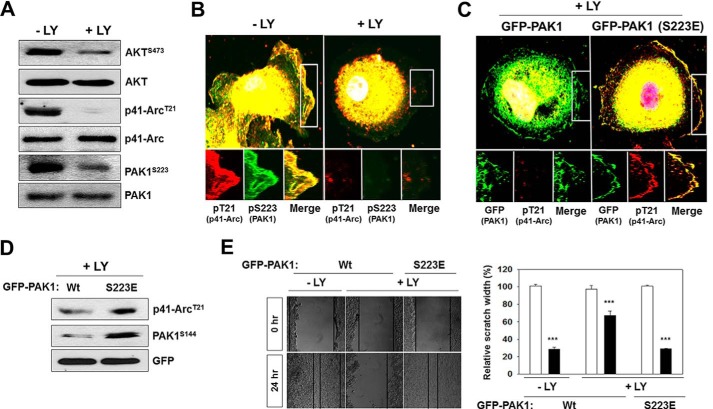
Given that PI3K acts as an activator of PAK1 (Figs. 4 and 5), we examined the effects of PI3K inhibition on the CK2-mediated phosphorylation and activation of PAK1. Akt is phosphorylated at Ser-473 by PI3K, thus this phosphorylation is used as a biochemical marker for PI3K activation (36). Our results show that LY294002 inhibits the phosphorylation of AktS473, PAK1S223, and p41-ArcT21 (Fig. 6A) and the EGF-induced colocalization of phospho-PAK1S223 and phospho-p41-ArcT21 to the membrane ruffles (Fig. 6B). However, expression of the constitutively active PAK1 (PAK1S223E) induces the phosphorylation of p41-ArcT21 (Fig. 6C) and PAKS144 (Fig. 6D) even in LY294002-treated PC3 cells. Furthermore, the migration defect of these cells is rescued by the expression of GFP-PAK1S223E (Fig. 6E). Thus, these observations indicate that PI3K is required for PAK1-mediated cell migration.
FIGURE 6.
Expression of a constitutively active PAK1 (PAK1S233E) bypasses the requirement for PI3K in EGF-induced PAK1 activation. PC3 cells were incubated and treated with EGF and LY294002 as described above (Fig. 4). A, whole-cell lysates of PC3 cells were blotted with antibodies to the indicated proteins. AKT phosphorylation at Ser-473 (AKTS473) was used as an indicator of PI3K activation. B, colocalization of phospho-PAK1S223 and phospho-p41-ArcT21 in PC3 cells. C, PC3 cells expressing GFP-PAK1 or GFP-PAK1S223E were costained with anti-phospho-p41-ArcT21 and anti-phospho-PAK1S223antibodies. D, whole-cell lysates of PC3 cells expressing GFP-PAK1 or GFP-PAK1S223E were blotted with antibodies against the indicated proteins. E, wound healing assay of PC3 cells expressing PAK1 or GFP-PAK1S223E. The results were expressed as the percentages of the remaining area as determined by normalizing the area of wound after 24 h (black bars) to the initial wound area at 0 h (white bars, set to 100%) (left). Each bar represents the mean ± S.E. of five fields counted (***, p < 0.001) (right). DNA was stained with DAPI in B and C.
CKIP-1 Is Required for PAK1-mediated Malignant Transformation of the Benign Prostate RWPE-1 Cells
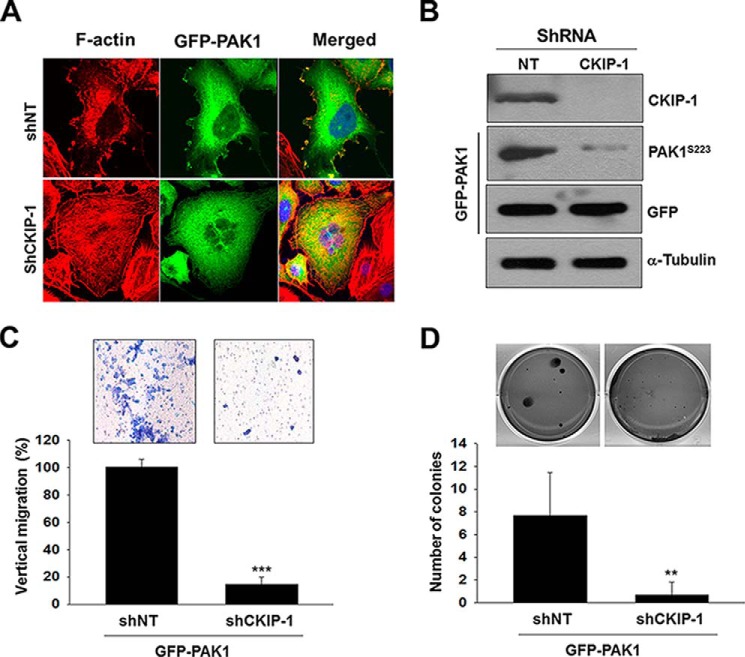
Overexpression of PAK1 induces malignant transformation of the benign prostate RWPE-1 cells in vitro and in vivo (26). Given that CKIP-1 regulates PAK1 activity (Figs. 2 and 3), we examined the effects of CKIP-1 silencing on the PAK1-mediated migration and invasion of RWPE-1 cells. Overexpression of PAK1 induces the formation of membrane ruffles, which, however, is inhibited by CKIP-1 knockdown (Fig. 7A). As observed in PC3 cells (Fig. 3), CKIP-1 knockdown decreases phosphorylation of PAK1 (Fig. 7B) and suppresses PAK1-induced migration (Fig. 7C) and proliferation (Fig. 7D) of RWPE-1 cells. These results suggest that CKIP-1 regulates prostate cancer migration and proliferation by modulating PAK1 activity.
FIGURE 7.
CKIP-1 is required for PAK1-mediated malignant transformation of the benign prostate RWPE-1 cells. A, RWPE-1 cells (shNT and shCKIP-1) expressing GFP-PAK1 were incubated in serum-containing medium and stained with rhodamine-labeled phalloidin. DNA was stained with DAPI (blue). B, whole-cell lysates of RWPE-1 cells expressing GFP-PAK1 were blotted with antibodies to the indicated proteins. C, invasion assay of RWPE-1 cells expressing GFP-PAK1. Cell invasiveness was assessed using a Transwell Boyden chamber assay (top), and the invasion rate was determined as described in Fig. 3G (bottom). D, anchorage-independent growth assay of RWPE-1 cells (shNT and shCKIP-1) expressing GFP-PAK1. GFP-PAK1-induced shNT or shCKIP-1 cells were visualized by phase-contrast microscopy (top). Quantitative analysis of colony numbers (mean ± S.D.) in week 3 is shown (n = 4) (bottom). **, p < 0.05 (as compared with control).
Discussion
PAK1 is a pluripotent kinase that regulates numerous cellular processes, including cell growth and proliferation, actin cytoskeleton organization, cell cycle, and apoptosis (37–40). It remains unknown, however, how PAK1 can perform such diverse functions remains unknown. It has been suggested that the fine control of localization and activation of PAK1 may be the mechanism used by the cell to activate the right PAK1-dependent pathway according to the cell cycle status or in response to extracellular stimuli (39). An early study showed that PI3K activation induces PAK1 kinase activity in a Cdc42/Rac1 or an Akt-independent mechanism (41). However, the underlying mechanism remains unknown. In this study, we establish the role of CKIP-1 in the PI3K activation of PAK1 in response to EGF. CKIP-1 was originally identified as a CK2α-interacting protein. However, it has not been demonstrated whether CKIP-1 is involved in phosphorylation of CK2 substrates (18, 19, 23). Our data show for the first time that CKIP-1 delivers CK2α to PAK1 in response to EGF, and that this process is regulated by PI3K, demonstrating a mechanistic link between CK2 and PI3K signaling.
Emerging evidence indicates that PI3K mediates plasma membrane association of CKIP-1 (23). CKIP-1 exhibits a very broad spectrum of binding to phosphoinositols through its PH domain (19). In myoblast C2C12 cells, CKIP-1 binds to PI3P (phosphoinositol-3-phosphate) at the plasma membrane in response to insulin and regulates muscle differentiation; however, PI3K inhibition or coexpression of PI3K (Δp85), a dominant negative mutant of PI3K, leads to nuclear accumulation of CKIP-1 (24). CKIP-1 bound to phosphoinositides via its PH domain regulates cell morphology and lamellipodia formation by recruiting the Arp2/3 complex to the plasma membrane (25). Subcellular localization of CKIP-1 is dependent on the cell type. CKIP-1 localizes in the nucleus in osteosarcoma Saos-2, mouse preosteoblastic MC-3T3, and rodent fibroblast Rat-1 cells, but is also found at the plasma membrane in monkey kidney COS-7 cells (18). Therefore, it has been suggested that CKIP-1, as a scaffolding protein, may shuttle between the nucleus and the plasma membrane and redistributes signaling complexes between these two compartments in a PI3K-dependent manner (24).
An intriguing finding in the present study may be that PI3K tightly controls PAK1 activity by regulating EGF-induced localization of CK2α and PAK1 to the plasma membrane. We show that CKIP-1, CK2α, and PAK1 are recruited to the leading edge of the cells in response to EGF (Fig. 1), and that this process is regulated by PI3K (Fig. 4). Accordingly, PAK1 phosphorylation of one of its plasma membrane substrates p41-ArcT21 occurs in PI3K-, CKIP-1- and CK2α-dependent manners. These findings reveal a novel signaling pathway by which PI3K activates PAK1 in response to EGF (PI3K → CKIP-1 → CK2 → PAK1). Our data provide evidence that PI3K regulates localization of PAK1 to the membrane ruffles (Fig. 4, D and E), but the underlying mechanism is unknown. PAK1, like CKIP-1, associates with the plasma membrane through phosphoinositides (42), and this process may be regulated by Nck and Grb2 (8, 9). PI3K/Akt also appears to regulate plasma membrane localization of PAK1; a mutation of the Akt phosphorylation site in PAK1 blocks PAK1 activation and inhibits PAK1 redistribution in response to chemoattractant stimulation, but LY294002 treatment results in a rapid loss of cell polarity and PAK1 redistribution (43). Thus, these observations are consistent with our results that PI3K regulates localization of PAK1 to the plasma membrane.
PAK1 acts as a convergence point of many signaling pathways that are frequently dysregulated in human cancers and thus is activated by various signaling factors, including p21 GTPases, thrombin, growth factors, and hormones (38, 40). Phosphorylation of PAK1 at Thr-423 in the kinase activation loop is crucial for enzymatic activity. Binding of GTPases and lipids, such as sphingosines, to PAK1 results in exposure of the activation loop of the kinase domain (44), making it accessible for Thr-423 phosphorylation by 3-phosphoinositide-dependent kinase 1 (PDK1) (45). It should be noted that PDK1 phosphorylation of PAK1 is not blocked by pretreatment with PI3K inhibitor wortmannin or when PDK1 was mutated to prevent phosphatidylinositol binding, indicating that this process is independent of PI3K activity (46). Therefore, Thr-423 phosphorylation by PDK1 may be necessary but not sufficient for the activation of PAK1. Our results have demonstrated that CK2 phosphorylation of Ser-223 is crucial for activation of PAK1 (26), and that this process is regulated by PI3K (this study). The kinase activity of a constitutively active PAK1 (PAK1H83,86L), postulated to mimic GTPase-induced structural changes (47), requires phosphorylation of Ser-223, suggesting that Ser-223 may become available for phosphorylation by CK2 after the conformational change (26). Therefore, PI3K-dependent, CKIP-1-mediated recruitment of CK2α to the plasma membrane is essentially required for the activation of PAK1.
PAK1 is the major isoform of PAKs expressed in most cancers (38, 48) and its expression is sufficient to promote prostate tumor growth (49). Given that Ser-223 of PAK1 is not conserved in other PAK family members, CK2-mediated Ser-223 phosphorylation is considered a unique regulatory mechanism for PAK1 activation (26). Activation of oncogenic kinases by elevated expression is a common feature of human primary tumors. However, there is no significant difference in PAK1 expression between prostate normal and malignant tissues (GeneCards, www.genecards.org). Instead, blocking Ser-223 phosphorylation leads to growth inhibition of prostate cancer PC3 cells (26). Therefore, PI3K-, CKIP-1-, and CK2-dependent phosphorylation of PAK1 plays a key role in malignant transformation of prostate cells. This concept is also supported by the observation that the silencing of CKIP-1 by shRNA inhibits PAK1-mediated migration and invasion of benign prostate epithelial RWPE-1 cells (Fig. 7). Aberrant activation of growth factor receptor signaling is associated with human cancer (38), and the EGF receptor is overexpressed in PC3 cells (50). These observations strengthen the view that CKIP-1-mediated CK2 phosphorylation of PAK1, which is triggered by PI3K activation, may be the principal mechanism for the activation of PAK1 in prostate cancer cells.
Author Contributions
Y. B. K. and Y. J. S. designed, performed experiments, and wrote the first draft of the manuscript. A. R. provided technical assistance and contributed to the preparation of the figures. J. H. K. conceived and coordinated the study and wrote the paper.
Acknowledgment
We thank Angela D. Dement for critical reading of this manuscript.
This work was supported by National Institutes of Health Grant GM087470 (to J. K.). The authors declare that they have no conflicts of interest with the contents of this article.
- PAK1
- p21-activated kinase 1
- AID
- autoinhibitory domain
- LY294002
- 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one
- DAPI
- 4′-6-diamidino-2-phenylindole
- CK
- casein kinase
- PH
- pleckstrin homology
- shRNA
- short hairpin RNA
- PM
- plasma membrane.
References
- 1. Manser E., Leung T., Salihuddin H., Zhao Z. S., Lim L. (1994) A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367, 40–46 [DOI] [PubMed] [Google Scholar]
- 2. Lei M., Lu W., Meng W., Parrini M. C., Eck M. J., Mayer B. J., Harrison S. C. (2000) Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell 102, 387–397 [DOI] [PubMed] [Google Scholar]
- 3. Parrini M. C., Lei M., Harrison S. C., Mayer B. J. (2002) Pak1 kinase homodimers are autoinhibited in trans and dissociated upon activation by Cdc42 and Rac1. Mol. Cell 9, 73–83 [DOI] [PubMed] [Google Scholar]
- 4. Morreale A., Venkatesan M., Mott H. R., Owen D., Nietlispach D., Lowe P. N., Laue E. D. (2000) Structure of Cdc42 bound to the GTPase binding domain of PAK. Nat. Struct. Biol. 7, 384–388 [DOI] [PubMed] [Google Scholar]
- 5. Bokoch G. M., Wang Y., Bohl B. P., Sells M. A., Quilliam L. A., Knaus U. G. (1996) Interaction of the Nck adapter protein with p21-activated kinase (PAK1). J. Biol. Chem. 271, 25746–25749 [DOI] [PubMed] [Google Scholar]
- 6. Lu W., Katz S., Gupta R., Mayer B. J. (1997) Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr. Biol. 7, 85–94 [DOI] [PubMed] [Google Scholar]
- 7. Daniels R. H., Hall P. S., Bokoch G. M. (1998) Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 17, 754–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Puto L. A., Pestonjamasp K., King C. C., Bokoch G. M. (2003) p21-activated kinase 1 (PAK1) interacts with the Grb2 adapter protein to couple to growth factor signaling. J. Biol. Chem. 278, 9388–9393 [DOI] [PubMed] [Google Scholar]
- 9. Galisteo M. L., Chernoff J., Su Y. C., Skolnik E. Y., Schlessinger J. (1996) The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J. Biol. Chem. 271, 20997–21000 [DOI] [PubMed] [Google Scholar]
- 10. Duncan J. S., Litchfield D. W. (2008) Too much of a good thing: the role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim. Biophys. Acta 1784, 33–47 [DOI] [PubMed] [Google Scholar]
- 11. Filhol O., Cochet C. (2009) Protein kinase CK2 in health and disease: Cellular functions of protein kinase CK2: a dynamic affair. Cell Mol. Life Sci. 66, 1830–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laramas M., Pasquier D., Filhol O., Ringeisen F., Descotes J. L., Cochet C. (2007) Nuclear localization of protein kinase CK2 catalytic subunit (CK2α) is associated with poor prognostic factors in human prostate cancer. Eur. J. Cancer 43, 928–934 [DOI] [PubMed] [Google Scholar]
- 13. Lin K. Y., Tai C., Hsu J. C., Li C. F., Fang C. L., Lai H. C., Hseu Y. C., Lin Y. F., Uen Y. H. (2011) Overexpression of nuclear protein kinase CK2 alpha catalytic subunit (CK2α) as a poor prognosticator in human colorectal cancer. PLoS ONE 6, e17193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cory G. O., Cramer R., Blanchoin L., Ridley A. J. (2003) Phosphorylation of the WASP-VCA domain increases its affinity for the Arp2/3 complex and enhances actin polymerization by WASP. Mol. Cell 11, 1229–1239 [DOI] [PubMed] [Google Scholar]
- 15. Xavier C. P., Rastetter R. H., Blömacher M., Stumpf M., Himmel M., Morgan R. O., Fernandez M. P., Wang C., Osman A., Miyata Y., Gjerset R. A., Eichinger L., Hofmann A., Linder S., Noegel A. A., Clemen C. S. (2012) Phosphorylation of CRN2 by CK2 regulates F-actin and Arp2/3 interaction and inhibits cell migration. Sci. Rep. 2, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang D., Jang D. J. (2009) Protein kinase CK2 regulates cytoskeletal reorganization during ionizing radiation-induced senescence of human mesenchymal stem cells. Cancer Res. 69, 8200–8207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pocha S. M., Cory G. O. (2009) WAVE2 is regulated by multiple phosphorylation events within its VCA domain. Cell Motil. Cytoskeleton 66, 36–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bosc D. G., Graham K. C., Saulnier R. B., Zhang C., Prober D., Gietz R. D., Litchfield D. W. (2000) Identification and characterization of CKIP-1, a novel pleckstrin homology domain-containing protein that interacts with protein kinase CK2. J. Biol. Chem. 275, 14295–14306 [DOI] [PubMed] [Google Scholar]
- 19. Olsten M. E., Canton D. A., Zhang C., Walton P. A., Litchfield D. W. (2004) The pleckstrin homology domain of CK2 interacting protein-1 is required for interactions and recruitment of protein kinase CK2 to the plasma membrane. J. Biol. Chem. 279, 42114–42127 [DOI] [PubMed] [Google Scholar]
- 20. Canton D. A., Olsten M. E., Kim K., Doherty-Kirby A., Lajoie G., Cooper J. A., Litchfield D. W. (2005) The pleckstrin homology domain-containing protein CKIP-1 is involved in regulation of cell morphology and the actin cytoskeleton and interaction with actin capping protein. Mol. Cell. Biol. 25, 3519–3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Canton D. A., Olsten M. E., Niederstrasser H., Cooper J. A., Litchfield D. W. (2006) The role of CKIP-1 in cell morphology depends on its interaction with actin-capping protein. J. Biol. Chem. 281, 36347–36359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang L., Xia X., Zhang M., Wang Y., Xing G., Yin X., Song L., He F. (2013) Integrated analysis of genomics and proteomics reveals that CKIP-1 is a novel macrophage migration regulator. Biochem. Biophys. Res. Commun. 436, 382–387 [DOI] [PubMed] [Google Scholar]
- 23. Nie J., Liu L., He F., Fu X., Han W., Zhang L. (2013) CKIP-1: a scaffold protein and potential therapeutic target integrating multiple signaling pathways and physiological functions. Ageing Res. Rev. 12, 276–281 [DOI] [PubMed] [Google Scholar]
- 24. Safi A., Vandromme M., Caussanel S., Valdacci L., Baas D., Vidal M., Brun G., Schaeffer L., Goillot E. (2004) Role for the pleckstrin homology domain-containing protein CKIP-1 in phosphatidylinositol 3-kinase-regulated muscle differentiation. Mol. Cell. Biol. 24, 1245–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baas D., Caussanel-Boude S., Guiraud A., Calhabeu F., Delaune E., Pilot F., Chopin E., Machuca-Gayet I., Vernay A., Bertrand S., Rual J. F., Jurdic P., Hill D. E., Vidal M., Schaeffer L., Goillot E. (2012) CKIP-1 regulates mammalian and zebrafish myoblast fusion. J. Cell Sci. 125, 3790–3800 [DOI] [PubMed] [Google Scholar]
- 26. Shin Y. J., Kim Y. B., Kim J. H. (2013) Protein kinase CK2 phosphorylates and activates p21-activated kinase 1. Mol. Biol. Cell 24, 2990–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shin Y. J., Kim J. H. (2012) The role of EZH2 in the regulation of the activity of matrix metalloproteinases in prostate cancer cells. PLoS ONE 7, e30393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shin Y. J., Kim E. H., Roy A., Kim J. H. (2013) Evidence for a novel mechanism of the PAK1 interaction with the Rho-GTPases Cdc42 and Rac. PLoS ONE 8, e71495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sells M. A., Boyd J. T., Chernoff J. (1999) p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J. Cell Biol. 145, 837–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edwards D. C., Sanders L. C., Bokoch G. M., Gill G. N. (1999) Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1, 253–259 [DOI] [PubMed] [Google Scholar]
- 31. Sanders L. C., Matsumura F., Bokoch G. M., de Lanerolle P. (1999) Inhibition of myosin light chain kinase by p21-activated kinase. Science 283, 2083–2085 [DOI] [PubMed] [Google Scholar]
- 32. Chong C., Tan L., Lim L., Manser E. (2001) The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J. Biol. Chem. 276, 17347–17353 [DOI] [PubMed] [Google Scholar]
- 33. Vadlamudi R. K., Li F., Barnes C. J., Bagheri-Yarmand R., Kumar R. (2004) p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep. 5, 154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hennessy B. T., Smith D. L., Ram P. T., Lu Y., Mills G. B. (2005) Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 4, 988–1004 [DOI] [PubMed] [Google Scholar]
- 35. Adam L., Vadlamudi R., Kondapaka S. B., Chernoff J., Mendelsohn J., Kumar R. (1998) Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J. Biol. Chem. 273, 28238–28246 [DOI] [PubMed] [Google Scholar]
- 36. Adi S., Wu N. Y., Rosenthal S. M. (2001) Growth factor-stimulated phosphorylation of Akt and p70(S6K) is differentially inhibited by LY294002 and Wortmannin. Endocrinology 142, 498–501 [DOI] [PubMed] [Google Scholar]
- 37. Bokoch G. M. (2003) Biology of the p21-activated kinases. Annu. Rev. Biochem. 72, 743–781 [DOI] [PubMed] [Google Scholar]
- 38. Kumar R., Gururaj A. E., Barnes C. J. (2006) p21-activated kinases in cancer. Nat. Rev. Cancer 6, 459–471 [DOI] [PubMed] [Google Scholar]
- 39. Parrini M. C. (2012) Untangling the complexity of PAK1 dynamics: The future challenge. Cell Logist 2, 78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Radu M., Semenova G., Kosoff R., Chernoff J. (2014) PAK signalling during the development and progression of cancer. Nat. Rev. Cancer 14, 13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Papakonstanti E. A., Stournaras C. (2002) Association of PI-3 kinase with PAK1 leads to actin phosphorylation and cytoskeletal reorganization. Mol. Biol. Cell 13, 2946–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Strochlic T. I., Viaud J., Rennefahrt U. E., Anastassiadis T., Peterson J. R. (2010) Phosphoinositides are essential coactivators for p21-activated kinase 1. Mol. Cell 40, 493–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chung C. Y., Potikyan G., Firtel R. A. (2001) Control of cell polarity and chemotaxis by Akt/PKB and PI3 kinase through the regulation of PAKa. Mol. Cell 7, 937–947 [DOI] [PubMed] [Google Scholar]
- 44. Zenke F. T., King C. C., Bohl B. P., Bokoch G. M. (1999) Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J. Biol. Chem. 274, 32565–32573 [DOI] [PubMed] [Google Scholar]
- 45. King C. C., Zenke F. T., Dawson P. E., Dutil E. M., Newton A. C., Hemmings B. A., Bokoch G. M. (2000) Sphingosine is a novel activator of 3-phosphoinositide-dependent kinase 1. J. Biol. Chem. 275, 18108–18113 [DOI] [PubMed] [Google Scholar]
- 46. King C. C., Gardiner E. M., Zenke F. T., Bohl B. P., Newton A. C., Hemmings B. A., Bokoch G. M. (2000) p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1). J. Biol. Chem. 275, 41201–41209 [DOI] [PubMed] [Google Scholar]
- 47. Sells M. A., Knaus U. G., Bagrodia S., Ambrose D. M., Bokoch G. M., Chernoff J. (1997) Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 7, 202–210 [DOI] [PubMed] [Google Scholar]
- 48. Ye D. Z., Field J. (2012) PAK signaling in cancer. Cell Logist 2, 105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goc A., Al-Azayzih A., Abdalla M., Al-Husein B., Kavuri S., Lee J., Moses K., Somanath P. R. (2013) P21 Activated Kinase-1 (Pak1) Promotes Prostate Tumor Growth and Microinvasion via Inhibition of Transforming Growth Factor beta Expression and Enhanced Matrix Metalloproteinase 9 Secretion. J. Biol. Chem. 288, 3025–3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gan Y., Shi C., Inge L., Hibner M., Balducci J., Huang Y. (2010) Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene 29, 4947–4958 [DOI] [PubMed] [Google Scholar]