Abstract
A female patient with non-small-cell lung cancer presented with a huge area of exposed bone in the mandible following spontaneous teeth loss. She was receiving multimodal chemotherapy containing bevacizumab. No previous treatment with bisphosphonates or comorbid conditions was reported. Pain medications and infection control were offered to the patient who was closely followed up. Initial imaging and histology of bone and surrounding mucosa (8 weeks after bevacizumab cessation) confirmed the clinical suspicion of avascular osteonecrosis of the mandible. Subsequent imaging and histology of bone and gingiva (12 weeks after bevacizumab cessation) revealed the initial sequestration of the mandible with a marked expansion of the mucosal vascular network. Spontaneous bone sequestration eventually occurred few months later, followed by stable and painless mucosal coverage of the mandibular bone. The patient remained disease-free up to 3 years of follow-up.
Background
This is a rare case of mandibular osteonecrosis occurred during bevacizumab treatment for lung cancer in the absence of any other recognised predisposing factors such as smoking, diabetes, vascular disease or concomitant treatment with bisphosphonates. What really adds interest to this report is that we describe the evolution of the osteonecrotic process over time. The disease process was studied by means of repeated clinical, radiological, histological and nuclear medicine investigations, observing that bevacizumab-associated osteonecrosis of the jaw is a self-limiting process that tends to remission following drug cessation.
Case presentation
In late August 2008, a 57-year-old woman presented to the outpatient clinic of the Unit of Oral and Maxillofacial Surgery of Verona with persistent oral pain and halitosis following spontaneous teeth loss. The patient had been diagnosed in March 2008 a bilateral non-small-cell lung cancer (NSCLC) with skeletal and thoracic lymph nodes dissemination and accordingly treated with gemcitabine, cisplatin and corticosteroid therapy until July 2008. No comorbid conditions were reported. In May 2008, she was also given 945 mg of intravenous bevacizumab every 21 days, a potent antiangiogenic drug. Four cycles were administered, the last one taking place in August 2008. The patient had not been previously treated with nitrogen-containing bisphosphonates (NBP).
At the end of June 2008, during chemotherapy and bevacizumab treatment, the patient came to see her dentist for the sudden onset of oral pain and halitosis with loosening of the partial denture fixed on the left mandible. The prosthesis was removed; nevertheless, pain persisted and spontaneous loss of two mandibular teeth ultimately occurred at the end of August. A 10-day cycle of oral amoxicillin-clavulanic acid (1 g three times a day) was administered by her general practitioner, who sent the patient to us for consultation. The oral examination showed a huge area (6×3 cm) of exposed necrotic bone in the left mandible; the gingival coverage was completely unwrapped from both the inner and outer cortices of the mandible down to the basal bone (figure 1A). A severe periodontal disease was present in both jaws.
Figure 1.
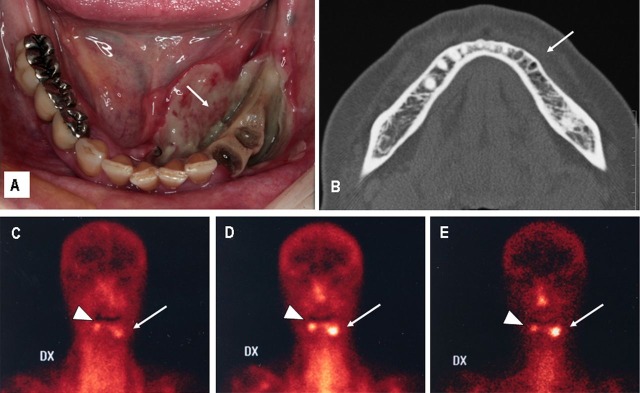
(A) Patient's intraoral view: large area of exposed bone involving the premolar region of the left mandible, with massive dehiscence of both the vestibular and lingual aspect of the oral mucosa (white arrow). (B) Axial CT scan (initial): no signs of bone disease of the left mandibular body are detected as compared with the healthy right side, except for the remnants of the alveolar sockets of the teeth spontaneously uprooted (white arrow). (C–E) Technetium 99 m-labeled leucocyte scintigraphy performed at patient's presentation (anterior views): persistent contrast uptake at 1 (C), 4 (D) and 24 h (E) at the level of left mandibular body (white arrow) and right premolar region (white arrow-head). The persistent contrast uptake at 24 h provides evidence of active mandibular bone infection.
Investigations
The CT initially performed did not display any sign of mandibular bone necrosis or inflammation (figure 1B). In contrast, 99 m Tc-labelled granulocyte scintigraphy performed in mid-September showed focal and persistent tracer uptake at the level of the exposed mandibular bone, suggestive for bone infection (figure 1C–E).
At the end of September the patient presented with a new episode of toothache due to a periodontal abscess in the right premolar region of the mandible. At that time, biopsies of the exposed bone and surrounding gingiva were obtained under local anaesthesia of the left side of the mandible and the patient was given oral lincomycin (500 mg bid) for 7 days. The pathology report confirmed the clinical suspicion of infected osteonecrosis. In addition, it showed an oral mucosa almost depleted of vessels with a scarce inflammatory infiltrate (figure 2). In mid-October, initial signs of mucosal healing were observed at the level of the exposed bone surface (figure 3A), and the CT scan showed the initial sequestration of the left alveolar process of the mandible (figure 3B). At that time, the deteriorated right premolars were extracted under local anaesthesia and a second mucosal biopsy was performed, which showed a marked expansion of the mucosal vascular network and the presence of diffuse inflammatory infiltrate (figure 3C,D). Even though stable mucosal healing of the extraction sites was achieved within 2 weeks, the patient experienced repeated episodes of painful swelling from the osteonecrotic site that required several cycles of antibiotics up to March 2009, when she presented with partial mucosal repair following spontaneous sequestration of a large necrotic bone fragment of the left mandible. One month later, the patient underwent a second 99 m Tc-labelled granulocyte scintigraphy, which showed a markedly reduced tracer uptake at the level of the left mandible, as compared to the initial one (figure 4A–C).
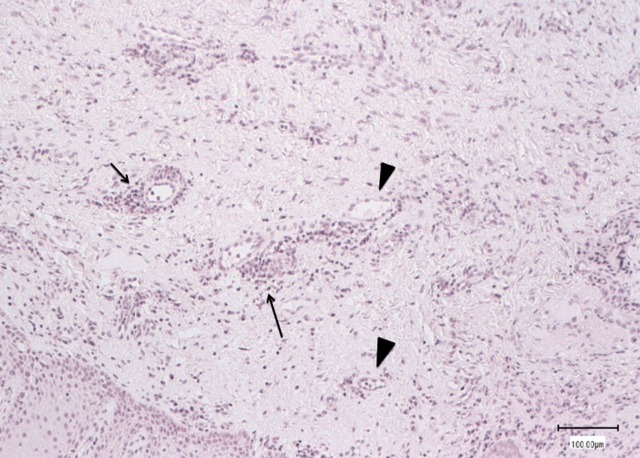
Figure 2.
Histological features of gingival biopsy taken in the area of exposed bone 8 weeks after bevacizumab cessation: poorly vascularised tissue with scanty inflammatory cells (arrows) surrounding the blood vessels (arrow-heads) (H&E stain).
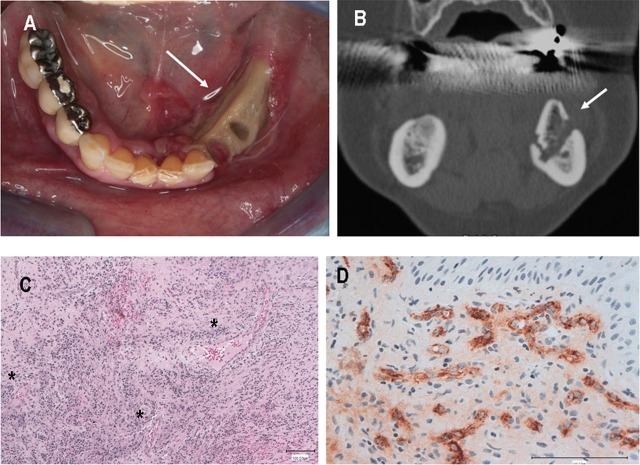
Figure 3.
(A) Intraoral view (mid-October): initial signs of mucosal healing with partial gingival coverage of both the lingual and the vestibular aspects of the exposed bone surface (arrow). (B) Coronal CT scan (mid-October): alveolar bone sequestration (white arrow) is clearly visible. (C) Gingival biopsy taken 12 weeks after bevacizumab cessation (H&E stain): marked inflammatory cells permeation surrounding perivasal spaces (asterisks). Note the expansion of the vascular network associated with exudate in the mucosal stroma. (D) Same sample at higher magnification, showing small vessels with swollen endothelial cells embedded with antigen stain, suggestive of mucosal angiogenic activity (factor-VIII-related antigen stain).
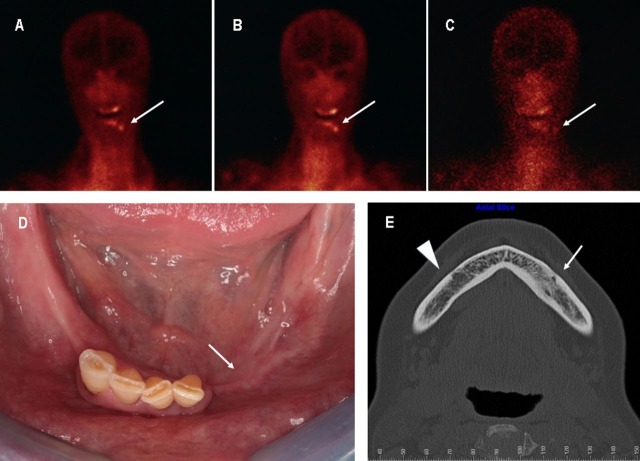
Figure 4.
(A–C) Technetium 99 m-labeled leucocyte scintigraphy (March 2009) (anterior views): almost complete absence of contrast uptake at 24 h (C) as compared with 1 (A) and 4 h (B) is suggestive of bone infection resolution. (D) Patient's intraoral view (3-year follow-up): healthy oral mucosa without signs of inflammation and bone exposure (white arrow). (E) Axial CT scan (3-year follow-up): a subtle bone marrow sclerosis of the left mandibular body is visible as compared with the opposite site (white arrow-head). Neither signs of bone necrosis nor sequestration can be seen.
Differential diagnosis
Bevacizumab is generally well tolerated but several related toxicities have been described, like thromboembolic events, mild-to-moderate haemorrhage, gastrointestinal perforation, wound-healing complications, proteinuria and hypertension.1 2
Recently, the occurrence of osteonecrosis of the jaws during bevacizumab treatment has been reported, especially in patients receiving concurrent therapy with NBP.3–5
Yet, few cases of jawbone necrosis have been reported in cancer patients receiving bevacizumab without any other recognised predisposing factors such as smoking, diabetes, vascular disease or concomitant treatment with NBP.6–10
The sudden onset of tooth loss, mucosal breakdown and massive alveolar bone exposure that occurs during bevacizumab treatment differs from the typical presentation of other osteonecrotic disorders (ie, bisphosphonate-related osteonecrosis of the jaw and osteoradionecrosis) that are normally more subtle.11–13
Treatment
Non-surgical therapy consisting of pain medications and antibiotics was offered to the patient in the beginning as the only possible strategy pending the end antiangiogenic effects of bevacizumab. Extraction of the right premolars was performed under local anaesthesia soon after the recovery of tissue vascularisation with an uncomplicated healing.
Outcome and follow-up
Therapy with bevacizumab was not resumed and NBP were never started during the follow-up. By the way, the patient was given a 150 mg-daily dose of Erlotinib (Tarceva; Roche, Welwyn Garden City, USA), because of NSCLC local progression. Neither clinical nor radiological signs of bone necrosis were observed up to the latest follow-up visit in September 2011 (figure 4D,E).
Discussion
Bevacizumab (Avastin; Roche, Welwyn Garden City, USA) is a recombinant humanised-IgG monoclonal antibody that binds vascular endothelial growth factor (VEGF) prior to its link to the cell receptors, and neutralises its activity.
A well-characterised activity of VEGF is the ability to promote the vascular endothelial cells proliferation inducing the formation of new blood vessels.14 Angiogenesis is involved in tumour development, growth and metastasis; consequently, the intracellular signalling pathways related to VEGF represents a potential target for anticancer therapy.15 16
Bevacizumab has shown antiangiogenic and antitumour activity in several preclinical models and randomised clinical trials.17 Bevacizumab, in combination with different chemotherapeutic agents, has been approved as a first-line treatment of advanced non-squamous NSCLC, metastatic colorectal and breast cancer.1 16 18
Upregulation of VEGF-mediated angiogenesis is a crucial step of inflammatory response also in wound healing.19 Bevacizumab inhibits tissue repair by preventing new blood vessel formation and the migration of inflammatory cells and essential nutrients to the diseased tissue.20 The histology of the oral mucosa obtained 4 weeks after the disease onset (ie, 8 weeks after bevacizumab cessation) showed an impaired neoangiogenesis with a scanty inflammatory response. We hypothesise that the reduced mucosal healing capacity caused by bevacizumab could precipitate the causative periodontal infection, which rapidly propagated to the underlying bone. The finding of bone infection at the first 99 m Tc-labelled granulocyte scintigraphy corroborates this hypothesis.
VEFG-dependent angiogenesis is also required either for bone remodelling and repair.21–23 It has been shown that VEGF may regulate osteoclast differentiation and directly stimulates the osteoclastic bone resorption, by enhancing the survival of mature rabbit osteoclasts.24
In bevacizumab users, osteoclasts inactivation and inhibition of neoangiogenesis could hamper the normal bone repair mechanism leading to the accumulation of avascular and non-viable bone. This might explain the fact that a large area of necrotic bone rapidly spread in our patient during anti-VEGF therapy.
On the contrary, the antiangiogenic and antiresorptive effects of bevacizumab are dose-dependent and time-dependent.25 This implies that angiogenesis, bone remodelling and healing processes should restart after drug cessation. The mucosal biopsy taken in our patient 12 weeks after bevacizumab suspension confirms this hypothesis. The restart of neoangiogenesis and inflammatory responses promoted the spontaneous sequestration of the necrotic bone while the complete mucosal healing achieved 3 months later.
The restoration of normal reparative bone mechanism was also confirmed by the fact that an oral surgical procedure was safely performed in the same patient 12 weeks after drug suspension.
The combined inhibitory effect of bevacizumab on mucosal healing and bone remodelling may be responsible for the initiation of the osteonecrotic process, in the presence of dental or periodontal infections. These worse effects are time-dependent and tend to normalise within 10–12 weeks following bevacizumab cessation. As a consequence, despite its destructive clinical presentation, bevacizumab-associated osteonecrosis of the jaw might have less detrimental effects on patients’ quality of life in the mid and long term as compared with bisphosphonate-related osteonecrosis.
In conclusion, bevacizumab-associated osteonecrosis of the jaw should be regarded as another, albeit infrequent, drug adverse reaction where the inhibition of angiogenesis seems to play an important pathogenetic role. It differs from other drug-related osteonecrotic diseases in that it is a self-limiting process that tends to remission following drug cessation.
Learning points
Consider bevacizumab as a potential cause of avascular osteonecrosis of the jaws.
Oral triggers (ie, dental and periodontal infection, pressure sores and tooth extraction) are in common with other forms of drug-related osteonecrosis of the jaws (ie, bisphosphonate and denosumab).
Bevacizumab treatment should be waived for at least 10 weeks prior to any invasive oral surgical procedure.
Thorough dental examination with elimination of potential infectious foci is highly recommended before the initiation of bevacizumab treatment.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Oxnard GR. Bevacizumab for non-small-cell lung cancer. N Engl J Med 2007;356:1373–75. [PubMed] [Google Scholar]
- 2.Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther 2006;28:1779–802. [DOI] [PubMed] [Google Scholar]
- 3.Aragon-Ching JB, Ning YM, Chen CC, et al. Higher incidence of osteonecrosis of the jaw (ONJ) in patients with metastatic castration resistant prostate cancer treated with anti-angiogenic agents. Cancer Invest 2009;27:221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christodoulou C, Pervena A, Klouvas G, et al. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology 2009;76:209–11. [DOI] [PubMed] [Google Scholar]
- 5.Guarnieri V, Miles D, Nicholas R, et al. Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res Treat 2010;122:181–8. [DOI] [PubMed] [Google Scholar]
- 6.Estilo CL, Fornier M, Farooki A, et al. Osteonecrosis of the jaw related to bevacizumab. J Clin Oncol 2008;26:4037–8. [DOI] [PubMed] [Google Scholar]
- 7.Greuter S, Schmid F, Ruhstaller T, et al. Bevacizumab-associated osteonecrosis of the jaw. Ann Oncol 2008;19:2091–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serra F, Paolantonio M, Spoto G, et al. Bevacizumab-related osteonecrosis of the jaw. Int J Immunopathol Parmacol 2009;22:1121–3. [DOI] [PubMed] [Google Scholar]
- 9.Dişel U, Beşen AA, Özylkan Ö, et al. A case report of bevacizumab-related osteonecrosis of the jaw: old problem, new culprit. Oral Oncol 2012;48:2–3. [DOI] [PubMed] [Google Scholar]
- 10.Hopp RN, Pucci J, Santos-Silva AR, et al. Osteonecrosis after administration of intravitreous bevacizumab. J Oral Maxillofac Surg 2012;70:632–5. [DOI] [PubMed] [Google Scholar]
- 11.Badros A, Weikel D, Salama A, et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol 2006;24:945–52. [DOI] [PubMed] [Google Scholar]
- 12.Ruggiero SL, Dodson TB, Assael LA, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws-2009 update. J Oral Maxillofac Surg 2009;67:2–12. [DOI] [PubMed] [Google Scholar]
- 13.Lazarovici TS, Yahalom R, Taicher S, et al. Bisphosphonate-related osteonecrosis of the jaws: a single-center study of 101 patients. J Oral Maxillofac Surg 2009;67:850–5. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun 2005;333:328–35. [DOI] [PubMed] [Google Scholar]
- 15.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23: 1011–27. [DOI] [PubMed] [Google Scholar]
- 16.Renk M, Crino’ L. Advances in anti-VEGF and anti-EGFR therapy for advanced non-small cell lung cancer. Lung Cancer 2009;63:1–9. [DOI] [PubMed] [Google Scholar]
- 17.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non small-cell lung cancer. N Engl J Med 2006;355:2542–50. [DOI] [PubMed] [Google Scholar]
- 18.Eskens FA, Sleijfer S. The use of bevacizumab in colorectal, ling, breast, renal and ovarian cancer: where does it fit? Eur J Cancer 2008;44:2350–6. [DOI] [PubMed] [Google Scholar]
- 19.Gordon CR, Rojavin Y, Patel M, et al. A review on bevacizumab and surgical wound healing. An important warning to all surgeons. Ann Plast Surg 2009;62:707–9. [DOI] [PubMed] [Google Scholar]
- 20.Scappaticci FA, Fehrenbacher L, Cartwright T, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol 2005;91:173–80. [DOI] [PubMed] [Google Scholar]
- 21.Peyruchaud O, Serre CM, NicAmhlaoibh R, et al. Angiostatin inhibits bone metastasis formation in nude mice through a direct anti-osteoclastic activity. J Biol Chem 2003;278:45826–32. [DOI] [PubMed] [Google Scholar]
- 22.Aldridge SE, Lennard TWJ, Williams JR, et al. Vascular endothelial growth factor receptors in osteoclast differentiation and function. Biochem Biophys Res Commun 2005;335:793–8. [DOI] [PubMed] [Google Scholar]
- 23.Yang Q, McHugh KP, Patntirapong S, et al. VEGF enhancement of osteoclast survival and bone resorption involves VEGF receptor-2 signaling and ß3 –integrin. Matrix Biol 2008;27:589–99. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa M, Kaneda T, Arakawa T, et al. Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts. FEBS Lett 2000;473:161–4. [DOI] [PubMed] [Google Scholar]
- 25.Yuan F, Chen Y, Dellian M, et al. Time-dependent vascular regression and permeability changes in established human tumour xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA 1996;93:14765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]