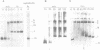Abstract
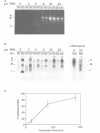
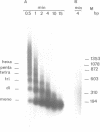
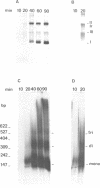
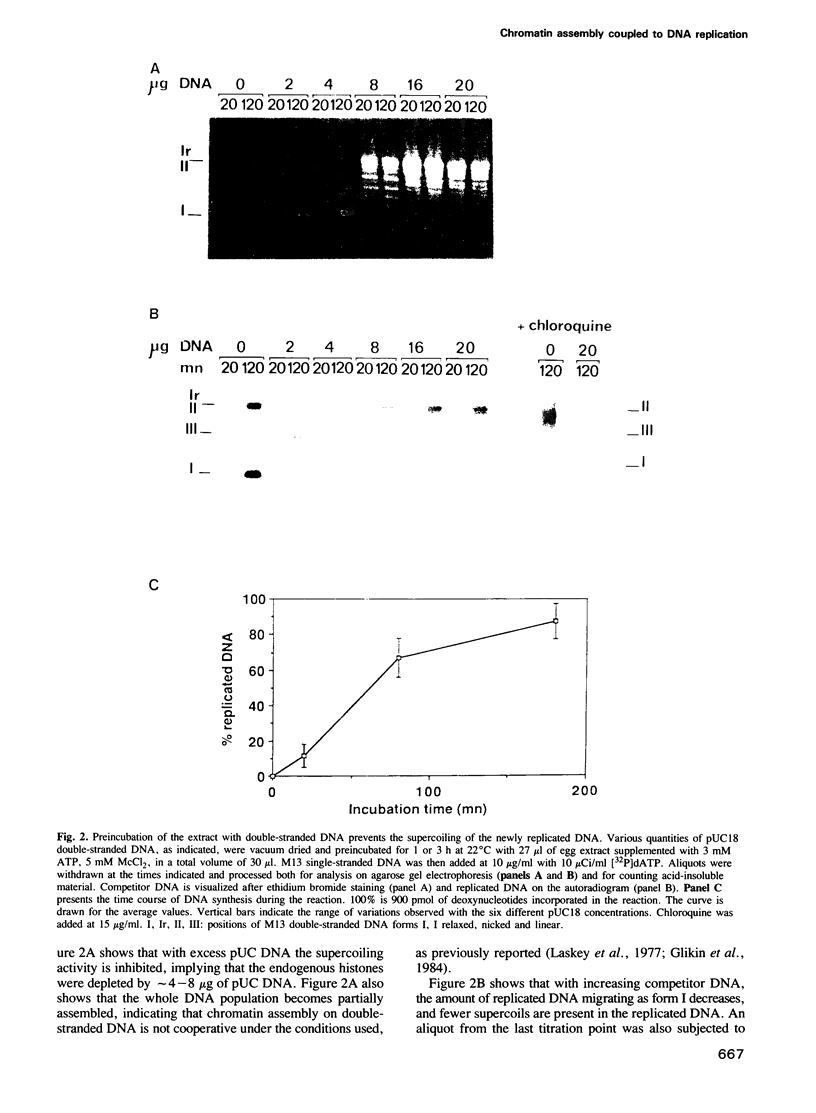
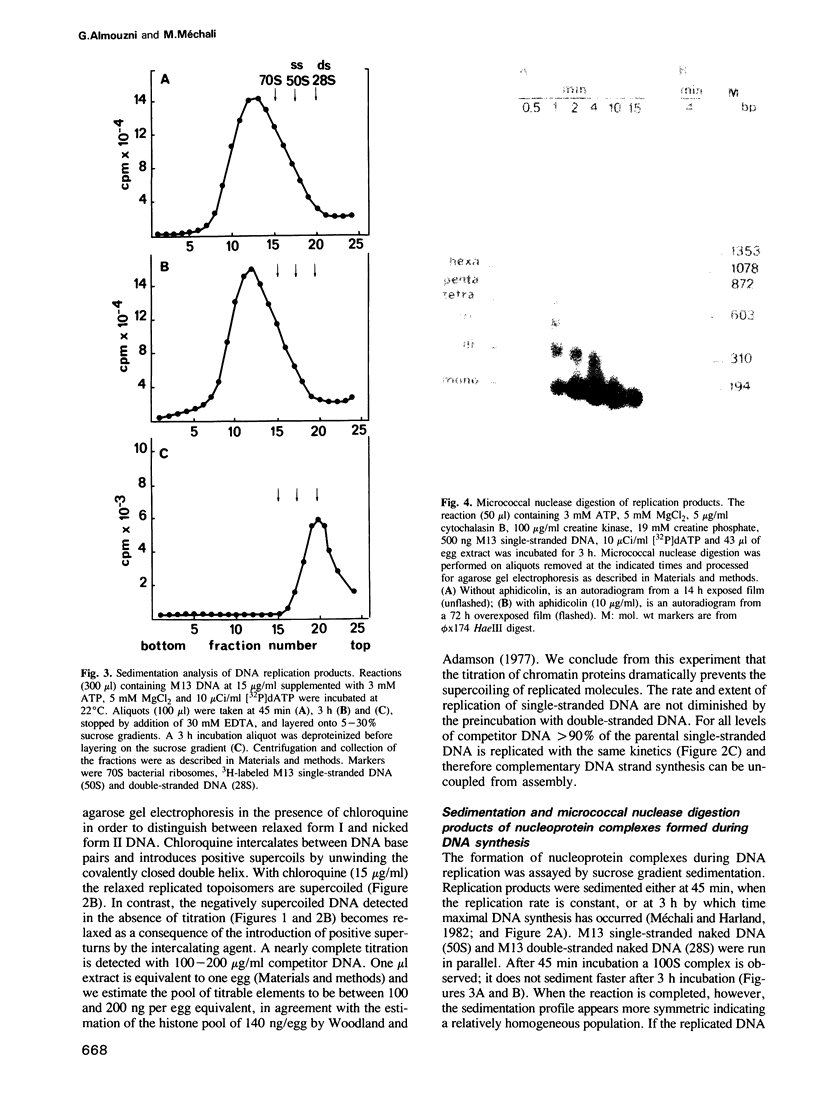
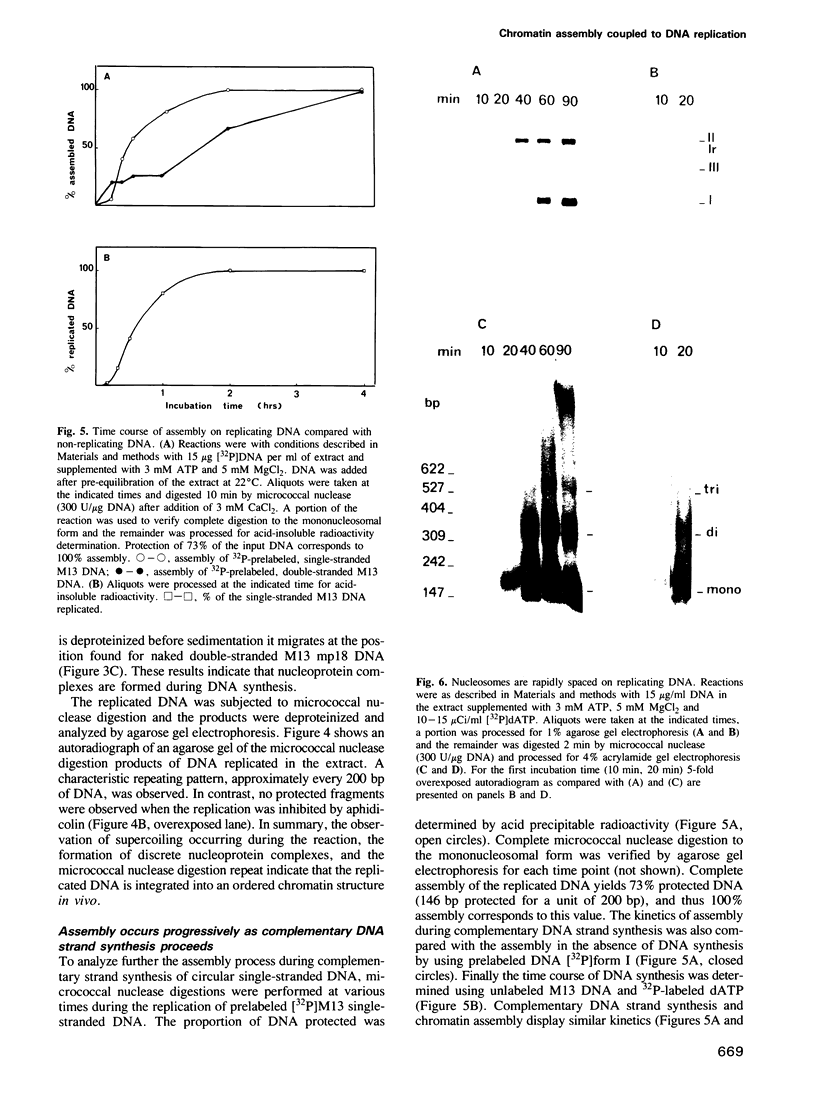
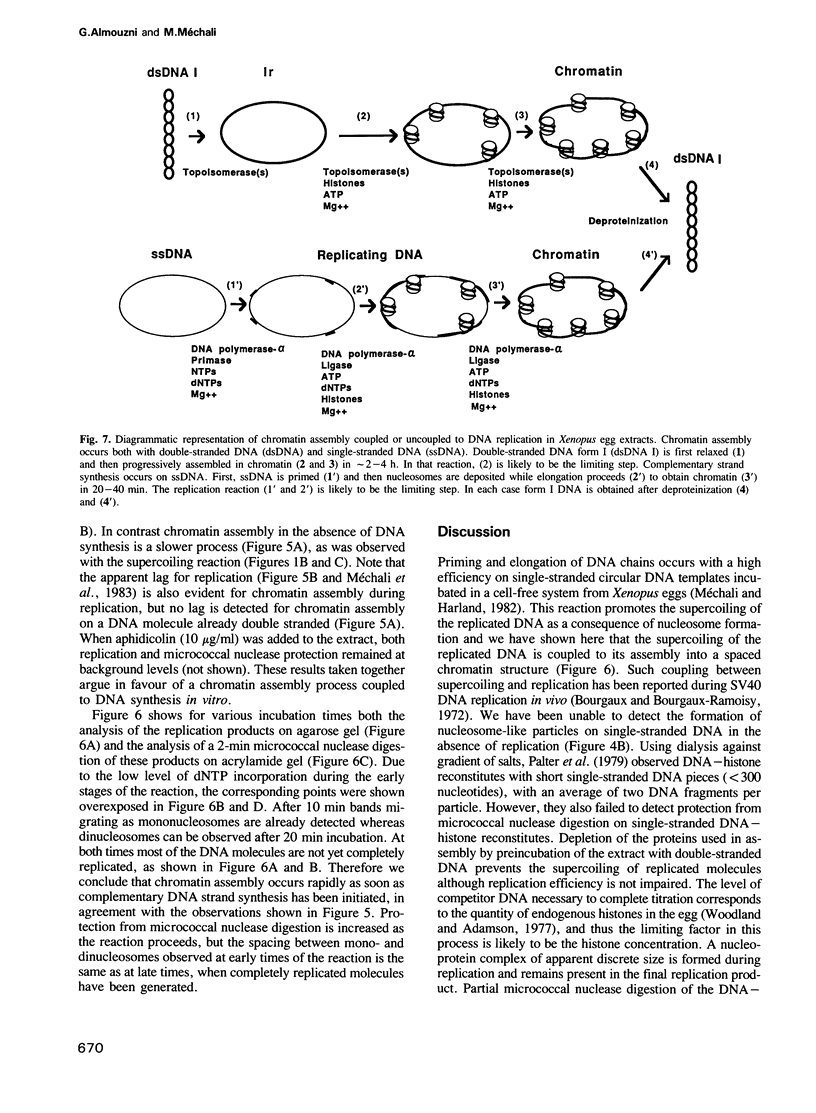
A cell-free system from Xenopus eggs mimics the reaction occurring at the eukaryotic replicative fork in vivo when chromatin assembly is coupled to complementary strand synthesis of DNA. DNA synthesis on single-stranded circular DNA promotes supercoiling and the replicated molecule sediments as a discrete nucleoprotein complex. Micrococcal nuclease digestion reveals a characteristic pattern of multiples of 200 bp of DNA. The kinetics of chromatin assembly and DNA synthesis are coincident and both processes occur with a rate comparable with chromosomal replication in vivo in early embryos. The DNA synthesis reaction can be uncoupled from the assembly reaction. Thus, titration of chromatin proteins by preincubation of the extract with double-stranded DNA prevents the supercoiling of replicated DNA without affecting the rate of synthesis. In contrast, chromatin assembly performed on unreplicated double-stranded DNA is a slower process as compared with the assembly coupled to DNA synthesis. Supercoiled molecules are detected after 30 min replication whereas at least 2 h are required to observe the first form I DNA with unreplicated double-stranded DNA. Such a system where chromatin assembly is promoted by DNA synthesis should be valuable for studying the interaction of specific factors with DNA during chromatin assembly at the replicative fork.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariga H., Sugano S. Initiation of simian virus 40 DNA replication in vitro. J Virol. 1983 Nov;48(2):481–491. doi: 10.1128/jvi.48.2.481-491.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow J. J., Laskey R. A. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell. 1986 Nov 21;47(4):577–587. doi: 10.1016/0092-8674(86)90622-7. [DOI] [PubMed] [Google Scholar]
- Blumenthal A. B., Kriegstein H. J., Hogness D. S. The units of DNA replication in Drosophila melanogaster chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:205–223. doi: 10.1101/sqb.1974.038.01.024. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D. Is a specific protein responsible for the supercoiling of polyoma DNA? Nature. 1972 Jan 14;235(5333):105–107. doi: 10.1038/235105a0. [DOI] [PubMed] [Google Scholar]
- Christiansen G., Griffith J. Salt and divalent cations affect the flexible nature of the natural beaded chromatin structure. Nucleic Acids Res. 1977 Jun;4(6):1837–1851. doi: 10.1093/nar/4.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crémisi C., Chestier A., Yaniv M. Assembly of SV40 and polyoma minichromosomes during replication. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):409–416. doi: 10.1101/sqb.1978.042.01.043. [DOI] [PubMed] [Google Scholar]
- Cusick M. E., DePamphilis M. L., Wassarman P. M. Dispersive segregation of nucleosomes during replication of simian virus 40 chromosomes. J Mol Biol. 1984 Sep 15;178(2):249–271. doi: 10.1016/0022-2836(84)90143-8. [DOI] [PubMed] [Google Scholar]
- Dawid I. B. Deoxyribonucleic acid in amphibian eggs. J Mol Biol. 1965 Jul;12(3):581–599. doi: 10.1016/s0022-2836(65)80313-8. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Eissenberg J. C., Cartwright I. L., Thomas G. H., Elgin S. C. Selected topics in chromatin structure. Annu Rev Genet. 1985;19:485–536. doi: 10.1146/annurev.ge.19.120185.002413. [DOI] [PubMed] [Google Scholar]
- Fowler E., Farb R., El-Saidy S. Distribution of the core histones H2A.H2B.H3 and H4 during cell replication. Nucleic Acids Res. 1982 Jan 22;10(2):735–748. doi: 10.1093/nar/10.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germond J. E., Rouvière-Yaniv J., Yaniv M., Brutlag D. Nicking-closing enzyme assembles nucleosome-like structures in vitro. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3779–3783. doi: 10.1073/pnas.76.8.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glikin G. C., Ruberti I., Worcel A. Chromatin assembly in Xenopus oocytes: in vitro studies. Cell. 1984 May;37(1):33–41. doi: 10.1016/0092-8674(84)90298-8. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Wickens M. P. The use of Xenopus oocytes for the expression of cloned genes. Methods Enzymol. 1983;101:370–386. doi: 10.1016/0076-6879(83)01028-9. [DOI] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978 Oct;15(2):317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Jackson V., Chalkley R. A new method for the isolation of replicative chromatin: selective deposition of histone on both new and old DNA. Cell. 1981 Jan;23(1):121–134. doi: 10.1016/0092-8674(81)90277-4. [DOI] [PubMed] [Google Scholar]
- Jackson V., Chalkley R. A reevaluation of new histone deposition on replicating chromatin. J Biol Chem. 1981 May 25;256(10):5095–5103. [PubMed] [Google Scholar]
- Jackson V., Granner D., Chalkley R. Deposition of histone onto the replicating chromosome: newly synthesized histone is not found near the replication fork. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2266–2269. doi: 10.1073/pnas.73.7.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein H. J., Hogness D. S. Mechanism of DNA replication in Drosophila chromosomes: structure of replication forks and evidence for bidirectionality. Proc Natl Acad Sci U S A. 1974 Jan;71(1):135–139. doi: 10.1073/pnas.71.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladiges W. C., Raff R. F., Brown S., Deeg H. J., Storb R. The canine major histocompatibility complex. Supertypic specificities defined by the primed lymphocyte test (PLT). Immunogenetics. 1984;19(4):359–365. doi: 10.1007/BF00345410. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D., Morris N. R. Assembly of SV40 chromatin in a cell-free system from Xenopus eggs. Cell. 1977 Feb;10(2):237–243. doi: 10.1016/0092-8674(77)90217-3. [DOI] [PubMed] [Google Scholar]
- Leffak I. M. Conservative segregation of nucleosome core histones. Nature. 1984 Jan 5;307(5946):82–85. doi: 10.1038/307082a0. [DOI] [PubMed] [Google Scholar]
- Leffak I. M., Grainger R., Weintraub H. Conservative assembly and segregation of nucleosomal histones. Cell. 1977 Nov;12(3):837–845. doi: 10.1016/0092-8674(77)90282-3. [DOI] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli G., Baldari C. T., Carri M. T., Di Cello G., Buongiorno-Nardelli M. An electron microscope study of chromosomal DNA replication in different eukaryotic systems. Exp Cell Res. 1982 Jan;137(1):127–140. doi: 10.1016/0014-4827(82)90015-5. [DOI] [PubMed] [Google Scholar]
- Méchali M., Harland R. M. DNA synthesis in a cell-free system from Xenopus eggs: priming and elongation on single-stranded DNA in vitro. Cell. 1982 Aug;30(1):93–101. doi: 10.1016/0092-8674(82)90015-0. [DOI] [PubMed] [Google Scholar]
- Nelson T., Hsieh T. S., Brutlag D. Extracts of Drosophila embryos mediate chromatin assembly in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5510–5514. doi: 10.1073/pnas.76.11.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982 Oct;30(3):675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Palter K. B., Foe V. E., Alberts B. M. Evidence for the formation of nucleosome-like histone complexes on single-stranded DNA. Cell. 1979 Oct;18(2):451–467. doi: 10.1016/0092-8674(79)90064-3. [DOI] [PubMed] [Google Scholar]
- Pospelov V., Russev G., Vassilev L., Tsanev R. Nucleosome segregation in chromatin replicated in the presence of cycloheximide. J Mol Biol. 1982 Mar 25;156(1):79–91. doi: 10.1016/0022-2836(82)90460-0. [DOI] [PubMed] [Google Scholar]
- Roufa D. J., Marchionni M. A. Nucleosome segregation at a defined mammalian chromosomal site. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1810–1814. doi: 10.1073/pnas.79.6.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russev G., Hancock R. Assembly of new histones into nucleosomes and their distribution in replicating chromatin. Proc Natl Acad Sci U S A. 1982 May;79(10):3143–3147. doi: 10.1073/pnas.79.10.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoji M., Worcel A. Structure of the two distinct types of minichromosomes that are assembled on DNA injected in Xenopus oocytes. Cell. 1985 Apr;40(4):923–932. doi: 10.1016/0092-8674(85)90352-6. [DOI] [PubMed] [Google Scholar]
- Seale R. L. Nucleosomes associated with newly replicated DNA have an altered conformation. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2717–2721. doi: 10.1073/pnas.75.6.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale R. L. Studies on the mode of segregation of histone nu bodies during replication in HeLa cells. Cell. 1976 Nov;9(3):423–429. doi: 10.1016/0092-8674(76)90087-8. [DOI] [PubMed] [Google Scholar]
- Seidman M. M., Levine A. J., Weintraub H. The asymmetric segregation of parental nucleosomes during chrosome replication. Cell. 1979 Oct;18(2):439–449. doi: 10.1016/0092-8674(79)90063-1. [DOI] [PubMed] [Google Scholar]
- Stein A., Whitlock J. P., Jr, Bina M. Acidic polypeptides can assemble both histones and chromatin in vitro at physiological ionic strength. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5000–5004. doi: 10.1073/pnas.76.10.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Gluzman Y. Replication and supercoiling of simian virus 40 DNA in cell extracts from human cells. Mol Cell Biol. 1985 Aug;5(8):2051–2060. doi: 10.1128/mcb.5.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanev R., Russev G. Distribution of newly synthesized histones during DNA replication. Eur J Biochem. 1974 Apr 1;43(2):257–263. doi: 10.1111/j.1432-1033.1974.tb03408.x. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Assembly of an active chromatin structure during replication. Nucleic Acids Res. 1979 Oct 10;7(3):781–792. doi: 10.1093/nar/7.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. Cooperative alignment of nu bodies during chromosome replication in the presence of cycloheximide. Cell. 1976 Nov;9(3):419–422. doi: 10.1016/0092-8674(76)90086-6. [DOI] [PubMed] [Google Scholar]
- Wobbe C. R., Dean F., Weissbach L., Hurwitz J. In vitro replication of duplex circular DNA containing the simian virus 40 DNA origin site. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5710–5714. doi: 10.1073/pnas.82.17.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. P., Brown D. D. DNA replication in vitro erases a Xenopus 5S RNA gene transcription complex. Cell. 1986 Oct 24;47(2):217–227. doi: 10.1016/0092-8674(86)90444-7. [DOI] [PubMed] [Google Scholar]
- Woodland H. R., Adamson E. D. The synthesis and storage of histones during the oogenesis of Xenopus laevis. Dev Biol. 1977 May;57(1):118–135. doi: 10.1016/0012-1606(77)90359-1. [DOI] [PubMed] [Google Scholar]
- Worcel A., Han S., Wong M. L. Assembly of newly replicated chromatin. Cell. 1978 Nov;15(3):969–977. doi: 10.1016/0092-8674(78)90280-5. [DOI] [PubMed] [Google Scholar]