Abstract
Acquired fistulas between the tracheobronchial tree and the gastrointestinal tract are rare but serious complications of laparoscopic sleeve gastrectomies with significant morbidity and mortality. With the rising popularity and widespread acceptance of bariatric surgery techniques, the occurrence of gastrobronchial fistulas is being increasingly recognised. We present the case of a 26-year-old woman who underwent laparoscopic sleeve gastrectomy for morbid obesity and presented later with a history of chronic productive cough. Upper gastrointestinal series showed the presence of a communicating fistula between the stomach and the lung, with extravasation of contrast into the lung. The aim of this paper is to highlight the importance of considering the diagnosis of a gastrobronchial fistula in cases of persistent respiratory infections in the postoperative period following bariatric surgery and to review its incidence, clinical manifestations and treatment.
Background
Laparoscopic sleeve gastrectomy (LSG) has become a standard procedure for the surgical treatment of patients with different degrees of obesity. The first LSG was performed by Gagner and Patterson as part of a duodenal switch procedure at Mount Sinai in New York in 1999. Since then, many surgeons and institutions have adopted this technique.1 Clinical advantages include no rerouting of intestine thereby eliminating the risk of late bowel obstruction from internal herniation while ensuring good weight loss, and unlike the gastric band, the risk of slippage and erosion is eliminated.2 There are three important adverse effects linked to this procedure: staple line bleeding, strictures—usually at the middle or distal portion of the residual stomach, and gastric leaks or fistulas which causes the greatest morbidity. It can result in abdominal sepsis, multiorgan failure and even death. Supportive measures such as antibiotic therapy, abdominal drainage, parenteral or enteral nutrition and high-dose proton pump inhibitors may be sufficient to control the systemic infection and heal the leakage. Some patients with gastric fistulas still evolve unsatisfactorily. The gastric leak, located on top of the stapler line, can rarely cause a primary subphrenic abscess and a secondary diaphragm rupture with the eventual occurrence of a gastrobronchial fistula (GBF).3 The occurrence of a GBF has rarely been reported and its true incidence is not known. The treatment of a GBF can be challenging and no consensus guidelines exist for the same.
Here, we present the case of a patient who presented with the complaints of a chronic cough approximately 20 months after undergoing a laparoscopic sleeve gastrectomy and was found to a have a GBF.
Case presentation
A 26-year-old woman presented to our hospital with the chief complaint of cough. She described the cough as being productive of yellowish sputum, small-to-moderate in amount, non-bloody and persistent for the past 1 year. She, however, noticed a recent worsening of symptoms over the past week. She denied any fever, chills, allergic symptoms, nausea, vomiting, recent travel, sick contacts, odynophagia, dysphagia or changes in her bowel habits. She was born in the USA and did not have a recent tuberculin skin test. The patient's history was significant for having undergone a laparoscopic sleeve gastrectomy for morbid obesity 20 months ago. Before the procedure, the patient had a body mass index of 40.2 kg/m² and she reported an 85 pound weight loss since then. Four months after the procedure, the patient was admitted to the hospital for fever, productive cough and abdominal pain. She was diagnosed as having a left lower lobe pneumonia and a small gastric leak as evident on upper gastrointestinal series. She was conservatively managed for the same with total parenteral nutrition and intravenous antibiotics. Repeat upper gastrointestinal (GI) series done a month later, showed no extravasation of contrast and free passage of gastrograffin into the duodenum. She continued to clinically improve, except for a mild persistent cough, and antibiotics were discontinued.
Family history was significant for bronchial asthma in her mother. Surgical history included the laparoscopic sleeve gastrectomy procedure. The patient was also a current smoker with a three-pack-year smoking history. She, however, denied the use of alcohol, illicit drugs or over-the-counter medications.
On physical examination, the patient appeared in no apparent distress. Vital signs revealed a blood pressure of 130/80 mm Hg, pulse rate of 88/min, temperature of 98.5 F and respiratory rate of 18/min. Cardiovascular system examination was within normal limits. Examination of the lungs revealed increased tactile fremitus and dullness to percussion in the left lung base, with decreased breath sounds and occasional ronchi. Abdomen was soft, non-tender, non-distended, with no organomegaly and normal bowel sounds.
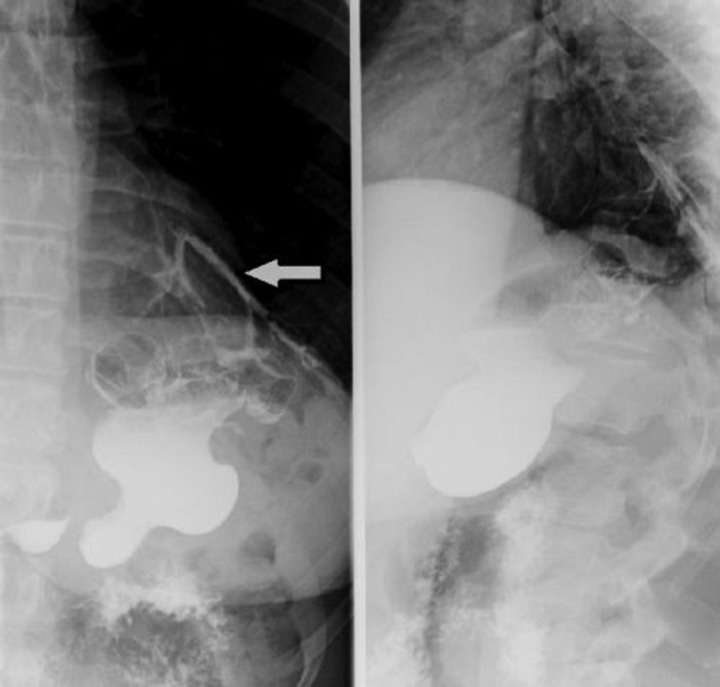
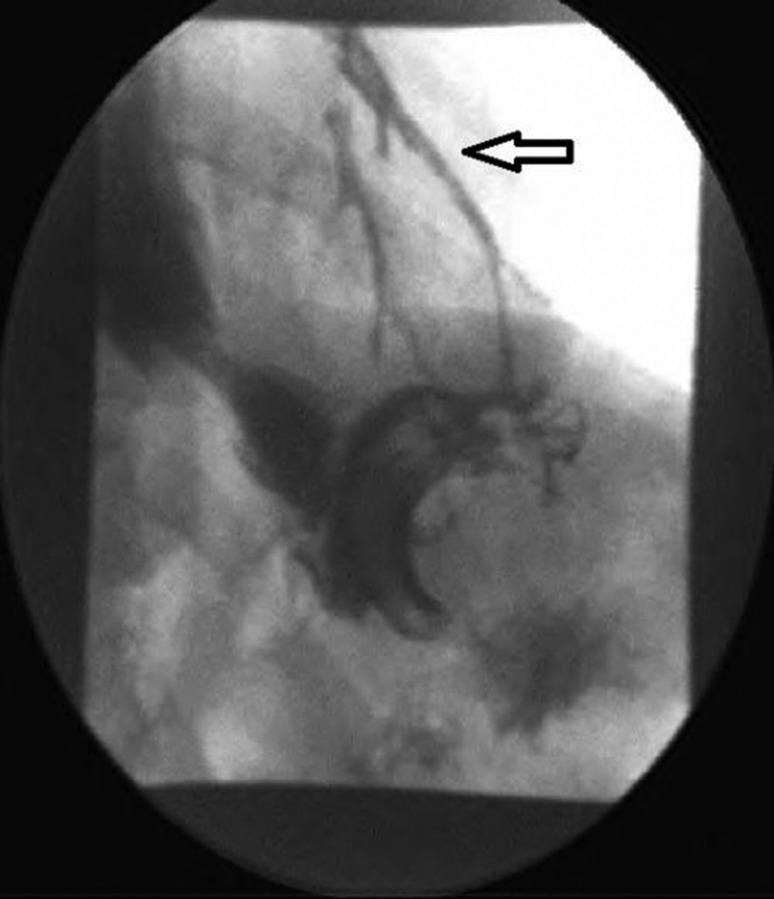
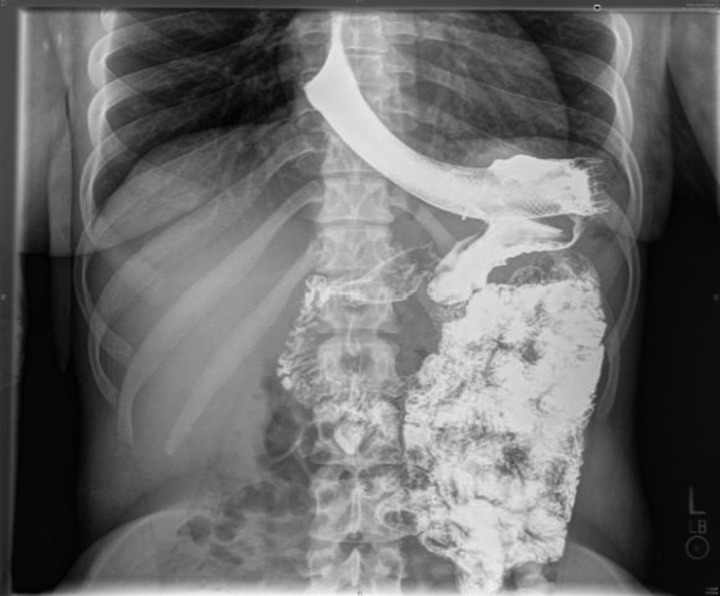
Laboratory investigations revealed a normal white blood cell count, haemoglobin and haematocrit. Liver-related tests, blood chemistry, amylase and lipase were within normal limits. CT of the thorax and abdomen with contrast showed a decrease in the size of the persistent chronic left lower lobe consolidation and a reduction in the size of the fluid collection from the leak as compared with the previous study. On upper gastrointestinal series, extravasation of contrast into the lung was noticed; consistent with a gastrobronchial fistula (figures 1 and 2). The patient was made nil per os and started on total parenteral nutrition. She then underwent esophagogastroduodenoscopy with ablation of the fistulous tract and placement of a self-expanding stent. Gastrograffin study postprocedure no longer showed evidence of a patent tract or extraluminal contrast leakage (figure 3).
Figure 1.
Upper gastrointestinal series with water-soluble contrast showing extravasation of contrast into the lungs.
Figure 2.
Fluoroscopic image showing the communicating fistula between stomach and lung.
Figure 3.
Upper gastrointestinal series after ablation of the fistulous tract and placement of a self-expanding stent.
Discussion
LSG is a relatively new and evolving surgical technique indicated in the treatment of morbid obesity. This procedure has been gaining widespread popularity among both patients and surgeons alike. Indications for LSG were validated with the recent First International Consensus Summit for Sleeve Gastrectomy.4 It induces early satiety by restricting the stomach size and decreases appetite by the resection of fundal ghrelin-producing cells.5 The most significant complications related to the procedure are bleeding of the staple line in nearly 2%, strictures of the middle and distal portion of the residual stomach in 1% and gastric leaks with incidence varying from 0.7% to 20%.6 Most leaks (up to 85.7%) occur in the proximal third of the stomach as compared with the distal portion.7 It has been postulated that most gastric fistulas and leaks occur not because of staple line dehiscence but owing to ischaemia in the gastric wall next to the staple line. Mechanical fistulas are usually discovered within the first 2 days after surgery whereas classic ischaemic fistulas tend to appear between 5 and 6 days after surgery, when the wall-healing process is between the inflammation and fibrotic phase.1 The fistulous secretion can then move into the subphrenic region and cause abdominal sepsis and a gastrocutaneous fistula. Less frequently, it can form a gastrogastric fistula. In rare circumstances, the subphrenic abscess can cross the diaphragm and eventually lead to a gastrobronchial fistula.8
Historically, GBF was first classified by Moeller and Carpenter in 1985. They classified the causes of GBF into five categories: (1) neoplasm, (2) prior oesophageal or gastric surgeries, (3) trauma, (4) gastric ulcers and (5) subphrenic abscesses.9 Prior gastro-oesophageal surgeries have been constantly implicated as the most common cause.
GBF secondary to LSG may have possible predisposing and perpetuating factors. Distal stenosis may decrease gastric emptying, thereby increasing the pressure in the stomach and directing the food and secretions into the fistula. This facilitates the persistent communication between the stomach and the respiratory tract. Inappropriate or inadequate drainage of the abscesses in the upper abdomen may result in chronic inflammation and irritation in the subphrenic region, thus predisposing to fistula formation.8 10
The clinical presentation of a GBF includes chronic productive cough, haemoptysis, fever and dyspnoea. Occasionally, vomiting and expectoration of food particles or other gastric contents may be seen. These symptoms may worsen in the supine position because the fistulous flow and bile reflux across the pylorus are increased in this position. Patients may also manifest with recurrent pneumonias or lung abscesses not responding to medical therapy. Persistent hypercapnia despite high minute ventilation has also been described by some authors. This has been explained on the basis of an increased physiological dead space due to the leakage of part of each breath into the stomach.3 10–12
A contrast study of the upper gastrointestinal tract (gastrograffin) is indicated in the diagnosis of a GBF. CT scan is also helpful in identification of abscesses, lung pathologies and can aid in performing drainage procedures. Other methods that have been used include methylene blue staining and measurement of bronchial secretion. Bronchoscopy is a much less definitive test due to the more distal bronchial or parenchymal location of majority of cases. However, bronchoscopy can suggest a fistulous tract between the stomach and the bronchus by visualisation of methylene blue in the bronchus after oral administration. Upper endoscopy does not diagnose GBF, but can identify the internal opening, can assess the anatomy of the stomach and can be used for therapeutic purposes, thus minimising the need for invasive surgery.10 11
The treatment of gastrobronchial fistulas should be tailored to the clinical state of the patient. In the absence of major signs of sepsis, an initial conservative management can be tried. Treatment with antibiotics is important in managing the coexistent lung infection. In cases of response failure, CT-guided aspiration and drainage of the abscess can be tried. Endoscopic balloon dilatation, self-expanding plastic stent placement and stricturotomy or septoplasty along with adequate nutritional support, are minimally invasive and effective techniques in resolving gastric stenosis which is considered the leading cause of perpetuation of the fistula. Some authors advocate a systematic but aggressive dilation every 30 days for a period of 3 months, even in the absence of obstructive symptoms for the prevention of recurrence of GBF. The use of endoscopic clip and fibrin or histoacryl glue for the closure of GBF has also been attempted by some. Surgical treatment with laparotomy and thoracotomy with the resection of the fistula, and rarely total gastrectomy and lobectomy may be required in some patients who fail to respond to a more conservative and endoscopic approach.3 10
In a recent multicentre retrospective study of 15 patients with established gastrobronchial fistula following bariatric surgery, the mean duration before the appearance of symptoms was found to be 6.7 months (1–30 months) with the main source of leak at the angle of his. Endoscopic management of GBF led to a 93.3% success rate in GBF closure without recurrence after an average follow-up of 27.3 months, with the average healing time of 4.4 months (range 1–10 months).10
In conclusion, GBF is a very rare complication after LSG and is difficult to diagnose. Physicians should be cognisant of this rare but extremely relevant diagnosis. Clinicians should have a high suspicion if patients have non-resolving pulmonary and abdominal symptoms in spite of optimal management. It is also essential that a joint evaluation is carried out by specialists, including pulmonologists, surgeons, radiologists and endoscopists, given the complexity of the obese patient and of the gastrobronchial infectious process.
Learning points.
Laparoscopic sleeve gastrectomy has become a standard procedure for the treatment of morbid obesity.
Gastrobronchial fistulas are rare but serious complications of laparoscopic sleeve gastrectomy.
The treatment of a gastrobronchial fistula can be challenging and requires a multidisciplinary approach.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Márquez MF, Ayza MF, Lozano RB, et al. Gastric leak after laparoscopic sleeve gastrectomy. Obes Surg 2010;20:1306–11. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen NT, Nguyen XMT, Dholakia C. The use of endoscopic stent in management of leaks after sleeve gastrectomy. Obes Surg 2010;20:1289–92. [DOI] [PubMed] [Google Scholar]
- 3.Fuks D, Dumont F, Berna P, et al. Case report: complex management of a postoperative bronchogastric fistula after laparoscopic sleeve gastrectomy. Obes Surg 2009;19:261–4. [DOI] [PubMed] [Google Scholar]
- 4.Deitel M, Crosby RD, Gagner M. The first International Consensus Summit for Sleeve Gastrectomy New York City. Obes Surg 2008;18:487–96, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Serra C, Baltasar A, Andreo L, et al. Treatment of gastric leaks with coated self-expanding stents after sleeve gastrectomy. Obes Surg 2007;17:866–72. [DOI] [PubMed] [Google Scholar]
- 6.Tan JT, Kariyawasam S, Wijeratne T, et al. Diagnosis and management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg 2010;20:403–9. [DOI] [PubMed] [Google Scholar]
- 7.Burgos AM, Braghetto I, Csendes A, et al. Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obes Surg 2009;19:1672–7. [DOI] [PubMed] [Google Scholar]
- 8.Campos JM, de Siqueira T, Meira MRDL, et al. Gastrobronchial fistula as a rare complication of gastroplasty for obesity. A report of two cases. J Bras Pneumol 2007;33:475–9. [DOI] [PubMed] [Google Scholar]
- 9.Moeller DD, Carpenter PR. Gastrobronchial fistula: case report and review of the English literature. Am J Gastroenterol 1985;80:538–41. [PubMed] [Google Scholar]
- 10.Campos JM, Pereira EF, Evangelista LF, et al. Gastrobronchial fistula after sleeve gastrectomy and gastric bypass: endoscopic management and prevention. Obes Surg 2011;21:1520–9. [DOI] [PubMed] [Google Scholar]
- 11.Jha PK, Deiraniya AK, Keeling-Roberts CS, et al. Gastrobronchial fistula—a recent series. Interact Cardiovasc Thorac Surg 2003;2:6–8. [DOI] [PubMed] [Google Scholar]
- 12.Stal JM, Hanly PJ, Darling GE. Gastrobronchial fistula: an unusual complication of esophagectomy. Ann Thorac Surg 1994;58:886–7. [DOI] [PubMed] [Google Scholar]