Abstract
Celiac disease or pulmonary haemosiderosis can be associated with several distinguished conditions. Pulmonary haemosiderosis is a rare, severe and fatal disease characterised by recurrent episodes of alveolar haemorrhage, haemoptysis and anaemia. Association of pulmonary haemosiderosis and celiac disease is extremely rare. We describe a case of celiac disease presented with dilated cardiomyopathy and pulmonary haemosiderosis without gastrointestinal symptoms of celiac disease. In addition, vitamin A deficiency was detected. This case suggests that celiac disease should be considered in patients with cardiomyopathy and/or pulmonary haemosiderosis regardless of the intestinal symptoms of celiac disease.
Background
Several associations have been reported in celiac disease (CD) and pulmonary haemosiderosis (PH). PH has been noted in congestive heart failure, dilated cardiomyopathy (CMP), myocarditis, Goodpasture syndrome, collagen vascular diseases or hypersensitivity to cow's milk. Similarly, CD may be associated with some other diseases, especially autoimmune ones (thyroid diseases, pernicious anaemia, autoimmune thrombocytopenia, sarcoidosis, insulin-dependent diabetes mellitus, dermatitis herpetiformis, alopecia). The incidence of CD was also found to be higher in Down syndrome, selective immunoglobulin A (IgA) deficiency and some neurological disorders such as ataxia, peripheral neuropathy, epilepsy and bilateral occipital calcifications.1
CMP or PH may be associated with CD.2 3 All the three may coexist rarely.4 Here, we present a girl with PH and CMP, who was detected CD and vitamin A deficiency additionally.
Case presentation
The patient, a 13-year-old girl, was referred to our department for further evaluation. Her medical history revealed hematemesis, severe anaemia (haemoglobin 3.4 gr/dl, mean corpuscular volume 68 fl) and pulmonary infiltration at 11 years of age. She had two attacks of haemoptysis since then. Her weight and height were within the percentiles of 10–25%. Rare crepitation sounds on her right side and 2/6 systolic murmur on the mitral area were heard. Laboratory tests revealed an iron-deficiency anaemia (haemoglobin 7.6 gr/dl). Her chest radiography and thorax CT delineated bilateral pulmonary infiltration and cardiomegaly (figure 1). Echocardiography revealed dilated CMP with decreased left ventricular ejection fraction (32%) and mild mitral regurgitation. With the help of her previous medical history and the results of sputum examination which indicated iron laden macrophages, she was diagnosed as PH. The possible causes of PH other than congestive heart failure were examined. Antigliadin antibodies (IgA and IgG) and antiendomysial antibody were positive. She was diagnosed as CD additionally. After intestinal biopsy that confirmed the diagnosis of CD, and gluten-free diet (GFD) was initiated. Her nutritional screening discovered hypovitaminosis A (plasma retinol: 8 mcg/dl). Vitamin A 200 000 IU was given once, and 3000 IU daily was maintained. Other laboratory tests (carnitine, Ig G, Ig A, Ig E, Ig M, C3, C4, dsDNA, and antithyroglobulin and antimicrosomal antibodies) were not significant. During her 2-year follow-up, anaemia was improved with oral iron supplementation. Anaemia and pulmonary haemorrhage did not recur. The control endoscopy and biopsy, 8 months later, showed improvement. Plasma vitamin A level was increased and remained in normal levels. Nevertheless, systolic function was decreased to an ejection fraction of 29%. Unfortunately, she died suddenly at home 2 years later.
Figure 1.
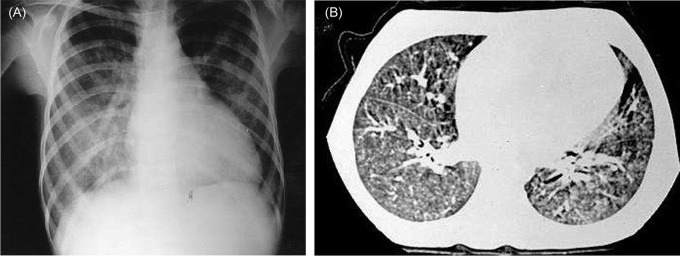
The patient's chest x-ray radiography (A) and an image from thorax CT (B) delineate bilateral pulmonary infiltration and cardiomegaly.
Treatment
Conservative treatment
Medical treatment
Diet treatment
Discussion
Both PH and CD have been associated with several diseases, some of which have been reported very rarely.4 The pathogenesis of this association is not well understood. It is either coincidental, or based on a cause-and-effect relationship. PH may result from either CD or CMP. CD may lead to both PH and CMP as well. Autoimmune mechanisms seem to be more likely in the pathogenesis of the associations of CD or PH because various organ involvements related to autoimmunity may occur in both CD and PH. Many organ-specific antibodies have been defined in CD. However, antimyosin antibody has not been studied in the patients of CD with/without CMP yet. We could not demonstrate antimyosin antibodies in our patient. Antimyosin antibodies might have become undetectable with the progression of CMP.5 Furthermore, some other antibodies against heart tissue might be the cause of CMP.6 Regarding the association of CD with PH, deposition of circulating immune complexes related to food allergens on the basement membrane of alveolar capillaries, or antireticulin antibodies reacting alveolar basement membrane may be the cause of PH in CD. However, no satisfying evidence explaining the involvement of an immune mechanism has been detected.7 As a result, a common immune-mediated mechanism has not been able to be demonstrated both in the cases in the literature and in our case.
Vitamin A deficiency, which was detected in our case, has been mentioned as the cause of ophthalmic complications and increased risk of oesophagus carcinoma in CD.8 However, it has not mentioned in pulmonary complications or associations of CD. Because vitamin A is very important for epithelial cells, its deficiency could influence pulmonary epithelial integrity, which may lead to blood leaking into alveoli and thus PH. As in the present case, vitamin A administration may be useful in the management of PH as an adjunctive therapy.
In a summary, the present case emphasises that CD should be considered in patients with CMP and/or PH even in the absence of gastrointestinal symptoms. Early diagnosis may be important for the prognosis of CMP/PH in CD because organ-specific antibodies were found less prevalent in the patients in which GFD was initiated earlier.9
Learning points.
Both cardiomyopathy and celiac disease have been associated with several diseases.
Cardiomyopathy or pulmonary haemosiderosis may be associated with celiac disease.
If the etiology is unclear, patients with cardiomyopathy or pulmonary haemosiderosis, celiac disease must be considered in differential diagnosis.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Lionetti P. The enteropathy of celiac disease. J Pediatr Gastroenterol Nutr 2002;34:18–21. [DOI] [PubMed] [Google Scholar]
- 2.Le Clainche L, Le Bourgeois M, Fauroux B, et al. Long-term outcome of idiopathic pulmonary hemosiderosis in children. Medicine (Baltimore) 2000;79:318–26. [DOI] [PubMed] [Google Scholar]
- 3.Curione M, Barbato M, De Biase L, et al. Prevalence of coeliac disease in idiopathic dilated cardiomyopathy. Lancet 1999;354:222–3. [DOI] [PubMed] [Google Scholar]
- 4.Yacoub M, Mahjoub H, Abroug S, et al. Idiopathic pulmonary hemosiderosis, celiac disease and cardiomyopathy. Arch Pediatr 1994;1:587–90. [PubMed] [Google Scholar]
- 5.Caforio AL, Goldman JH, Baig MK, et al. Cardiac autoantibodies in dilated cardiomyopathy become undetectable with disease progression. Heart 1997;77:62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makhdoom ZA, Randall NW. Dilated cardiomyopathy due to anticardiolipin syndrome in association with celiac sprue. J Clin Gastroenterol 2000;31:91–2. [DOI] [PubMed] [Google Scholar]
- 7.Ploier R, Emhofer J, Dorninger L, et al. Immunological aspects of a child with idiopathic pulmonary hemosiderosis and celiac disease. Klin Padiatr 1998;210:409–12. [DOI] [PubMed] [Google Scholar]
- 8.Alwitry A. Vitamin A deficiency in coeliac disease. Br J Ophthalmol 2000;84:1079–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ventura A, Neri E, Ughi C, et al. Gluten-dependent diabetes-related and thyroid-related autoantibodies in patients with celiac disease. J Pediatr 2000;137:263–5. [DOI] [PubMed] [Google Scholar]


