Abstract
Papillary thyroid carcinoma is an extraintestinal manifestation of patients with familial adenomatous polyposis, mainly occurring in young women. Recent publications highlight that familial adenomatous polyposis-associated papillary thyroid carcinoma represents a distinct type of follicular cell neoplasm histologically characterised by cribriform-morular aspects, the incidence of which has probably been underestimated so far. We report a case history of familial adenomatous polyposis-associated papillary thyroid carcinoma occurring in a 55-year-old man with Gardner syndrome, underscoring the importance of careful ultrasound screening examination of the thyroid gland in this condition.
Background
The case presented here highlights that, although familial adenomatous polyposis (FAP)-related papillary thyroid carcinoma (PTC) is a rare event in men, it should not be disregarded. Lastly, the peculiar morphology of cribriform-morular variant of PTC should alert pathologists about the possibility of an underlying FAP syndrome. An effective communication with clinicians is mandatory, since in up to a third of the cases, thyroid cancer may represent the first manifestation of this autosomal dominant disease.
Case presentation
Introduction
FAP is an inherited autosomal-dominant syndrome caused by germline mutations in the APC gene, characterised by hundreds of colonic adenomas already developing in young patients. PTC is a well-known extraintestinal manifestation of FAP, mostly occurring in young women.1–3 Recent studies highlight that clinical pathological and molecular features of thyroid cancer in FAP differ from the sporadic form.4–7 In addition, an increasing body of evidence suggests that the prevalence of PTC in the context of the FAP syndrome has been underestimated so far.8
We describe a patient who was diagnosed with FAP-associated thyroid carcinoma, discovered during routine surveillance for FAP. This case underlines both the appropriateness of thyroid screening in this condition and the role of pathologists in characterisation of the histological features of this particular form of familial non-medullary thyroid cancer, especially when the neoplasm appears before intestinal or other extraintestinal manifestations of FAP.
Case
A 55-year-old man was diagnosed with Gardner's syndrome in his 30s, as a member of a well-documented and genetically characterised FAP family (APC mutation in exon 15). Since then, he underwent regular gastrointestinal endoscopy examinations, but no duodenal or colonic polyps were seen, with the exception of recurrent multiple fundic gland polyps of the stomach. In 1988, the patient underwent surgery for fibromatosis (desmoid tumour) of the thoracic wall, and subsequent adjuvant radiotherapy was delivered. In January 2009, he complained of abdominal pain; the CT scan revealed a mass of the ileal mesentery of 14×6 cm. Concomitantly, screening thyroid ultrasonography revealed the presence of a well-circumscribed nodule in the left lobe, 3.5 cm in diameter (figure 1). Fine needle aspiration (three passes) was not representative (Thy1, according to British Guidelines for the management of thyroid cancer in adults). The patient underwent simultaneous segmental ileal resection and left hemithyroidectomy. The poorly circumscribed mesenteric mass was consistent with a desmoids, characterised by elongated, spindle cells of uniform appearance infiltrating the surrounding mesenterial fat and the intestinal wall, while the thyroid nodule was consistent with a cribriform-morular variant of PTC (figure 2A). The neoplastic cells formed several small nests dispersed in an encapsulated nodule characterised by highly hyalinized fibrous stroma with calcifications and bona metaplasia. Immunohistochemical staining revealed strong nuclear and cytoplasmic of beta-catenin expression in both PTC (figure 2B) and fibromatosis, but it was restricted to the membrane of the normal follicular cells. Both the surrounding thyroid tissue and the subsequently resected thyroid right lobe were free of tumour.
Figure 1.
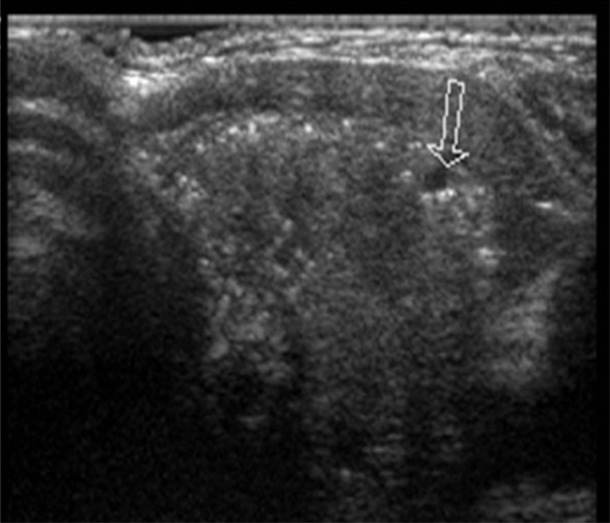
Thyroid sonogram: heterogeneous nodule with marked hypoechogenicity and punctate microcalcifications (arrow) in the left lobe.
Figure 2.
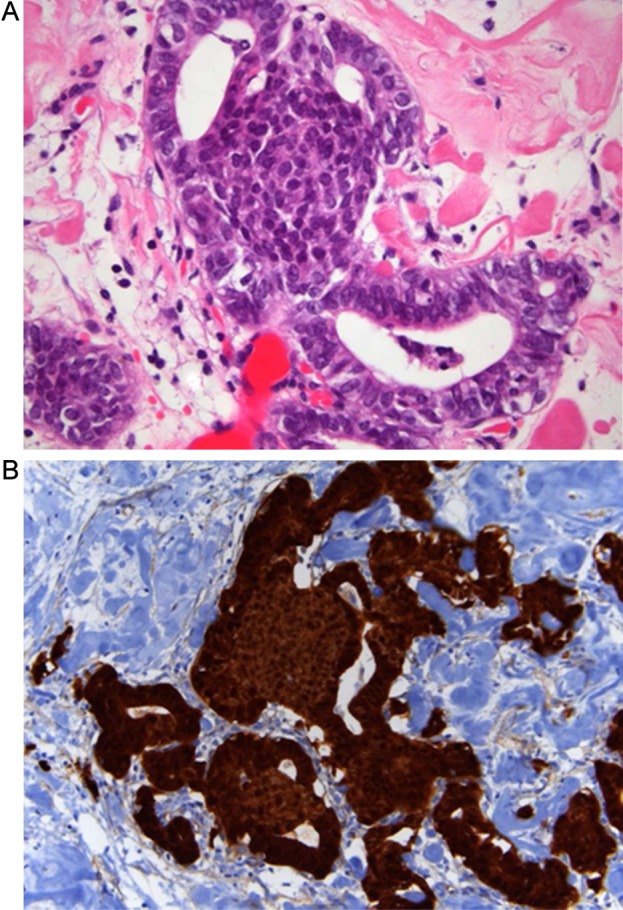
(A) Cribriform-morular variant of papillary thyroid carcinoma in a familial adenomatous polyposis patient displaying characteristic morular structures (H&E). (B) Immunohistochemistry: strong nuclear and cytoplasmatic expression of β-catenin.
Discussion
Extracolonic manifestations of FAP include osteomas, epidermal cysts, desmoids, upper gastrointestinal tract polyps/hamartomas, congenital hypertrophy of the retinal pigmented epithelium, hepatoblastomas and thyroid tumours. The pathogenetic mechanisms underlying extracolonic disease are the same as those driving the development of adenomatous colonic polyps. Germline mutations of the APC gene lead to a truncated protein that lacks the β-catenin binding site. As a consequence, β-catenin cannot be degraded and accumulates in the nucleus, as highlighted by immunohistochemical analysis, where it mediates enhancement of transcriptional activities. The reported lifetime risk of developing thyroid cancer in patients with FAP approaches 2%. However, recent works suggest a significantly higher prevalence of 12% in patients screened with ultrasound.8 Young women with FAP carry a particular risk of developing thyroid cancer, which is usually bilateral and multifocal. The histological features of FAP-associated thyroid cancers were initially described by Harach et al,9 and subsequently confirmed by other authors.4 Up to 60–90% exhibit features consistent with the cribriform-morular variant of PTC, a subtype accounting only for 0.1–0.2% of all PTC. The tumours are usually encapsulated and may display extensive fibrosis. They show solid, cribriform and papillary growth patterns with peculiar intervening morular structures. The neoplastic cells are cuboidal to tall and do not usually show characteristic nuclear features of sporadic PTC, such as nuclear grooving, clearing, overlapping and intranuclear inclusions. Most of FAP patients with thyroid cancers bear germline mutations of APC gene in exon 15; however, before codon 1220 and outside the mutation cluster region located between codons 1286 and 1513.5 6 Clinically, the cribriform-morular variant behaves as classical PTC, with approximately 10% of cases developing metastatic disease.
Our report underscores a number of aspects with important clinical implications. First, it urges clinicians to pay attention to familial non-medullary thyroid carcinomas which have been recognised only recently,1–3 but account for approximately 5% of all non-medullary thyroid cancers. In addition to FAP, thyroid neoplasms may occur in a variety of familial tumour syndromes with a preponderance of non-thyroid tumours such as PTEN hamartoma tumour syndrome (PHTS; Cowden syndrome), Carney complex, Peutz-Jeghers syndrome, Werner syndrome and multiple endocrine neoplasia two. Second, it emphasises the importance of thyroid screening ultrasound examination in individuals with FAP. This aspect is of particular importance for gastroenterologists who usually follow these patients by regular endoscopy and though well aware of extracolonic manifestations such as desmoids or gastric fundic gland polyps, but tend to underestimate the prevalence of thyroid cancers. The case presented here highlights that, although FAP-related PTC is a rare event in men, it should not be disregarded. Lastly, the peculiar morphology of cribriform-morular variant of PTC should alert pathologists about the possibility of an underlying FAP syndrome. An effective communication with clinicians is mandatory, since in up to a third of the cases, thyroid cancer may represent the first manifestation of this autosomal-dominant disease.10 11
For the moment there are insufficient data regarding the optimal screening for thyroid cancer in FAP. In our opinion it is reasonable to recommend an ultrasound examination of the thyroid gland at initial diagnosis. Young women must be observed with particular care; it remains unclear, however, whether the detection of APC mutations outside the mutation cluster region should influence screening strategies. We suggest that any thyroid nodule in FAP individuals be considered suspicious until proven otherwise. A ‘negative’ fine needle aspiration should be interpreted with caution, due to the extensive fibrosis and lack of characteristic nuclear changes of the cribriform-morular variant of PTC. As recently reported, a negative 99 mTc-sestaMIBI thyroid scan rules out malignancy in cold nodules with non-diagnostic cytology, with a 100% negative predictive value, whereas histology is mandatory in an MIBI-positive nodule.12 As a consequence, 99 mTc-sestaMIBI thyroid scanning should be considered a complementary diagnostic tool in patients with FAP and thyroid nodules.
Additional studies are also warranted to clarify the clinical significance of the recognition of the cribriform-morular variant of PTC in patients not known to be FAP carriers. So far it is not possible to distinguish between sporadic and familial forms of this variant: when diagnosed, we suggest to perform both endoscopic examination of the gastrointestinal tract and radiological exams in order to assess the coexistence of FAP, whereas search for APC germline mutations should probably be restricted to individuals with a family history suspicious for FAP syndrome.
Learning points.
To urge clinicians to pay attention to familial non-medullary thyroid carcinomas which have been recognised only recently.
To emphasise the importance of thyroid screening ultrasound examination in individuals with FAP.
Thyroid cancer may represent the first manifestation of FAP; the peculiar morphology of cribriform-morular variant of PTC should alert pathologists about the possibility of an underlying FAP syndrome.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Dotto J, Nosé V. Familial thyroid carcinoma. A diagnostic algorithm. Adv Anat Pathol 2008;15:332–49. [DOI] [PubMed] [Google Scholar]
- 2.Nosé V. Familial thyroid cancer: a review. Mod Pathol 2011;4(Suppl 2):S19–33. [DOI] [PubMed] [Google Scholar]
- 3.Nosé V. Familial non-medullary thyroid carcinoma: an update. Endocr Pathol 2008;19:226–40. [DOI] [PubMed] [Google Scholar]
- 4.Cameselle-Teijeiro J, Chan JK. Cribriform-morular variant of papillary carcinoma: a distinctive variant representing the sporadic counterpart of familial adenomatous polyposis-associated thyroid carcinoma? Mod Pathol 1999;12:400–11. [PubMed] [Google Scholar]
- 5.Cetta F, Montalto G, Gori M, et al. Germline mutations of the APC gene in patients with Familial Adenomatous Polyposis-associated thyroid carcinoma: results from a European Cooperative Study. J Clin Endocrinol Metab 2000;85:286–92. [DOI] [PubMed] [Google Scholar]
- 6.Soravia C, Sugg SL, Berk T, et al. Familial adenomatous polyposis associated thyroid cancer: a clinical pathological and molecular genetics study. Am J Pathol 1999;154:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito Y, Miyauchi A, Ishikawa H, et al. Our experience of treatment of cribriform morular variant of papillary thyroid carcinoma; difference in clinicopathological features of FAP-associated and sporadic patients. Endocr J 2011;58:685–9. [DOI] [PubMed] [Google Scholar]
- 8.Herraiz M, Barbesino G, Faquin W, et al. Prevalence of thyroid cancer in familial adenomatous polyposis syndrome and the role of screening ultrasound examination. Clin Gastroenterol Hepatol 2007;5:367–73. [DOI] [PubMed] [Google Scholar]
- 9.Harach HR, Williams GT, Williams ED. Familial adenomatous polyposis associated thyroid carcinoma: a distinct type of follicular cell neoplasm. Histopathology 1994;25:549–61. [DOI] [PubMed] [Google Scholar]
- 10.Chung DC, Maher MM, Faquin WC. Case 37–2006: a 19-year-old women with thyroid cancer and lower gastrointestinal bleeding. N Engl J Med. 2006;355:2349–57. [DOI] [PubMed] [Google Scholar]
- 11.Schaeffer DF, Yoshida EM, Owen DA, et al. Familial adenomatous polyposis-rendering a diagnosis based on recognition of an unusual primary thyroid neoplasm. Case Rep Med 2011;2011:767610 Epub 2011 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giovanella L, Suriano S, Maffioli M, et al. 99mTc-sestaMIBI scanning in thyroid nodules with non-diagnostic cytology. Head Neck 2010;32:607–11. [DOI] [PubMed] [Google Scholar]


