Abstract
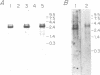
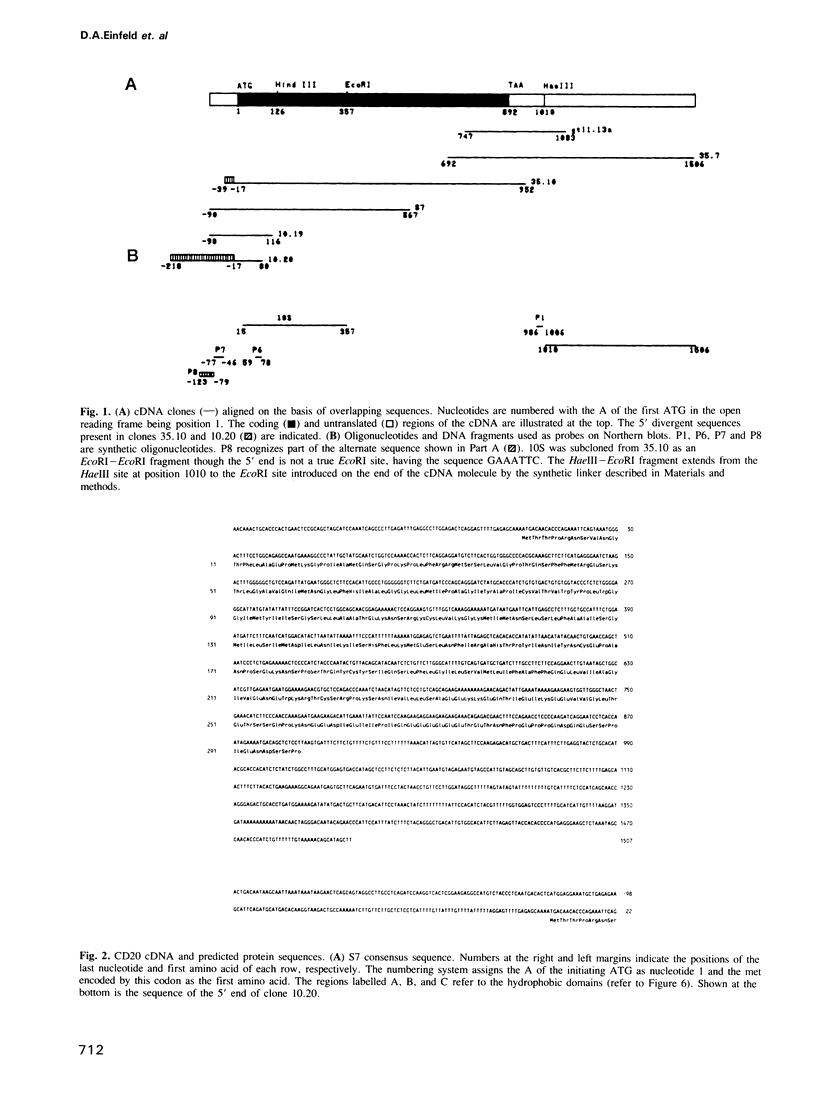
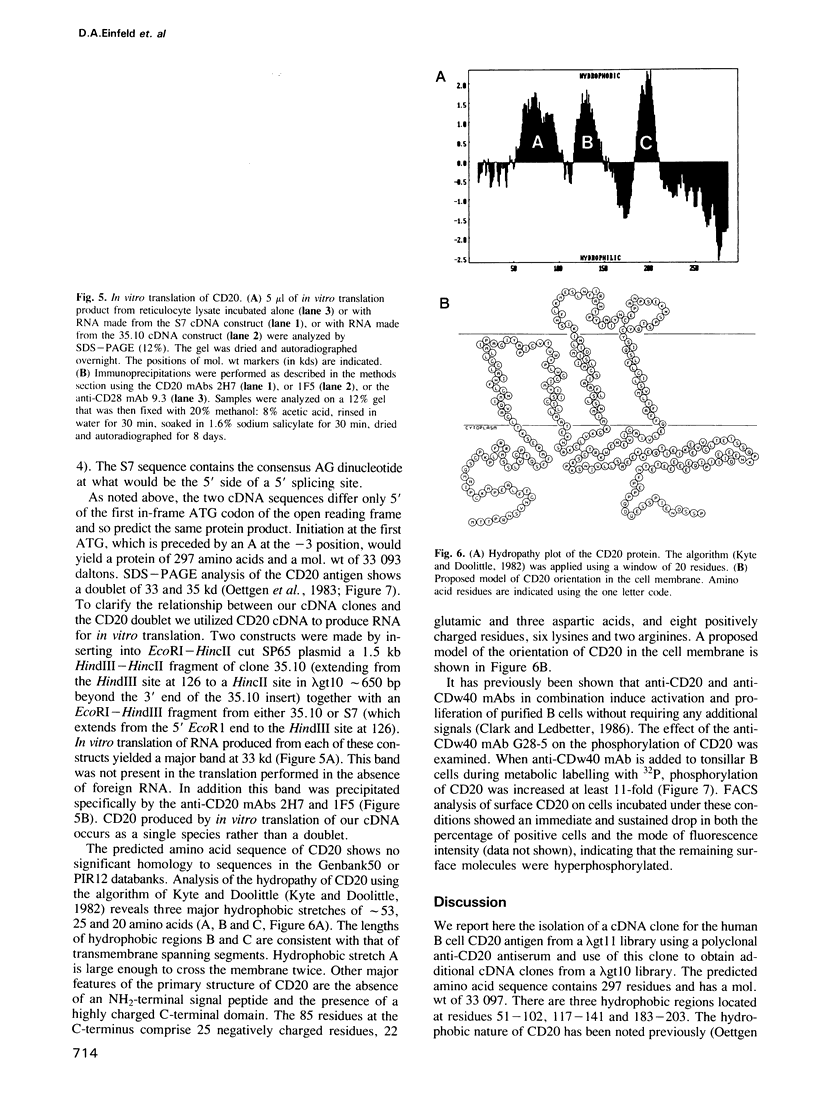
CD20 is an antigen expressed on normal and malignant human B cells that is thought to function as a receptor during B cell activation. Here we report the isolation of a CD20-specific cDNA clone from a lambda gt11 library using a polyclonal antiserum raised against purified CD20 antigen. Additional cDNA clones were then isolated from a lambda gt10 library. Alignment of the sequences of overlapping lambda clones reveal a single consensus sequence except for a divergence that preceded the first methionine within the open reading frame. Normal B cells and B cell lines contain a prominent 2.6 kb mRNA and a lower level of a 3.3 kb mRNA. An oligonucleotide derived from one of the divergent sequences hybridized to the 3.3 kb mRNA only, indicating that the two mRNA species are derived from an alternative splicing mechanism. The predicted amino acid sequence of CD20 reveals three major hydrophobic regions of approximately 53, 25 and 20 amino acids. CD20 lacks an NH2-terminal signal peptide and contains a highly charged COOH-terminal domain. Although CD20 is immunoprecipitated as a doublet of 33 and 35 kd proteins from B cells, in vitro translation of CD20 cDNA produced a single 33 kd protein that was specifically immunoprecipitated with monoclonal CD20 antibodies. CD20 was strongly phosphorylated on resting B cells after CDw40 stimulation, suggesting that CD20 may be functionally regulated by a protein kinase(s).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballivet M., Patrick J., Lee J., Heinemann S. Molecular cloning of cDNA coding for the gamma subunit of Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4466–4470. doi: 10.1073/pnas.79.14.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4494–4498. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Shu G. Activation of human B cell proliferation through surface Bp35 (CD20) polypeptides or immunoglobulin receptors. J Immunol. 1987 Feb 1;138(3):720–725. [PubMed] [Google Scholar]
- Clark E. A., Shu G., Ledbetter J. A. Role of the Bp35 cell surface polypeptide in human B-cell activation. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1766–1770. doi: 10.1073/pnas.82.6.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers-Thiery A., Giraudat J., Bentaboulet M., Changeux J. P. Complete mRNA coding sequence of the acetylcholine binding alpha-subunit of Torpedo marmorata acetylcholine receptor: a model for the transmembrane organization of the polypeptide chain. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2067–2071. doi: 10.1073/pnas.80.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Fennewald S., van Santen V., Kieff E. Nucleotide sequence of an mRNA transcribed in latent growth-transforming virus infection indicates that it may encode a membrane protein. J Virol. 1984 Aug;51(2):411–419. doi: 10.1128/jvi.51.2.411-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer-Moore J., Stroud R. M. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):155–159. doi: 10.1073/pnas.81.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. L., Boublik M., Kaback H. R. Structure of the lac carrier protein of Escherichia coli. J Biol Chem. 1983 Jan 10;258(1):31–34. [PubMed] [Google Scholar]
- Golay J. T., Clark E. A., Beverley P. C. The CD20 (Bp35) antigen is involved in activation of B cells from the G0 to the G1 phase of the cell cycle. J Immunol. 1985 Dec;135(6):3795–3801. [PubMed] [Google Scholar]
- Kopito R. R., Lodish H. F. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature. 1985 Jul 18;316(6025):234–238. doi: 10.1038/316234a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T., Fukuda K., Mikami A., Maeda A., Takahashi H., Mishina M., Haga T., Haga K., Ichiyama A., Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986 Oct 2;323(6087):411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Clark E. A. Surface phenotype and function of tonsillar germinal center and mantle zone B cell subsets. Hum Immunol. 1986 Jan;15(1):30–43. doi: 10.1016/0198-8859(86)90315-0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Martin P. J., Spooner C. E., Wofsy D., Tsu T. T., Beatty P. G., Gladstone P. Antibodies to Tp67 and Tp44 augment and sustain proliferative responses of activated T cells. J Immunol. 1985 Oct;135(4):2331–2336. [PubMed] [Google Scholar]
- Ledbetter J. A., Shu G., Gallagher M., Clark E. A. Augmentation of normal and malignant B cell proliferation by monoclonal antibody to the B cell-specific antigen BP50 (CDW40). J Immunol. 1987 Feb 1;138(3):788–794. [PubMed] [Google Scholar]
- Liebowitz D., Wang D., Kieff E. Orientation and patching of the latent infection membrane protein encoded by Epstein-Barr virus. J Virol. 1986 Apr;58(1):233–237. doi: 10.1128/jvi.58.1.233-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983 Oct;34(3):807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Oettgen H. C., Bayard P. J., Van Ewijk W., Nadler L. M., Terhorst C. P. Further biochemical studies of the human B-cell differentiation antigens B1 and B2. Hybridoma. 1983;2(1):17–28. doi: 10.1089/hyb.1983.2.17. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull G. E., Schwartz A., Lingrel J. B. Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature. 1985 Aug 22;316(6030):691–695. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- Smeland E. B., Beiske K., Ohlsson R., Holte H., Ruud E., Blomhoff H. K., Jefferis R., Godal T. Activation of human B cells: alternate options for initial triggering and effects of nonmitogenic concentrations of anti-IgM antibodies on resting and activated cells. J Immunol. 1987 May 15;138(10):3179–3184. [PubMed] [Google Scholar]
- Smeland E., Godal T., Ruud E., Beiske K., Funderud S., Clark E. A., Pfeifer-Ohlsson S., Ohlsson R. The specific induction of myc protooncogene expression in normal human B cells is not a sufficient event for acquisition of competence to proliferate. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6255–6259. doi: 10.1073/pnas.82.18.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980 Oct;125(4):1678–1685. [PubMed] [Google Scholar]
- Tedder T. F., Boyd A. W., Freedman A. S., Nadler L. M., Schlossman S. F. The B cell surface molecule B1 is functionally linked with B cell activation and differentiation. J Immunol. 1985 Aug;135(2):973–979. [PubMed] [Google Scholar]
- Tedder T. F., Forsgren A., Boyd A. W., Nadler L. M., Schlossman S. F. Antibodies reactive with the B1 molecule inhibit cell cycle progression but not activation of human B lymphocytes. Eur J Immunol. 1986 Aug;16(8):881–887. doi: 10.1002/eji.1830160802. [DOI] [PubMed] [Google Scholar]
- Webb N. R., Rose T. M., Malik N., Marquardt H., Shoyab M., Todaro G. J., Lee D. C. Bovine and human cDNA sequences encoding a putative benzodiazepine receptor ligand. DNA. 1987 Feb;6(1):71–79. doi: 10.1089/dna.1987.6.71. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]