Abstract
In this case report, a patient is described with an unusual cause of renal artery stenosis (RAS). The patient presented with acute anuric renal failure and hypertensive urgency, following a nephrectomy, which was complicated by massive blood loss. Because the acute renal failure was first presumed to be due to acute tubular necrosis, the diagnosis of a nearly complete iatrogenic RAS was not made until 6 weeks after surgery. The stenosis was caused by five misplaced surgical clips on the artery of the remaining kidney. The hypertension was initially treated with ACE inhibitor. Eight weeks after the initial surgery, a successful revascularisation procedure was performed, leading to the recovery of kidney function.
Background
The classic symptoms of a bilateral severe renal artery stenosis (RAS) are usually refractory hypertension, chronic kidney failure and sometimes ‘flash’ pulmonary oedema. The stenosis of the renal arteries causes reduction in renal perfusion, resulting in volume expansion due to reduced diuresis.1 There is also an increased renin release from the under-perfused kidney.2 Whether treatment of RAS is beneficial for improvement of renal function remains controversial.3 4 5 In the general population, RAS is mostly caused by atherosclerosis or fibromuscular dysplasia.6 In this case report we describe a patient who presented with symptoms classic of severe RAS, but with an unexpected cause. Moreover, after 8 weeks of severe impaired kidney perfusion, with anuric renal failure, a successful revascularisation procedure was performed. This led to the recovery of kidney function.
Case presentation
A 54-year-old woman was referred to the department of internal medicine, complaining of back pain, loss of appetite and weight loss. She had no medical history, besides essential hypertension, which was well regulated with amlodipine and metoprolol. A CT scan of the abdomen revealed a mass in the left kidney, suggestive of renal cell cancer. Multiple small lesions in bones and lungs were suggestive of pulmonary and bone metastases.
Different treatment options were discussed. Finally, a proposal was made for a palliative nephrectomy, followed by treatment with sunitinib (a multitargeted receptor tyrosine kinase inhibitor).
Preoperatively, the patient had a good kidney function, with a serum creatinine of 53 μmol/l (0.59 mg/dl) (estimated glomerular filtration rate (GFR) 104 ml/min/1.73 m2).
Surgery was complicated by hypotensive episodes due to massive bleeding. The surgeon could not immediately determine the source of the bleeding and had to explore the abdomen. There was an estimated total blood loss of 3 litres (lowest blood pressure 70/30 mm Hg). The subsequent hypovolaemic shock was treated with multiple transfusions with washed erythrocytes, fresh frozen plasma and hydroxyl-ethyl-starch. The diagnosis of renal cell carcinoma in the removed kidney was confirmed by histopathological examination.
After surgery, the patient was admitted to the intensive care unit. She was stable in haemodynamic condition with a mean arterial pressure between 60 and 70 mm Hg. After 24 h, she was successfully extubated. However, it soon became apparent that she was anuric. A duplex ultrasound of the remaining kidney, made on the intensive care the first day after surgery, showed no signs of hydronephrosis and suggested a normal blood flow in the renal artery. It was concluded that the patient most likely suffered from acute tubular necrosis in combination with possible nephrotoxicity of infused hydroxyl-ethyl-starch.7 8 After 48 h, she was discharged to the internal medicine ward. Conventional haemodialysis was started on the fourth day postsurgery.
In the days thereafter, the patient developed hypertension (blood pressure 180/100 mm Hg). Her antihypertensive medications, which were stopped immediately after surgery, were reintroduced. Several increases in dosage over the following days did not alleviate the elevated blood pressure. Efforts to raise the ultrafiltration rate during dialysis were unsuccessful, resulting in vomiting and muscle cramps, but not in lowering of the blood pressure.
Four weeks after surgery, the patient complained of blurred vision and headaches. The ophthalmologist diagnosed a hypertensive retinopathy grade 3. The blood pressure at this time was 176/88 mm Hg. Lisinopril 10 mg daily was started, which immediately resulted in a lowered blood pressure. She remained anuric with a maximum urine production of 50 cc/24 h. After adding up the acute anuric kidney failure after nephrectomy, the severe hypertension and the strong antihypertensive effect of ACE inhibition, we presumed that the patient was suffering from renovascular hypertension due to a (maybe pre-existent) stenosis in the remaining renal artery.
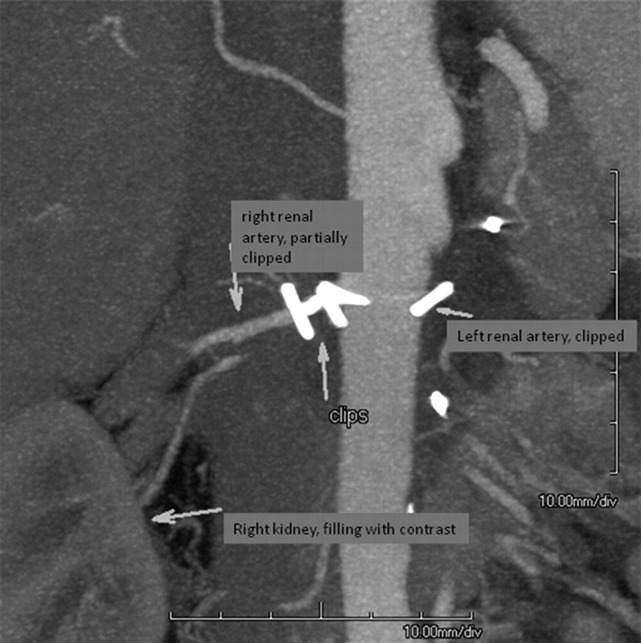
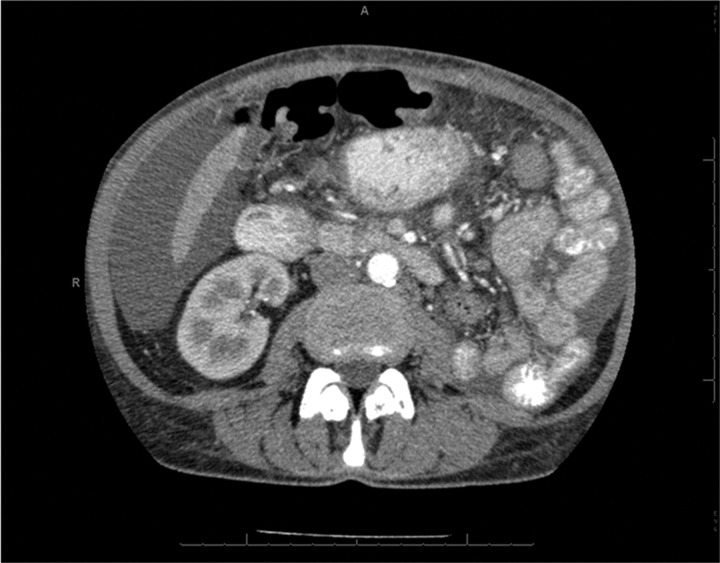
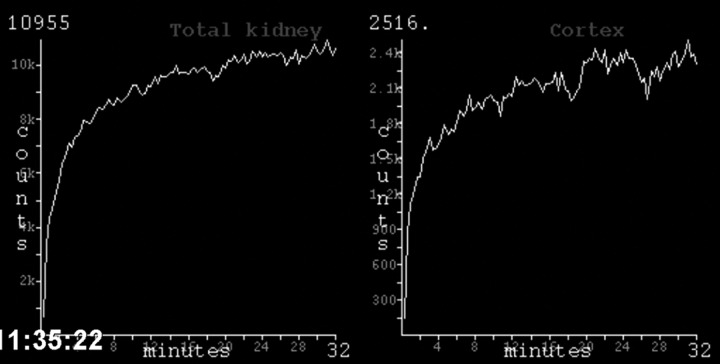
Six weeks after the initial surgery a CT angiography was performed which indeed showed a 95% stenosis of the remaining renal artery. The stenosis was not caused by pre-existing atherosclerosis or fibromuscular dysplasia, but by five misplaced surgical clips (figure 1). The scan also showed that the artery was not completely occluded; the cortex of the kidney was still slightly enhanced by contrast (figure 2). Perfusion of the kidney was confirmed by renal scintigraphy (figure 3). A percutaneous angiographic revascularisation procedure was initiated, but was unsuccessful. Eventually, the patient was referred to a university medical centre to explore the options for surgical revascularisation.
Figure 1.
CT angiography with röntgen contrast showing a 95% stenosis of the remaining renal artery, caused by five misplaced surgical clips.
Figure 2.
CT abdomen with röntgen contrast showing a not-complete occlusion of the renal artery; the cortex of the kidney is still slightly enhanced by contrast.
Figure 3.
Renal scintigraphy confirming perfusion of the kidney.
After ample discussion between the surgical team and the consulting nephrologists about the expected benefit of revascularisation after such a long time of renal ischaemia, they finally decided to give it a chance. Therefore, 2 months after the initial nephrectomy, a hepatorenal bypass was created.
Outcome and follow-up
Diuresis started immediately after surgery. Three weeks later, the blood pressure had normalised, the urinary production exceeded 2 litres/24 h and patient was free from dialysis. Renal function stabilised on an estimated GFR of 32 ml/min/1.73 m2 (serum creatinine 130 μmol/l).
One year after the revascularisation procedure, the patient is still alive and being treated with sunitinib without any complications. Kidney function remains stable and she is normotensive without any antihypertensive drugs. The tumour does not show any signs of progression.
Discussion
This case report describes an unusual cause of RAS. The patient presented with acute anuric renal failure and severe hypertension after unilateral nephrectomy, suggestive of occlusion of the remaining renal artery. The RAS was due to misplaced surgical clips, which were probably randomly placed in a period of massive bleeding. However, there was a diagnostic delay. A duplex ultrasound performed on the intensive care the first day postsurgery suggested a normal flow in the renal artery. For the diagnosis of RAS, echography has low diagnostic accuracy.9 Since acute tubular necrosis was suspected and because of the risk of contrast-induced nephropathy, a diagnostic CT angiography was not performed until 6 weeks after surgery.
The weeks following surgery, the patient developed hypertension, which proved only treatable with ACE inhibition. This presentation is strongly suggestive for RAS. Due to the decreased blood flow behind the stenosis, the kidney senses a low blood pressure, resulting in secretion of renin, which causes severe hypertension. In bilateral RAS, or unilateral RAS in a one-kidney patient, administration of an ACE inhibitor could further compromise renal function because of reduced kidney perfusion.10 11
It is known from abdominal aortic surgery that the maximum time the kidney can be deprived of blood is approximately 50 min.12 The same is true for kidney transplants.13 14 In this case report, a subtotal severe occlusion of renal blood flow, leading to anuric acute renal failure for more than 8 weeks, was successfully treated with revascularisation surgery. Apparently, the renal blood flow was too low to permit filtration pressure, but enough to save the metabolic demands of the kidney to keep it viable.
This case demonstrates three important findings. First, when there is an unexplained acute renal failure after abdominal surgery, iatrogenic occlusion of the renal artery should be considered. The appropriate diagnostic step should be an angiography, for which general precautions should be taken to prevent contrast-induced nephropathy.15
Second, revascularisation of RAS can be beneficial for both kidney function and blood pressure control in certain cases, especially when the stenosis causes acute renal failure.
Third, a warm ischaemic time of several weeks in a non-total occlusion of the renal artery can be tolerated by the kidney.
In conclusion, we describe a patient with an iatrogenic, subtotal RAS, where a successful revascularisation procedure was performed 2 months after the initial event, resulting in the recovery of kidney function.
Learning points.
When there is an unexplained acute renal failure after abdominal surgery, iatrogenic occlusion of the renal artery should be considered. The appropriate diagnostic step should be an angiography.
Revascularisation of renal artery stenosis (RAS) can be beneficial for both kidney function and blood pressure control in certain cases, especially when acute renal failure is caused by RAS.
In a non-total occlusion of the renal artery, a warm ischaemic time of several weeks can be tolerated by the kidney.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Rabbia C, Pini R. Evidence-based medicine in renal artery stenting. J Cardiovasc Surg 2010;51:755–63. [PubMed] [Google Scholar]
- 2.Ruby ST, Burch A, White WB. Unilateral renal artery stenosis seen initially as severe and symptomatic hypokalemia. Pathophysiologic assessment and effects of surgical revascularization. Arch Surg 1993;128:346–8. [DOI] [PubMed] [Google Scholar]
- 3.Valluri A, Severn A, Chakraverty S. Do patients undergoing renal revascularization outside of the ASTRAL trial show any benefit? Results of a single-centre observational study. Nephrol Dial Transplant 2012;27:734–8. [DOI] [PubMed] [Google Scholar]
- 4.Wheatly K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009;361:1953–62. [DOI] [PubMed] [Google Scholar]
- 5.Van de Berg DT, Deinum J, Postma CT, et al. The efficacy of renal angioplasty in patients with renal artery stenosis and flash oedema or congestive heart failure: a systematic review. Eur J Heart Fail 2012;14:773–81. [DOI] [PubMed] [Google Scholar]
- 6.Vagaonescu TD, Dangas G. How to diagnose, how to treat: renal artery stenosis—diagnosis and management. J Clin Hypertens (Greenwich) 2002;4:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickenmann M, Oettl T, Mihatsch MJ. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis 2008;51:491–503. [DOI] [PubMed] [Google Scholar]
- 8.Groeneveld AB, Navickis RJ, Wilkes MM. Update on the comparative safety of colloids: a systematic review of clinical studies. Ann Surg 2011;253: 470–83. [DOI] [PubMed] [Google Scholar]
- 9.Drieghe B, Madaric J, Sarno GO, et al. Assessment of renal artery stenosis: side-by-side comparison of angiography and duplex ultrasound with pressure gradient measurements. Eur Heart J 2008;29:517–24. [DOI] [PubMed] [Google Scholar]
- 10.Hannedouche T, Godin M, Fries D, et al. Acute renal thrombosis induced by angiotensin-converting enzyme inhibitors in patients with renovascular hypertension. Nephron 1991;57:230–1. [DOI] [PubMed] [Google Scholar]
- 11.Hackam DG, Spence JD, Garg AX, et al. Role of renin-angiotensin system blockade in atherosclerotic renal artery stenosis and renovascular hypertension. Hypertension 2007;50:998–1003. [DOI] [PubMed] [Google Scholar]
- 12.Wahlberg E, DiMuzio PJ, Stoney RJ. Aortic clamping during elective operations for infrarenal disease: the influence of clamping time on renal function. J Vasc Surg 2002;36:13–18. [DOI] [PubMed] [Google Scholar]
- 13.Gok MAS, Asher JF, Shenton BK, et al. Graft function after kidney transplantation from non-heartbeating donors according to Maastricht category. J Urol 2004;172:2331–4. [DOI] [PubMed] [Google Scholar]
- 14.Lledo-Garcia E, Subira-Rios D, Rodriguez-Martinez D, et al. Sildenafil as a protecting drug for warm ischemic kidney transplants: experimental results. J Urol 2009;182:1222–5. [DOI] [PubMed] [Google Scholar]
- 15.Bader BD, Berger ED, Heede MB, et al. What is the best hydration regimen to prevent contrast media-induced nephrotoxicity? Clin Nephrol 2004;62:1–7. [DOI] [PubMed] [Google Scholar]