Abstract
We report an 8-year-old boy who developed high fever and low-back pain. He was diagnosed as having a paravertebral abscess with severe disseminated Staphylococcus aureus infection. He received intravenous antibiotics and drainage of the abscess. Afterwards, he developed thrombosis, endocarditis and empyema thoracis. He received further intravenous antibiotics and proper drainages for his conditions. He eventually recovered well. This case highlights the need for rapid diagnosis and appropriate treatment of severe S aureus infection to prevent serious complications.
Background
Staphylococcus aureus is the most common cause of infection of the skin and soft tissue with severe sepsis being a major sequel that leads to high morbidity and mortality.1 In the preantibiotic era, S aureus was associated with death rates of more than 80%.2 Today, death rates range from 20–40% in patients with S aureus bacteraemia (SAB), and despite improvements in diagnostic and therapeutic tools, these rates have remained unchanged over the past several decades.3 4 SAB commonly causes complications that can involve any part of the body with frequencies ranging from 11% to 53%.5 6 However, certain complications are especially important because of their notoriously poor outcomes and because early diagnosis can be difficult. Recently, many studies especially from the USA and Europe reported that the Panton-Valentine leukocidin (PVL) gene is a major virulence factor in community-associated methicillin-resistant S aureus (CA-MRSA).7–9 Most severe deep soft tissue infections caused by PVL MRSA showed a higher morbidity and mortality. However, a study from the northeast of Thailand found that MRSA isolates did not have the PVL gene but carried the SCC mec type III complex.10 This case highlights the need for rapid diagnosis, appropriate treatment and drainage in order to prevent serious complications.
Case presentation
An 8-year-old boy from northern Thailand presented at our hospital with chief complaint of high fever and low back pain for 7 days. He had no history of major trauma, except that of minor injuries during football games, and no history of underlying diseases. His birth history was unremarkable. He had no known drug allergies and his vaccination was completed. Upon admission to our hospital, he looked sick and had dyspnoea. Physical examination of the boy revealed a high-grade fever (39°C), heart rate of 150 beats/min, and respiratory rate of 68 breaths/min. He had a mildly injected pharynx, and suprasternal and subcostal retraction. Fine crepitation was heard at his left lower lung. His heart sound was normal. His upper abdomen was distended with voluntary guarding and moderate tenderness, and no rebound tenderness. His liver was mildly enlarged (span: 7 cm), mildly tender and firm with sharp margins. His spleen was normal.
Investigations
A complete blood count was notable for haemoglobin concentration of 9.8 g/dl, haematocrit of 28.4%, and a white blood cell count of 23 700 cells/mm3 with 78.7% neutrophils, 8.9% lymphocytes and 12.1% monocytes. The platelet count was 241 000 cells/mm3. A liver function test revealed a total protein level of 5.4 g/dl, albumin of 2 g/dl, globulin of 3.4 g/dl, alkaline phosphatise of 60 U/l, cholesterol of 84 mg/dl, aspartate aminotransferase of 42 U/l, alanine transaminase of 21 U/l, total bilirubin of 0.43 mg/dl and direct bilirubin of 0.21 mg/dl.
Chest radiography showed bilateral haziness of both lungs, and there was no free air under the diaphragm (figure 1). Abdominal radiography showed small bowel dilatation at the left lower quadrant, and soft tissue density at the mid-abdomen. A CT scan showed a paravertebral abscess (figure 2); multiple consolidations in the bilateral basal lungs with minimal left pleural effusion; and thrombosis along the inferior vena cava (IVC) (figure 3), left common iliac vein (CIV) and proximal left internal iliac vein (IIV); and a small liver abscess (figure 4). A bone scan showed osteomyelitis at the fifth lumbar (L5) vertebral body.
Figure 1.
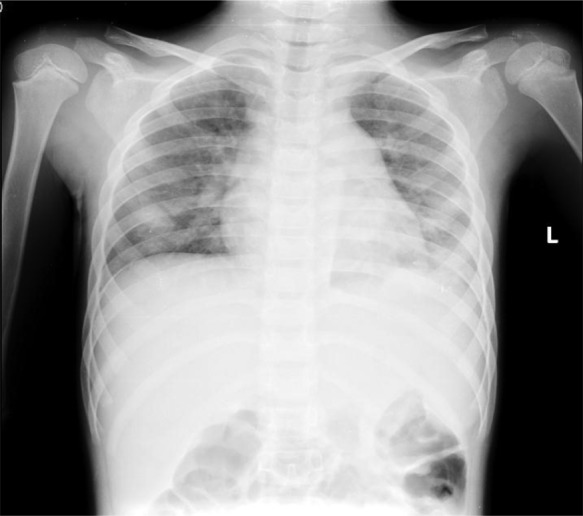
Chest radiograph showing bilateral haziness and no free air under diaphragm.
Figure 2.
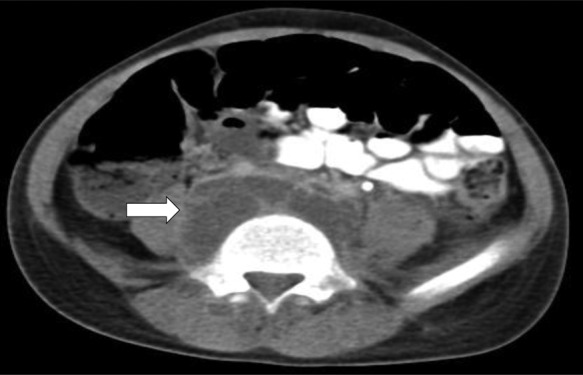
CT scan of abdomen showing paravertebral abscess (arrow).
Figure 3.
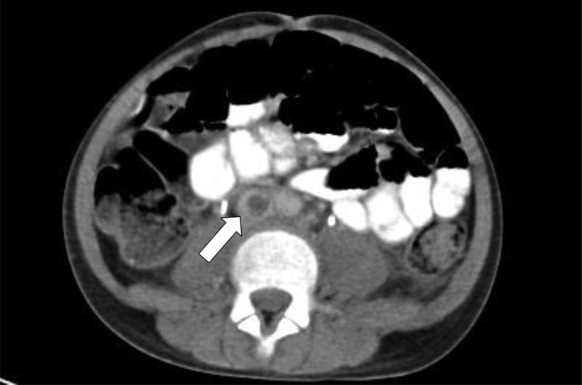
CT scan of abdomen showing thrombosis along inferior vena cava (arrow).
Figure 4.
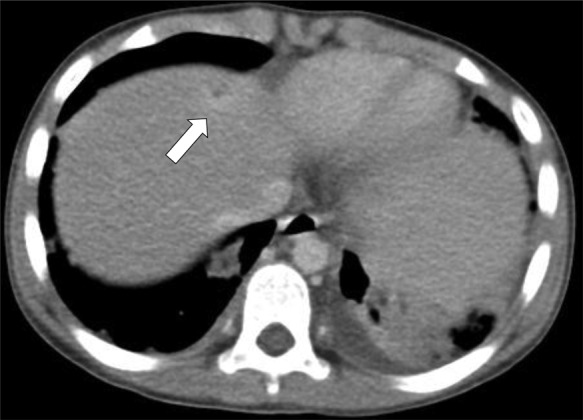
CT scan of abdomen showing small liver abscess (arrow).
A test for anticardiolipin IgM and IgG was negative. Laboratory results revealed normal protein C level of 115.8% (70–130%), normal protein S level of 70% (60–140%) and a normal antithrombin III level of 92.6% (75–125%). Two specimens of blood culture showed S aureus and were sensitive to oxacillin. Tests for PVL gene and mec types were not carried out since these tests are not available in our hospital and are not routine tests in Thailand.
Differential diagnosis
Severe disseminated S aureus infection (DSAI).
Treatment
The boy received intravenous antibiotics (cloxacillin, clindamycin and gentamicin). The paravertebral abscess was drained 3 days after the initiation of antibiotics. The pus culture was positive for S aureus, which was sensitive to oxacillin and clindamycin.
Outcome and follow-up
One day after he was drained of his abscess, the boy had high-grade fever and progressive dyspnoea. Physical examination showed suprasternal, subcostal and intercostal retraction; fine crepitation at the left lower lung; and decreased breath sounds of both lungs. A chest x-ray showed pleural effusion of both lungs. An echocardiogram showed a clot in the IVC and right atrium, vegetation at anterior leaflet of mitral valve and a large amount of fluid and fibrin in the left lung tissue (figure 5). A CT scan of the chest showed evidence of empyema thoracis.
Figure 5.
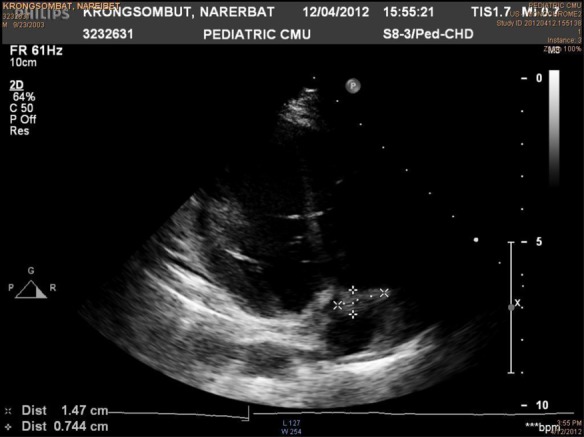
Echocardiogram showing vegetation at anterior leaflet of mitral valve (arrow).
We consulted a chest surgeon to retain the left intercostal drainage (ICD). A follow-up CT scan of the chest (5 days after retained ICD) showed that the massive left pleural effusion had decreased slightly. A CT scan of the upper abdomen showed that the large abscess along the paravertebral region and intraluminal thrombus in the distal IVC, left CIV and proximal left IIV had decreased in size. A paediatric surgeon performed drainage through a retroperitoneal approach and found that the boy also had a 4 cm long bilateral paravertebral abscess at the iliac crest level. The drainage contained a turbid fluid of 30 ml with fibrin, and the gram stain of the pus showed many Gram-positive cocci in clusters. Afterwards, left thoracotomy was performed to remove the anterior basal segment of the left lower lung and lingular lobe.
A follow-up echocardiogram 5 days after the first echocardiogram was normal. Twelve days after treatment with intravenous antibiotics and drainage, his fever had gone. We administered gentamicin for 14 days for its synergistic effect and continued intravenous cloxacillin and clindamycin for 6 weeks. A CT scan at follow-up showed that the lung abscesses and left pleural effusion had dramatically decreased in size. The boy ambulated well and went back to school.
Discussion
An 8-year-old boy presented with severe DSAI. He had a paravertebral abscess, pneumonia, endocarditis, liver abscess and thrombosis. Antibiotic therapy alone is generally not sufficient in patients with severe DSAI without drainage of the abscesses. Loculated collections of purulent material should be relieved by incision and drainage.11 Empirical parenteral antibiotic therapy should be provided immediately to severely ill patients, as in this case. For community-associated infections in Thailand, the likelihood of MRSA is low. In general, Thai physicians thus prescribe β-lactam antibiotics as an empirical therapy instead of vancomycin. In severe cases, aminoglycosides such as gentamicin should be added to synergise with β-lactam antibiotics.1 In addition, when a patient has signs of toxic shock syndrome, clindamycin should be added to suppress bacterial toxic synthesis.
Observational studies indicated that bone and joint infections were the most common source of community-acquired SAB in children, accounting for approximately 60% of cases.12 13 In this case, the boy presented with paravertebral abscess and osteomyelitis at the L5 vertebral body. We, therefore, prescribed cloxacillin, gentamicin and clindamycin as an empirical treatment. However, the abscess needed further drainage, which was performed 3 days after initiation of antibiotic therapy. Afterwards, he developed complications of pulmonary infection, infective endocarditis (IE), liver abscess and septic thrombosis. IE is actually much less frequent in children than in adults (1.4% of cases occur in children whereas up to 30% occur in adults) 13 but had occurred in our case. There are many reports of pulmonary and cardiovascular complications in children, as in our case.14–18 Paterson et al14 who reviewed the records of 1156 patients treated for acute staphylococcal osteitis or septic arthritis over a 12-year period found that 33 patients showed metastatic staphylococcal pneumonia and 10 patients had cardiovascular complications such as myocarditis, pericarditis and pericardial effusion. Wang et al15 reported a case of a 10-year-old boy with multiple staphylococcal arthritis, deep vein thrombosis, pulmonary embolism, pericardial effusion and occlusion of the anterior parietal branch of the right middle cerebral artery.
In this case, since the patient suffered from severe complications of S aureus, we were aware of the possibility of PVL CA-MRSA as a causative agent. As recommended by clinical practice guidelines,10 we therefore prescribed cloxacillin along with gentamicin and clindamycin as an empirical treatment until we knew the results of the bacteria culture. Even though the pus culture turned to methicillin-sensitive strain S aureus, we continued cloxacillin along with clindamycin because we found that he had severe deep tissue infection that involved many vital organs. After proper drainage of all sources of infection, the boy's clinical condition improved.
Learning points
Carefully take the patient's history of any traumas or minor injuries, which will help to diagnose Staphylococcus infection.
Promptly provide a diagnosis and proper antibiotic treatment once Staphylococcus infection is suspected.
Closely monitor and follow-up patients with Staphylococcus infection in order to discover any further hidden metastatic sites of infection.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Lowell GS, Daum RS. Staphylococcus aureus infections. In: Long SS, Pickering LK, Prober CG. Principles and practice of pediatric infectious disease. 3rd edn Philadelphia: Elsevier, 2008:679–93. [Google Scholar]
- 2.Skinner D, Keefer CS. Significance of bacteremia caused by Staphylococcus aureus. A study of 122 cases and a review of the literature concerned with experimental infection in animals. Arch Intern Med 1941;68:851–75. [Google Scholar]
- 3.Mylotte JM, McDermott C, Spooner JA. Prospective study of 114 consecutive episodes of Staphylococcus aureus bacteremia. Rev Infect Dis 1987;9:891–907. [DOI] [PubMed] [Google Scholar]
- 4.Wyllie DH, Crook DW, Peto TE. Mortality after Staphylococcus aureus bacteraemia in two hospitals in Oxfordshire, 1997–2003: cohort study. BMJ 2006;333:281–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998;339:520–32. [DOI] [PubMed] [Google Scholar]
- 6.Fowler VG, Justice A, Moore Cet al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis 2005;40:695–703. [DOI] [PubMed] [Google Scholar]
- 7.Boyle-Vavra S, Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Paton-Valentine leukocidin. Lab Invest 2007;87:3–9. [DOI] [PubMed] [Google Scholar]
- 8.Wannet W.2003. Virulent MRSA strains containing the Panton–Valentine leukocidin gene in the. Netherlands. Euro Surveill (weekly serial online) (cited 10 September 2012); 7:pii=2173. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2173 (accessed 10 September 2012).
- 9.Lopez-Aguilar C, Perez-Roth E, Mendez-Alvarez S, et al. Association between the presence of the Panton-Valentine leukocidin-encoding gene and a lower rate of survival among hospitalized pulmonary patients with Staphylococcal disease. J Clin Microbiol 2007;45:274–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Bayer A, Cosgrove SEet al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011;52:285–92. [DOI] [PubMed] [Google Scholar]
- 11.Todd JK. Staphylococcus aureus. In: Kliegman RM, Stanton BF, Geme JW, Schor NF, Behrman RE. Nelson Textbook of Pediatrics. 19th edn Philadelphia: Elsevier, 2011:904–8. [Google Scholar]
- 12.Hill PC, Wong CG, Voss LMet al. Prospective study of 125 cases of Staphylococcus aureus bacteremia in children in New Zealand. Pediatr Infect Dis J 2001;20:868–73. [DOI] [PubMed] [Google Scholar]
- 13.Suryati BA, Watson M. Staphylococcus aureus bacteraemia in children: a 5-year retrospective review. J Paediatr Child Health 2002;38:290–4. [DOI] [PubMed] [Google Scholar]
- 14.Paterson MP, Hoffman EB, Roux P. Severe disseminated staphylococcal disease associated with osteitis and septic arthritis. J Bone Joint Surg 1990;72:94–7. [DOI] [PubMed] [Google Scholar]
- 15.Wang CF, Lee GH, Wang CL. Disseminated staphylococcal disease: report of one case. Acta Paediatr Taiwan 2005;46:87–90. [PubMed] [Google Scholar]
- 16.Dastjerdi MS, Rostami M, Mirbagheri M. Disseminated staphylococcal infection with polyarthritis in a 12 year old boy. JRMS 2007;12:45–8. [Google Scholar]
- 17.Hieber JP, Nelson AJ, McCracken GH. Acute disseminated staphylococcal disease in childhood. Am J Dis Child 1977;131:181–5. [DOI] [PubMed] [Google Scholar]
- 18.Miles F, Voss L, Segedin E, et al. Review of Staphylococcus aureus infections requiring admission to a paediatric intensive care unit. Arch Dis Child 2005;90:1274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


