Abstract
A 63-year-old man presented to our hospital with amoebic liver abscess and was treated successfully for the same. During the course of his treatment, he developed syncopal attacks and was found to have Torsades de Pointes on electrocardiogram. The patient was treated with intravenous magnesium and direct current cardioversion. Hypokalaemia, chloroquine and sepsis were suspected to have precipitated the arrhythmia. The patient remained arrhythmia-free following the correction of these factors.
Background
Torsades de Pointes (TdP) is a polymorphic ventricular tachycardia occurring in patients with long QT intervals.1 Various drugs, electrolyte disturbances, endocrine factors and so on are known to precipitate this arrhythmia and may lead to sudden cardiac death. Identification of the possible causative factors and their swift correction is important. Cardiac manifestations in amoebiasis are rare, and to the best of our knowledge, one case of ventricular arrhythmia in hepatic amoebic abscess has been reported, and none in the post-emetine era. We report this case so as to sensitise the treating physicians about a potentially fatal event due to a commonly used drug and a frequently encountered electrolyte imbalance.
Case presentation
A 63-year-old man was troubled with high-grade fever with chills associated with right hypochondriac dull aching pain for 15 days. He had been treated on an outpatient basis with a course of chloroquine and paracetamol for 3 days. However, his abdominal pain increased in intensity. He also developed loose motions associated with abdominal cramps and mucus discharge. For these complaints, he was admitted to another hospital, where upon examination, he was febrile and tachycardic. Abdominal palpation revealed an enlarged and tender liver. Ultrasonography showed an ill-defined rounded lesion of mixed echogenecity of size 10×11 cm in the right lobe of the liver, suggestive of a partially liquefied liver abscess. He received treatment with intravenous piperacillin-tazobactam and metronidazole and oral chloroquine for 10 days, resulting in marked clinical improvement. However, on 11th day of therapy, he developed two successive episodes of tonic posturing movements, frothing at the mouth associated with a transient loss of consciousness. The ECG monitor showed broad complex tachycardia. In view of haemodynamic instability, he was cardioverted with a synchronised direct current (DC) shock, initiated on amiodarone and magnesium sulphate drips and transferred to our centre. The patient had no previous admissions for ischaemic heart disease or cerebrovascular disease. He was not previously detected to be diabetic or hypertensive. He had never had any syncopal episodes in the past. He was a teetotaller and did not smoke.
The patient was received with an endotracheal tube in situ, breathing room air; his vitals were stable. Apart from mild right hypochondrial tenderness, his systemic physical examinations were normal.
Investigations
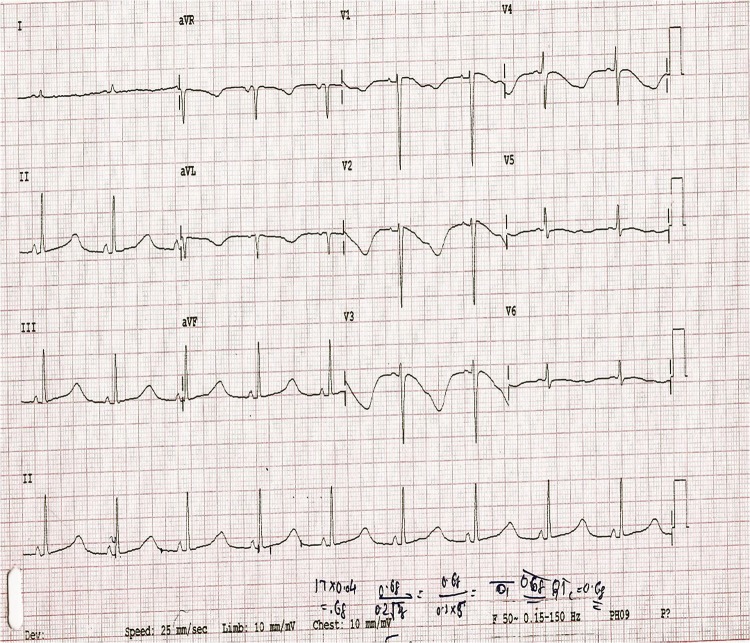
His ECG on admission showed normal sinus rhythm and axis, with T-wave inversions in frontal leads V2–V4. There was delayed repolarisation with a prolonged corrected QT interval (680 ms; figure 1). During the day, he had four more episodes of ventricular tachycardia with haemodynamic compromise, each requiring termination by DC cardioversion. The ECGs during these episodes showed polymorphic ventricular tachycardia with twisting around the axis (figure 2). Oral long-acting propranolol was initiated at 40 mg once a day. His investigations revealed a picture of sepsis with total leucocyte count of 51 300/mm3 and haemoglobin 11.2. His electrolyte panel revealed the following results (in mEq/l): Na+ 138, K+ 2.1, Cl− 104, Mg++ 2.4, Ca++ 7.5. His liver functions were mildly deranged: alanine transaminase was elevated to 167 IU/l, serum albumin 2.1 g/dl and prothrombin time prolonged to 17.6 s compared with a control of 13. The liver abscess of volume 220–260 ml, partially liquefied, was pigtailed under sonographic guidance. The aspirated fluid showed a polymorphonuclear cell predominance, and his entameba immunohaemagglutination test was positive with significant titres (>1 : 320). A transthoracic echocardiography was carried out while the patient was in sinus rhythm. It revealed a left ventricular ejection fraction of 55% and no valvular or regional wall motion abnormalities.
Figure 1.
ECG on admission showing sinus rhythm and normal axis, with T-wave inversions in frontal leads V2–V4. There was delayed repolarisation with a prolonged corrected QT interval (680 ms).
Figure 2.
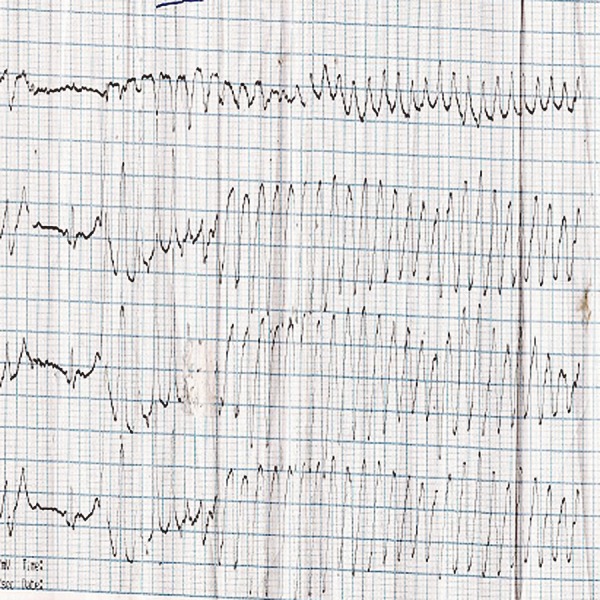
ECG during syncopal episode showing polymorphic ventricular tachycardia with twisting around the axis.
Treatment
He was treated with intravenous metronidazole and oral diloxanide furoate for 10 days, and his loose motions were relieved on the fourth day of therapy. His hypokalaemia resolved after treatment, rising from an initial 2.1–3.7 mEq/l in 4 days, and his leucocyte counts dropped gradually to normal levels (10 600/mm3) in 10 days. Following the withdrawal of chloroquine and correction of electrolyte imbalance, the patient remained arrhythmia free after day 2 of admission, and he was discharged after 12 days of indoor stay. The patient was counselled to be cautious regarding the use of QT prolonging drugs. The corrected QT interval at discharge was 440 ms (figure 3). The patient's coronary angiography, done 8 weeks following discharge, was normal. Repeat ECGs at 6 and 8 weeks following discharge showed corrected QT intervals of 422 ms and 426 ms, respectively. Oral propranolol was stopped 6 weeks after discharge. After 6 months of follow-up, the patient did not have any episodes of syncope and torsades.
Figure 3.
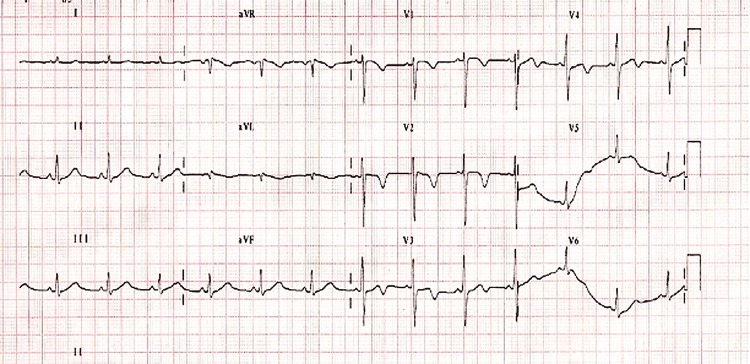
ECG at discharge showing normalisation of the QT interval to 440 ms and T inversion in leads V1–V4.
Discussion
TdP is an uncommon and distinctive polymorphic ventricular tachycardia with a characteristic electrocardiographic pattern of continuously changing morphology of the QRS complex, so named since the complexes seem to ‘twist’ around a baseline axis. It occurs in the setting of delayed myocardial repolarisation, that is, prolongation of QT interval on ECG.1 The arrhythmia can occur in the congenital long QT syndromes (Jervell/Lange-Nielsen syndrome, Romano-Ward syndrome, sporadic long QT syndrome) or as a consequence of therapy with QT-prolonging drugs. It may also be brought on by clinical circumstances under which repolarisation is delayed (eg, electrolyte disturbances such as hypokalaemia and hypomagnesemia, bradycardia, endocrinopathies and nutritional disorders. The electrocardiographic hallmark of both the congenital and acquired forms of the long QT syndrome is marked QT(U) lability, particularly as a function of HR.
Congenital long QT syndrome is known to be caused by mutations of the genes for cardiac potassium, sodium or calcium ion channels. TdP is widely thought to be triggered by reactivation of calcium channels, reactivation of a delayed sodium current, or a decreased outward potassium current that results in early afterdepolarisation, in a condition with enhanced TDR (transmural dispersion of repolarisation) usually associated with a prolonged QT interval. TDR serves as a functional re-entry substrate to maintain TdP. The underlying mechanism is thought to be triggered activity arising as a consequence of early afterdepolarisation.2 At the cellular level, the repolarisation phase of the myocytes is driven predominantly by outward movement of potassium ions.
Two important K+ currents participating in ventricular repolarisation are the subtypes of the delayed rectifier current, IKr (‘rapid’) and IKs (‘slow’). Blockade of either of these outward potassium currents may prolong the action potential. IKr is the most susceptible to pharmacological influence and the blockade of IKr current by these drugs is in part responsible for their pro-arrhythmic effect. Blockade of the IKr current manifests clinically as a prolonged QT interval and as the emergence of other T-wave or U-wave abnormalities on the surface ECG. When accompanied by the presence of a notably increased dispersion of repolarisation, this may induce re-entry and provoke TdP, which is then sustained by further re-entry or spiral wave activity.
Diagnostic criteria for long QT syndrome
A presentation with syncope or sudden cardiac death, in combination with a long QT on an ECG, typically suggests long QT syndrome (LQTS).In 1993, Schwartz et al3 suggested diagnostic criteria that still serve as the best criteria for clinicians. In their model, the criteria are divided into three main categories, as shown in table 1 below. The maximum score is 9, with a score of greater than 3 indicating a high probability of LQTS.
Table 1.
| Criterion | Points |
|---|---|
| ECG findings* | |
| QTc (ms)† | |
| >480 | 3 |
| 460–469 | 2 |
| 450–459 in male patient | 1 |
| Torsade de pointes‡ | 2 |
| T-wave alternans | 1 |
| Notched T wave in 3 leads | 1 |
| Low heart rate for age§ | 0.5 |
| Clinical history | |
| Syncope | |
| With stress | 2 |
| Without stress | 1 |
| Congenital deafness | 0.5 |
| Family history | |
| A. Family members with definite LQTS# | 1 |
| B. Unexplained sudden cardiac death <30 years in an immediate family member | 0.5 |
*In the absence of medications or disorders known to affect these electrocardiographic features.
†QTc calculated by Bazett's formula.
‡Mutually exclusive.
§Resting heart rate below the second percentile for the age.
#Definite LQTS is defined by an LQTS score of more than 3 (≥4).
LQTS, long QT syndrome.
Antiarrhythmic drugs are the most common causes of TdP in developed countries. Various drugs which are not usually classified as having any cardiovascular actions have been associated with TdP, and usually are part of three major classes: neuroleptics, antibiotics and non-sedating antihistaminics.
List of QT prolonging drugs
| Antiarrhythmics | Class 1: dihydroquinidine, disopyramide, encainide, flecainide, mexiletine, procainamide, propafenone and quinidine |
| Class 3: amiodarone, azimilide, bretylium, dofetilide, dronedarone, d-sotalol, ersentilide, ibutilide, nifekalant, sematilide and sotalol | |
| Anti-anginals/vasodilators | Bepridil, lidoflazine, prenylamine, ranolazine, terodiline and vardenafil |
| Anti-hypertensives | Indapamide, isradipine, moexipril/hydrochlorthiazide and nicardipine |
| Antihistamines | Astemizole, azelastine, diphenhydramine, ebastine, hydroxyzine and terfenadine |
| Serotonin agonists and antagonists | Cisapride, dolasetron, granisetron, ketanserin and ondansetron |
| Antimicrobials | Macrolide antibiotics: azithromycin, clarithromycin, erythromycin, roxithromycin, spiramycin and telithromycin |
| Quinolone antibiotics: ciprofloxacin, gatifloxacin, gemifloxacin, grepafloxacin, levofloxacin, moxifloxacin, ofloxacin and sparfloxacin | |
| Antifungals: cotrimoxazole, fluconazole (caution with itraconazole), ketoconazole and voriconazole | |
| Others: pentamidine and trimethoprim sulfa (bactrim) | |
| Antiviral: foscarnet (HIV) | |
| Antimalarials | amantidine, chloroquine, halofantrine and quinine. |
| Psychiatric drugs | Tricyclic antidepressants: amitriptyline, amoxapine, clomipramine, desipramine, doxepin, imipramine, nortriptyline, protriptyline and trimipramine. |
| Phenothiazines: chlorpromazine, fluphenazine, prochlorperazine, thioridazine and trifluoperazine. | |
| Others: atomoxetine, citalopram and clozapine, droperidol, fluoxetine, haloperidol, levomethadyl, lithium, maprotiline, mesoridazine, methadone, paroxetine, pericycline, pimozide, quetiapine, risperidone, sertindole, sertraline, trazodone, venlafaxine, zimeldine and ziprasidone. | |
| Anticonvulsant | Felbamate and fosphenytoin (prodrug of phenytoin). |
| Anti-migraine | Naratriptan, sumatriptan and zolmitriptan |
| Anti-cancer | Arsenic trioxide, geldanamycin, sunitib, tacrolimus and tamoxifen |
| Stimulant drugs | Some cold remedies contain these drugs, so it is important always to check the label. Adrenaline (epinephrine), amphetamine, cocaine, dexmethylphenidate, dobutamine, dopamine, ephedrine, fenfluramine, isoprenaline (isoproterenol), levalbuterol, metaproterenol, methylphenidate, midodrine and norepinephrine (noradrenaline), phentermine, phenylephrine, phenylpropanolamine, pseudoephidrine, ritodrine, salbutamol (albuterol), salmeterol, sibutramine and terbutaline. |
| Others | Alfuzosin, chloral hydrate, clobutinol, domperidone, galantamine, octreotide, organophosphates, perflutren lipid microspheres, probucol, solifenacin, tizanidine, tolterodine and vasopressin. |
Chloroquine is a drug widely used in infectious diseases like malaria and amoebiasis and inflammatory conditions such as rheumatoid arthritis and lupus erythematosus. The use of chloroquine has been associated with many cardiovascular side effects such as hypotension, rhythm abnormalities and cardiac failure. The proarrhythmic effects of chloroquine are well documented, with reports of the drug causing prolonged QRS and QTc intervals and TdP.4 A slowing in ventricular conduction and an excessive lengthening in the QT interval have been proposed as mechanisms of the proarrhythmic effects of chloroquine.5 Its effect on various ion channels produces arrhythmias; blockade of inward sodium current (INa) has been suggested as the principal cause of impaired ventricular conduction and acquired long QT syndrome is usually caused by blockade of one or more potassium currents. Decreased extracellular potassium will cause prolonged action potential duration; that is, prolonged QT interval, as well as increase at the rate of spontaneous phase four depolarisation (increased automaticity), and hyperpolarise the resting membrane potential.6 Isolated hypokalaemia has been reported to induce TdP.7 Two or more risk factors often come together to precipitate the arrhythmia, namely choloroquine therapy and colitis-related hypokalaemia in our patient. Sepsis itself has been postulated to precipitate arrhythmias in critically ill patients, especially in the presence of an arrhythmogenic substrate. However, no concrete evidence in this causality relationship exists.8 Amoebiasis has been known to present with cardiac manifestations, most commonly arrhythmias due to cardiotoxicity of the now outmoded drugs emetine and dihydroemetine. Seshadri et al9 have reported one case of multiple episodes of ventricular tachycardia in a patient of amoebic liver abscess receiving emetine and chloroquine. Rarely, an amoebic liver abscess may spread contiguously to involve the pericardium (amoebic pericarditis).10 As per our knowledge, TdP precipitated by chloroquine and hypokalaemia in liver and intestinal amoebiasis has hitherto not been reported. The management of patients with drug-induced TdP includes identifying and withdrawing the offending drug(s), replenishing the potassium concentration to 4.5–5 mmol/l and infusing intravenous magnesium (1–2 g). Intravenous magnesium is a safe and effective drug to abort an episode of TdP. It remains the primary line of therapy, even in the presence of normal serum magnesium levels.11 In resistant cases, temporary cardiac pacing may be needed to increase the heart rate and shorten the QT interval.
Patients who have developed this arrhythmia remain at risk for sudden cardiac death, and the management must also include drug lists, counselling and avoidance of vigorous activity. Drug-induced long QT syndromes are most often caused by antiarrhythmics, but drugs in common use such as antibiotics (erythromycin and azithromycin), prokinetics (cisapride), antihistaminics (terfenadine) and some antipsychotics have been known to precipitate ventricular storm. Although these must be strictly avoided in patients with previously detected long QT, care should be taken if they are prescribed in combination. In the management of a ventricular storm in the acute setting, β-blockers have been found to play a major role, non-selective β-blockers have greater efficiency in preventing the arrhythmic effects of catecholamines.12 Other agents include amiodarone, class I antiarrhythmic agents such as lidocaine and short-acting general anaesthesia.13 Non-pharmacological therapy such as intra-aortic balloon counter-pulsation may be used during stabilisation and electrophysiological studies with radiofrequency mapping are indicated if the arrhythmia is sustained after removal of precipitating factors, recurrent or not managed with pharmacological agents.14
Guidelines for the management of patients with LQTS, as suggested by the American College of Cardiology, the American Heart Association and the European Society of Cardiology, in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society are as follows.14
No participation in competitive sports for patients with the diagnosis established by means of genetic testing only.
β-Blockers should be given to patients who have QTc-interval prolongation (>460 ms in women and >440 ms in men).
An implantable cardioverter-defibrillator (ICD) should be used in survivors of cardiac arrest and is recommended for patients with syncope while receiving β-blockers; ICD therapy can be considered for primary prevention in patients with characteristics that suggest high risk (QTc interval >500 ms).
Some cases of drug-induced arrhythmias depend on genetic predisposition. Molecular screening may allow identification among family members of gene carriers potentially at risk if treated with I(Kr) blockers. Evolving technology may lead to rapid screening for mutations of candidate genes that cause drug-induced life-threatening arrhythmias and allow early identification of individuals at risk. In our patient, genetic screening was not possible owing to lack of finances and resources.15
Learning points
Torsades de Pointes must be borne in mind in a patient presenting with syncope, haemodynamic compromise or sudden death.
It is imperative to recognise the arrhythmia and the underlying QT prolongation, because treatment and prognosis differ from other ventricular tachycardias. Once diagnosed, it is eminently preventable through a close scrutiny over patients’ drug intake.
Torsades may appear in a relatively common infective condition such as amoebiasis, and drugs in widespread use such as chloroquine can precipitate it.
The electrolyte milieu of the myocardium is an important consideration while working up the arrhythmia, and prompt correction of dyselectrolytemias may prevent it.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Haverkamp W, Hördt M, Chen X, et al. Torsade de pointes. Z Kardiol 1993;82:763–74. [PubMed] [Google Scholar]
- 2.Roden DM. Torsade de pointes. Clin Cardiol 1993;16:683–6. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz PJ, Moss AJ, Vincent GM. Diagnostic criteria for the long QT syndrome: an update. Circulation 1993;88:782–4. [DOI] [PubMed] [Google Scholar]
- 4.Harris L, Downar E, Shaikh NA, et al. Antiarrhythmic potential of chloroquine: New use for an old drug. Can J Cardiol 1988;4:295–300. [PubMed] [Google Scholar]
- 5.Sánchez-Chapula J, Salinas-Stefanon E, Torres-Jácome J. Blockade of currents by the antimalarial drug chloroquine in feline ventricular myocytes. JPET 2001;297:437–45. [PubMed] [Google Scholar]
- 6.Roden DM, Iansmith DHS. Effects of low potassium or magnesium concentrations on isolated cardiac tissue. Am J Med 1987;82:18–23. [DOI] [PubMed] [Google Scholar]
- 7.Curry P, Fitchett D, Stubbs W, et al. Ventricular arrhythmias and hypokalaemia. Lancet 1976;2:231–3. [DOI] [PubMed] [Google Scholar]
- 8.Reising S, Kusumoto F, Goldschlager N. Life-threatening arrhythmias in the intensive care unit. J Intensive Care Med 2007;22:3–13. [DOI] [PubMed] [Google Scholar]
- 9.Seshadri MS, John L, Varkey K, et al. Ventricular tachycardia in a patient on dehydroemetine and chloroquine for amoebic liver abscess. Med J Aust 1979;5:406–7. [DOI] [PubMed] [Google Scholar]
- 10.Adeyemo AO. Intrathoracic complications of amoebic liver abscess. J R Soc Med 1984;77:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzivoni D, Banai S, Schuger C, et al. Treatment of torsade de pointes with magnesium sulfate. Circulation 1988;77:392–7. [DOI] [PubMed] [Google Scholar]
- 12.Tsagalou EP, Kanakakis J, Rokas S, et al. Suppression by propranolol and amiodarone of an electrical storm refractory to metoprolol and amiodarone. Int J Cardiol 2005;99:341–2. [DOI] [PubMed] [Google Scholar]
- 13.Burjorjee JE, Milne B. Propofol for electrical storm; a case report of cardioversion and suppression of ventricular tachycardia by propofol. Can J Anaesth 2002;49:973–7. [DOI] [PubMed] [Google Scholar]
- 14.Zipes DP, Camm AJ, Borggrefe M, et al. European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death). J Am Coll Cardiol 2006;48: e247–346. [DOI] [PubMed] [Google Scholar]
- 15.Napolitano C, Schwartz PJ, Brown AM, et al. Evidence for a cardiac ion channel mutation underlying drug-induced QT prolongation and life-threatening arrhythmias. J Cardiovasc Electrophysiol 2000;11:691–6. [DOI] [PubMed] [Google Scholar]