Abstract
The Klippel-Trenaunay syndrome is a rare congenital disorder that affects one or more limbs. It is characterised by cutaneous vascular nevi, venous malformations and hypertrophy of soft tissues and bone. There are very few cases reported in pregnant women, so the level of uncertainty is high when it appears during gestation. It is a disease that increases obstetric risk and can exacerbate complications, mainly thromboembolic and haemorrhagic. We report below the case of a pregnant woman diagnosed with this syndrome and the multidisciplinary management held in our centre.
Background
The Klippel-Trenaunay syndrome (KTS) is a rare congenital disorder of unknown origin and variable expression1 affecting one or more extremities and characterised by a triad consisting of vascular skin nevus, varicose veins or venous malformations and hypertrophy of soft tissue and bone.1–5 This syndrome was first described in 1900 by Klippel and Trenaunay, who described a syndrome of osteohypertrophic varicose nevus.1 3 4 Subsequently, Parkes and Weber added to the description a similar condition with the presence of arteriovenous fistula.1 3 4 These two diseases must be differentiated because they do not share aetiology or treatment. However, there is often confusion between the two, which is even called Klippel-Trenaunay-Weber syndrome (KTWS).2 4
Regarding the origin and aetiology of this disease, it should be noted that it is a rare birth defect4 5 (with a reported incidence of up to one case in every 27 500 newborns).3 Its expression is variable and its origin unknown.1 4–6
When attempting to approach its aetiopathogenesis, we should know that it is included in the neurocutaneous syndromes, which are numerous and complex and within these among those secondary to alterations in embryonic development of the nervous system and skin.3 It is therefore a neurocutaneous syndrome with vascular involvement.3
It is characterised by the presence of a clinical triad consisting of a flat angioma or nevus flammeus as the most common finding, varicose veins or venous malformations and hypertrophy of bone and soft tissue1 4 which is usually asymmetrical, congenital or early onset, and can affect one or both extremities.3 At least two of the three main symptoms should be present to accept the diagnosis of this syndrome.4 Oduber proposed in 2008 a definition of KTS based on two major features: congenital vascular (capillary, venous, arteriovenous or lymphatic) malformation and disturbed growth of bone or soft tissue.5
The most characteristic feature of the syndrome is venous malformations, which increase during pregnancy.4 Hypertrophy of soft tissue and bone leads to a difference in size between the legs.4 In addition, other pigmented skin abnormalities may be found,3 such as pigmented and warty nevus, blue spots, nevus spilus, etc. Pelvic and intra-abdominal extension of vascular malformations is common,1 and visceral compromise is occasionally seen in cases of KTWS and Sturge-Weber angiomatosis.3
The diagnosis of this disease is usually started by clinical suspicion in childhood given the early onset of the symptoms mentioned.1 3
Morbidity from this syndrome is based on the fact that the potential complications that may occur are related on one hand with presence of vascular anomalies that may result in venous insufficiency, cellulitis, ulcers, thrombophlebitis, thromboembolic disease1 and on the other, on the frequency of extension of vascular malformations to the pelvic and intra-abdominal level, as well as to the skin of the lower abdomen and pelvis, external genitalia.1 6–9
Case presentation
The patient is a 25-year-old woman who was diagnosed at birth of KTS. She was referred to our high-risk obstetric unit in the 32nd week of her first pregnancy to prepare delivery. The patient belonged to Jehova's Witnesses community (also her husband, mother and siblings) and explicitly refused the use of blood transfusions (even autotransfusions) or any other blood product. Clinically, the patient presented from birth a dystrophic left leg, grossly enlarged, with significant varicose syndrome when compared with a contralateral limb (figure 1).
Figure 1.

Detail of the patient's leg.
Third trimester blood count showed 4.54 × 106 red cells/mm3, 11.8 g/dl haemoglobin, 37.8% haematocrit, 168 × 103 platelets/mm3, 10 954 leucocytes/mm3, 2.49% reticulocytes/mm3, 43 mg/dl iron and 11 ng/ml ferritin. In order to increase haemoglobin levels and cope better baseline bleeding, she was treated with iron and subcutaneous erythropoietin. To achieve a target haemoglobin of 15 g/dl we calculated a need of 1110 mg of intravenous iron. We administered five doses of 200 mg intravenous iron, every other day. To enhance haematopoiesis we also administered four doses of 40 000 IU subcutaneous erythropoietin. As a pregnant KTS patient she had a very high haemorrhagic risk and her religious beliefs limited treatment options in the case of bleeding.
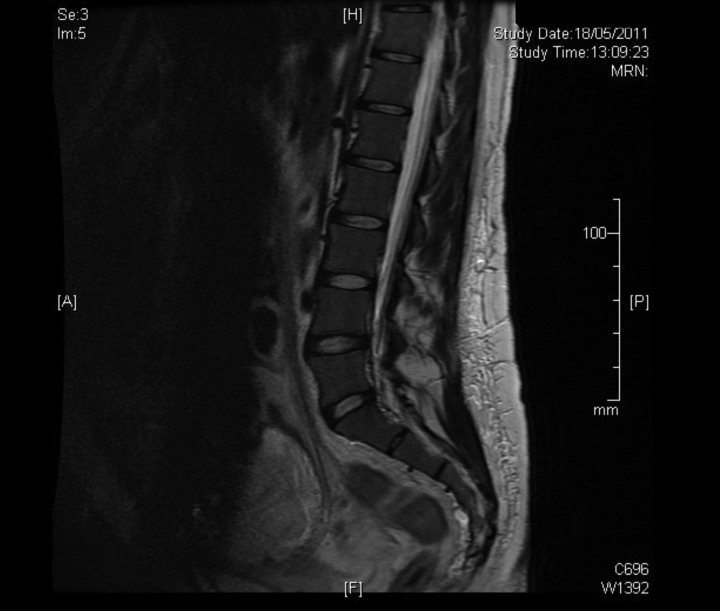
A multidisciplinary approach was followed, with the assistance of cardiovascular surgeons, anaesthesiologists, interventional radiologists, intensive care unit doctors, haematologists, neonatologists and gynaecologists. Abdominopelvic MRI was performed to assess any other pelvic or uterine gross vascular malformations. MRI showed multiple deep varicose veins in the left thigh, left inguinal area and vulvar region that drained into the great saphenous vein and the common femoral vein. The most posterior varicose veins crossed gluteal muscles and drained into iliac vein and other posterior venous plexuses. MRI also showed the absence of varicosities in the lower uterine segment and anterior abdominal wall. The spinal region was also free of varicose veins (figure 2).
Figure 2.
Patient's spinal region MRI.
From the obstetric point of view, fetal growth was consistent with gestational age, and a fetal weight of 2500 g was estimated in the 36th week, placenta located in the anterior uterine wall and there was no olygohidramnios.
We decided the date and the way of delivery (induction of labour versus elective caesarean section) by involving the patient and her partner actively in final decision, after a medical multidisciplinary counselling. Vaginal delivery added risks related to the rupture of proximal varicose plexuses extremely difficult to solve. On the contrary, caesarean section added surgical and anaesthetics risks but offered greater guarantees to solve a hypothetical heavy bleeding.
It was decided to schedule an elective caesarean section at 38 weeks gestation. Both vascular surgeons and interventional radiologists were ready to act in case of vascular complications. It was also decided to use a cell recovery system during caesarean section in order to retrieve, filter and store blood spilled into the operative field.
The patient got a preoperative 14.7 g/dl haemoglobin concentration and 44% haematocrit. A caesarean section, under spinal anaesthesia, was performed at a gestational age of 38 weeks and 3 days. Both, the anaesthetic procedure and the operation were uneventful and as it was expected, no vascular malformation was seen at the uterine segment. A 2700 g female was born with normal Apgar scores. During caesarean section a volumen of 900 cc of blood was recovered what involved 300 cc of red cell concentrate.
The postoperative course was uneventful, with a 12 g/dl postoperative haemoglobin concentration and a 38% haematocrit. She was discharged from the hospital on day 4 after surgery.
Discussion
We know that the normal physiological changes of pregnancy (increased venous pressure, oedema in lower extremities, venous stasis and increased cardiac output) aggravate the clinical signs of this syndrome, which means that we will find an increased risk of mainly thromboembolic and bleeding complications in these pregnant women.1 6 7 There have also been reports of rectal, vaginal or vulvar bleeding as well as obstruction vulvovaginal varicose veins in the vaginal introitus.6
The main complication described in pregnancy and postpartum of patients with this syndrome is coagulopathy, including deep vein thrombosis and other thromboembolic problems,6 7 with a 10-times greater risk than in the normal population.5 Although there are no prospective studies on the use of anticoagulants during pregnancy in this syndrome, use of thromboembolic prophylaxis with low-molecular-weight heparin is generally recommended, mainly in the postpartum.4 6
The presence of vascular abnormalities at the neuroaxial level may complicate the performance of local and regional anaesthesia, so it is recommended to perform MRI of structures close to the spinal cord in these cases to prevent a traumatic puncture of these vessels.6
Regarding the course of KTS in pregnancy, there are still few data owing to the uncommonness of its coexistence. As this disease greatly increases obstetric risk and may exacerbate its complications (mainly thromboembolic and haemorrhagic) throughout pregnancy, pregnancy is usually advised against.1
It is therefore a rare syndrome with an unknown incidence in pregnancy as there are few reports of pregnancy in patients with this syndrome.1 6 Therefore, little information is available about obstetric care to be followed in these patients.1 6 It should be recommended to perform a complete general and obstetric history thoroughly assessing past history, prior complications of patient, etc. It is very important to carry out a careful examination assessing lesions and their potential extension to the genital and pelvic level, etc. The literature on this regard should be reviewed when presented with a case of these characteristics in an attempt to determine appropriate management.
The choice of delivery route is not easy and will depend on multiple circumstances such as the previous history of the patient (previous complications in the history may indicate potential future complications), the specific and thorough examination of the pregnant woman involved with adequate assessment of the possible extension of the lesions and various additional data, for which we will use the physical examination by inspection of the lesions, bimanual palpation and use of the precise complementary techniques.
We should know that though a caesarean section may allow us access and direct vision of possible vascular malformations damaging the pelvic organs, its use as route of direct termination in these pregnant women does not eliminate the risks of bleeding because though venous malformations affect primarily and principally the extremities, they can also, to a lesser extent, affect abdominal and even thoracic structures.4 5 Thus, the indication for caesarean section should be the standard obstetric indication,5 or an individualised indication. In the postpartum period, prophylactic anticoagulation is recommended.4 5
Regarding anaesthesia in delivery or caesarean section of these patients, the prior history should be evaluated to know whether there were previous episodes of access to the peridural route without complications. Complementary imaging techniques may also be used for assessing the presence of vascular malformations at this level. However, it is sometimes preferred to resort to general anaesthesia because of the concern about the presence of a haemangioma or vascular malformations in the epidural space that may have been overlooked.6
For a pregnant woman with KTS, it is necessary to individualise the case and perform management through a multidisciplinary team including obstetricians, anaesthetists, cardiovascular surgeons, interventional radiologists, haematologists and paediatricians.5 Thus, with an adequate history, examination and assessment of the patient which allows individualised action and joint management by a multidisciplinary team, we can anticipate potential complications and control them better.6
Learning points.
Multidisciplinary approach is needed in Klippel-Trenaunay syndrome pregnant patients.
Preoperative/prelabour improvement of haemoglobin levels is mandatory in Jehova witness patients.
Elective caesarean section was the most secure option for this patient.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Klippel M, Trenaunay P. Du noevus variquex osteohypertrophique. Arch Gen Med 1990;185:641–72. [Google Scholar]
- 2.Torres-Farías E, Torres-Gómez LG, Burciaga-Sepúlveda AS. Síndrome de Klippel-Trenaunay y embarazo. Comunicación de un caso. Ginecol Obstet Mex 2010;78:287–90. [PubMed] [Google Scholar]
- 3.Lindenauer SM. The Klippel-Trenaunay syndrome: varicosity, hypertrophy and hemangioma with no arteriovenous fistula. Ann Surg 1965;162:303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reyes Puentes LM, Fuentes Camargo MJ, Pérez Martínez C, et al. Diagnóstico prenatal ecográfico del Síndrome Klippel-Trenaunay-Weber: a propósito de un caso. Rev Ciencias Médicas 2010;14:256–61. [Google Scholar]
- 5.Oduber CE, Van Der Horst CM, Hennekam RC. Klippel-Trenaunay syndrome. Diagnostic criteria and hypothesis on etiology. Ann Plas Surg 2008;60:217–23. [DOI] [PubMed] [Google Scholar]
- 6.Güngor Gündoğan T, Jacquemyn Y. Klippel-Trenaunay syndrome and pregnancy. Obstet Gynecol Int 2010;2010:706850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fait G, Daniel Y, Kupferminc MJ, et al. Klippel-Trénaunay-Weber syndrome associated with fetal growth restriction. Hum Reprod 1996;11:2544–5. [DOI] [PubMed] [Google Scholar]
- 8.Rebarber A, Roman A, Roshan D, et al. Obstetric management of Klippel-Trenaunay syndrome. Obstet Gynecol 2004;104:1205–8. [DOI] [PubMed] [Google Scholar]
- 9.Stein SR, Perlow JH, Sawai SK. Klippel-Trenaunay-type syndrome in pregnancy. Obstet Gynecol Surv 2006;61:194–20. [DOI] [PubMed] [Google Scholar]