Abstract
Sarcoidosis is a systemic disease characterised by non-caseating granulomas affecting mainly the lung and lymphatics. Literature reveals that 5% of the patients of sarcoidosis have involvement of the nervous system. Various neurological complications of sarcoidosis commonly reported are cranial nerve palsies, aseptic meningitis, myelopathy and intracranial masses. We report unusual case of neurosarcoidosis who presented with combination of right-sided vision loss and complete ophthalmoplegia with extensive leptomeningitis. This patient was suffering from resistant diabetes which leads to therapeutic problems.
Background
Sarcoidosis is known to be idiopathic granulomatous disease which frequently involves lungs, ocular system and lympathics.1 Neurological involvement in sarcoidosis occurs in 2–5% of patients. Neurological disorders due to neurosarcoidosis reported in the literature are cranial neuropathies, aseptic meningitis, hydrocephalus, intracranial masses, encephalopathies, seizures and peripheral neuropathies. There can be varied presentations due to meningeal involvement.1 2 Patients suffering from neurosarcoidosis have leptomeningeal and intraparenchymal infiltration of granulomas that leads to cranial nerve palsies, basal meningitis and endocrine dysfunction. We report an unusual case of neurosarcoidosis presenting as acute complete right-sided ophthalmoplegia and vision loss with interesting MRI features of extensive meningeal infiltration. This patient also poses therapeutic problems as steroids further leads to bad diabetic status.
Case presentation
A 55-year-old woman, known diabetic for last 10 years developed low-to-moderate grade, continuous headache for previous 1.5 months. After few days she manifested with swelling of the right eyelid. At the same time she developed drooping of the right eyelid with almost no movement of the eyeball in any direction (figure 1). Patient was also recently diagnosed as a case of hypothyroidism. There was no history of prolonged fever, cough with or without expectoration, haemoptysis or nasal bleeding. The patient denied history of rash, arthralgia and urinary problems.
Figure 1.
Photograph of the patient showing right-sided ptosis and ophthalmoplegia.
General examination showed pulse rate 86/min, regular and blood pressure 110/80 mm Hg with no features of dysautonomia. Thyroid was not enlarged. Higher mental state disclosed drowsy state. Patient had complete ophthalmoplegia on the right side along with ptosis (figure 1). Acuity of vision assessment revealed absence of light perception on the right side. On the left side vision was 6/9 by the Snellen chart examination. Pupils were semidilated and sluggishly reacting on the right side while normal size and normal reaction on the left side. There was loss of pinprick sensation on V1 and V2 segment on the right side of the face. Motor system was normal and distal deep tendon reflexes were absent. Plantars were bilaterally flexor. Rest of the neurological examination did not reveal any abnormality.
Investigations
Lab assessment revealed following findings. Haemoglobin 8 g%, total lymphocyte count 6700/m3, P 68%, L 25%, serum creatinine 0.6 mg/dl, serum Na/K 131/4.2 mEq/l. Fasting/postprandial blood sugar was 216 and 342 mg/dl, respectively. Cerebrospinal fluid (CSF) examination revealed cells 32/m3 with predominant lymphocytes, protein 56 mg/dl and sugar 142 mg/dl (corresponding blood sugar 326 mg/dl). CSF was negative for Gram staining and acid-fast bacilli. The CSF PCR for mycobacteria was negative. The India ink preparation for fungi showed negative results. Cryptococcus antigen was not found. The sera for HIV were non-reactive. The vasculitic profile including antinuclear antibody and rheumatoid factor depicted negative study. Thyroid assessment was suggestive of hypothyroidism. Endoscopic biopsy of maxillary sinus showed non-caseating granulomas along with chronic inflammatory cells including plasma cells. Serum ACE enzyme was significantly elevated 96 units/l (normal range 8–52 units/l). CT chest did not reveal any abnormality. MRI of cranium with contrast revealed on axial section extensive leptomeningitis starting from tentorium cerebelli traversing anteriorly through cavernous sinus to right retro-orbital area also involving optic nerve(figure 2). There is also dural enhancement of the right temporal region. On coronal section T1 contrast there is thickening and enhancement of right tentorium cerebelli (figure 3).
Figure 2.
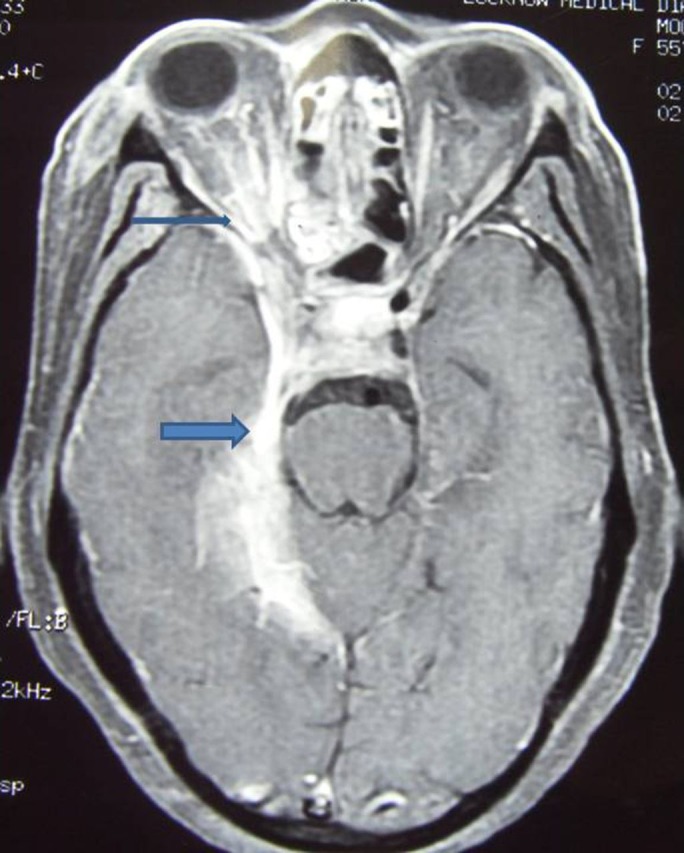
MRI T1 contrast on axial section revealing extensive leptomeningitis from tentorium cerebelli to right orbital area.
Figure 3.
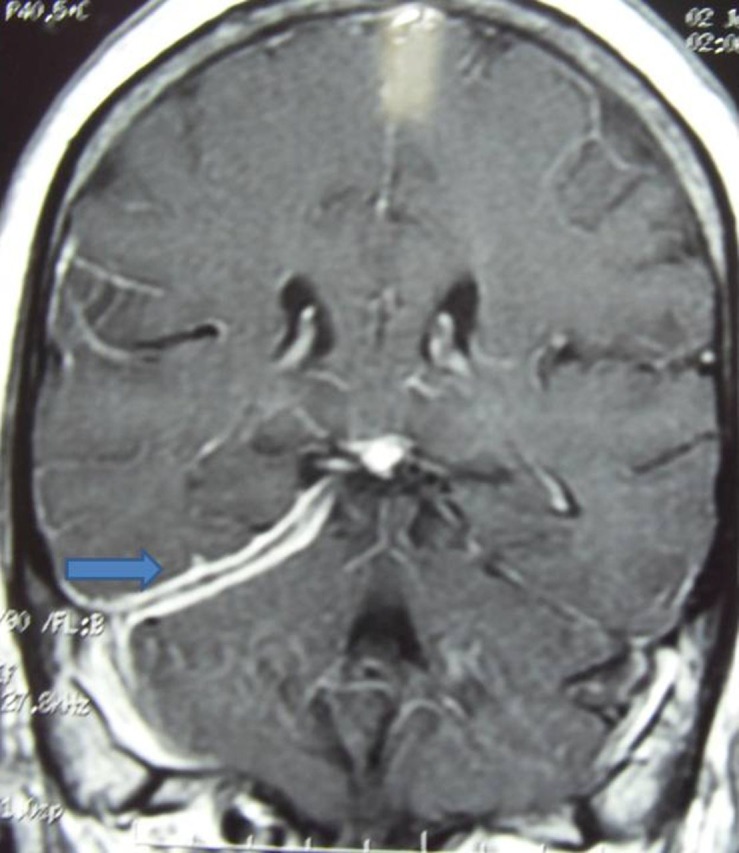
T1 contrast on coronal section showing thickening and enhancement of right tentorium cerebelli.
Treatment
So based on laboratory findings and MRI results, diagnosis of neurosarcoidosis was considered. Patient was started steroids (prednisolone 1 mg/kg body weight per day) with best effort to control blood sugar.
Outcome and follow-up
On follow-up after a month the patient showed poor response and finally immunosuppresants were started. The 6 months follow-up revealed partial recovery.
Discussion
Neurosarcoidosis is relatively uncommon disorder with varied clinical manifestations. Common neurological presentations are craniopathies, aseptic meningitis and hypothalamic dysfunction.3
The diagnosis of neurosarcoidosis is based on compatible clinical features, pathological findings and excluding alternative diagnosis. Zajicek et al4 proposed a diagnostic classification for neurosarcoidosis into definite, probable and possible categories based on tissue evidence of non-caseating granulomas and supportive evidence of sarcoid pathology in laboratory and imaging studies. On the basis of retrospective case analysis in a study regarding ocular features in neurosarcoidosis authors found that cranial nerve involvement was the commonest presentation. Among 27 patients with cranial neuropathy lower motor neuron type of facial palsy was commonly found. Other ocular manifestations were diplopia and anterior uveitis.5
Chang et al in their study reported about cavernous sinus syndrome due to neurosarcoidosis. The patient had complete ophthalmoplegia on the left side. MRI revealed gadolinium enhancing lesion on left cavernous sinus. They concluded that neurosarcoidosis should be included in the differential diagnosis of cavernous sinus syndrome with neuroophthalmological signs.6
MRI findings of neurosarcoidosis are quite variable. It can be grouped into two parts, intraparenchymal and leptomeningeal involvement.7 Intraparenchymal lesions commonly present as multiple non-enhancing periventricular white matter T2 hyperintense lesions. Enhancing parenchymal masses are also frequently reported which can be mistaken for tumour. Leptomeningeal enhancement is more typical radiological manifestation of neurosarcoidosis. This is usually seen as thickening and enhancement of leptomeninges.8
In our case patient had complete ophthalmoplegia with vision loss on the right side along with uncontrolled diabetes mellitus. The patient received steroids with poor response. Sarcidosis is known for spontaneous remission but central nervous system involvement has more resistance course. The initial therapy in neurosarcoidosis is steroids administration; nevertheless, good number of patients require immunosupressants and other drugs due to side effects or non-responsiveness to steroids. As per literature, 25% of the patients will still have refractory course with steroid treatment and more alarming is 20–40% of those refractory patients will not respond to any level of current conventional immunosuppression.9
A case was reported of refractory neurosarcoidosis who failed on steroids and conventional immunosuppressive agents. They tried thalodimide in this difficult to treat patient and got promising results. They concluded that thalodimide should be used in intractable neurosarcoidosis.10
Learning points.
Neurosarcoidosis is a rare disorder with varied neurological manifestations.
The cranial nerve palsy, aseptic meningitis and hypothalamic dysfunction are usual clinical presentation of neurosarcoidosis.
MRI of the cranium mainly demonstrated meningeal infiltration due to non-caseating granulomas.
The early diagnosis is desirable to have best clinical outcome.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Souliere CR, Jr, Kava CR, Barrs DM, et al. Sudden hearing loss as the sole manifestation of neurosarcoidosis. Otolaryngol Head Neck Surg 1991;105:376–81. [DOI] [PubMed] [Google Scholar]
- 2.Stern BJ, Krumholz A, Johns C, et al. Sarcoidosis and neurological manifestations. Arch Neurol 1985;42:909–17. [DOI] [PubMed] [Google Scholar]
- 3.Spencer TS, Campellone JV, Maldonado I, et al. Clinical and magnetic resonance imaging manifestations of neurosarcoidosis. Semin Arthritis Rheum 2005;34:649–61. [DOI] [PubMed] [Google Scholar]
- 4.Zajicek JP, Scolding NJ, Foster O, et al. Central nervous system sarcoidosis–diagnosis and management. QJM 1999;92:103–17. [DOI] [PubMed] [Google Scholar]
- 5.Menezo V, Lobo A, Yeo TK, et al. Ocular features in neurosarcoidosis. Ocul Immunol Inflamm 2009;17:170–8. [DOI] [PubMed] [Google Scholar]
- 6.Chang CS, Chen WL, Li C, et al. Cavernous sinus syndrome due to sarcoidosis: a case report. Acta Neurol Taiwan 2009;18:37–41. [PubMed] [Google Scholar]
- 7.Dumas JL, Valeyre D, Belin C, et al. Central nervous system sarcoidosis: follow-up at MR imaging during steroid therapy. Radiology 2000;214:411–20. [DOI] [PubMed] [Google Scholar]
- 8.Nowak DA, Widenka DC. Neurosarcoidosis: a review of its intracranial manifestation. J Neurol 2001;248:363–72. [DOI] [PubMed] [Google Scholar]
- 9.Hoitsma E, Faber CG, Drent M, et al. Neurosarcoidosis: a clinical dilemma. Lancet Neurol 2004;3:397–407. [DOI] [PubMed] [Google Scholar]
- 10.Hoyle J, Newton H, Katz S. Prognosis of refractory neurosarcoidosis altered by thalidomide: a case report. J Med Case Reports 2008;2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]



