Abstract
Disorders of sexual desire affect an estimated 30% of women in North America and Europe, with etiologies based on interpersonal, personal, and physiological factors. There are currently no pharmacological agents approved for use in the treatment of female sexual dysfunction. This is due, in part, to a focus on the effects of experimental drugs on reflexive components of sexual behavior, such as lordosis, in animal models. Here we report that PT-141, a peptide analogue of α-melanocyte-stimulating hormone that binds to central melanocortin receptors, selectively stimulates solicitational behaviors in the female rat. This occurs without affecting lordosis, pacing, or other sexual behaviors. PT-141 did not cause generalized motor activation, nor did it affect the perception of sexual reward. A selective pharmacological effect on appetitive sexual behavior in female rats has not been reported previously, and indicates that central melanocortin systems are important in the regulation of female sexual desire. Accordingly, PT-141 may be the first identified pharmacological agent with the capability to treat female sexual desire disorders.
Neuropeptides derived from proopiomelanocortin (POMC), including β-endorphin, corticotropin (ACTH), and α-melanocyte-stimulating hormone (α-MSH), have pronounced effects on the sexual behavior of rats (1–3). In female rats, α-MSH has been shown to facilitate or inhibit the sexually receptive posture lordosis depending on the hormonal status of the animals and whether they are in a low or high state of sexual receptivity, respectively (4–7). Estradiol increases α-MSH levels in hypothalamic brain regions associated with female sexual behavior (8, 9), suggesting that α-MSH release may be one of several intermediaries of estrogen action. Recently, PT-141, a peptide analogue of α-MSH, was reported to be erectogenic in men (10, 11) by an action believed to occur at central melanocortin type 3 or 4 receptors (12). Given the prevalence of sexual arousal and desire disorders in women (13) and the lack of available pharmacological treatments, we asked whether PT-141 might facilitate appetitive aspects of sexual behavior in the female rat. Accordingly, the present study examined the dose–response effects of PT-141 on proceptive sexual behaviors, such as solicitation, hops and darts, and pacing, and receptive sexual behaviors, such as lordosis, in ovariectomized female rats tested under different hormone priming regimens in bilevel chambers (14) and unilevel pacing chambers (15, 16) (shown in Fig. 1).
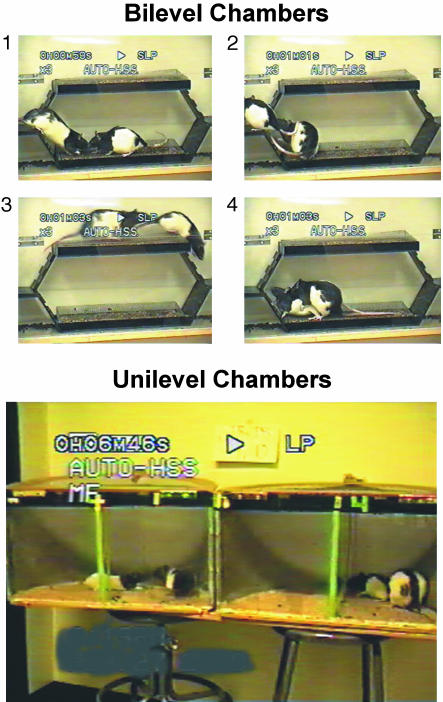
Fig. 1.
Pacing chambers used to assess female sexual behavior in the present experiments. (Upper) Copulation in bilevel chambers, initiated by solicitation (1), followed by anogenital investigation of the female (2), a runaway by the female to the other level (3), enticing the male to chase her, and finally the display of lordosis by the female (4), which allowed the male to mount with vaginal intromission. (Lower) Copulation in unilevel pacing chambers. The female on the left is crawling through a hole in a central partition to initiate copulation with the male. The female on the right has just been mounted.
Methods
Animals. Ovariectomized Long–Evans rats received 10 preliminary tests of sexual behavior in bilevel or unilevel pacing chambers with sexually vigorous Long–Evans male rats before tests with PT-141. This was done to acclimate the females to the handling and testing procedures, and has been shown previously to induce stable baseline rates of sexual behavior (14). Tests were conducted at 4-day intervals during the middle third of the rats' dark circadian cycle. Full sexual receptivity was induced by s.c. injections of estradiol benzoate (E; 10 μg in 0.1 ml of sesame oil) and progesterone (P; 500 μg in 0.1 ml of sesame oil) 48 and 4 h before each test, respectively. For tests involving only E priming, the sesame oil vehicle was administered 4 h before rats were tested.
Drug Administration. PT-141, a cyclic heptapeptide melanocortin analogue (Palatin Technologies, Cranbury, NJ), was dissolved in saline to obtain doses of 50, 100, and 200 μg/kg/ml, and injected s.c. 5 min before each behavioral test. An equal volume of saline was administered to control animals.
Behavioral Testing. Bilevel chambers. Females (n = 40) were administered E and P and assigned randomly to receive one of four doses of PT-141 (0, 50, 100, or 200 μg/kg). Females were placed into the bilevel chambers (Fig. 1) along with a sexually vigorous male for a 30-min test of copulation. All tests were videotaped and scored by using a computerized event recorder. The latency and frequency of solicitations (headwise orientation to the male followed by an abrupt runaway to another level), hops and darts, pacing (number of level changes), rejection responses, and lordosis quotients were scored for each female. A different group of females treated with E alone (n = 20) were assigned to one of two doses of PT-141 (0 or 200 μg/kg) in a randomized repeated-measures design, such that they were used as their own controls. The same behavioral measures were scored for those females.
Unilevel pacing chambers. A different group of females (n = 40) was administered E and P and assigned randomly to one of four doses of PT-141 (0, 50, 100, or 200 μg/kg). The chambers were bisected with Plexiglas dividers with four square holes (4 cm × 4 cm) cut out of the bottom. The size of the holes allowed each female free access to either side, but were too small for the male to enter (Fig. 1). Females were placed into the chamber along with a sexual vigorous male for a 30-min test of copulation. All tests were videotaped and scored by using a computerized event recorder. The same behavioral measures were scored as in bilevel chambers, with the addition of female–male mounting and the latencies to leave and return to the side containing the male after a mount, intromission, or ejaculation. Solicitation was defined here as a headwise orientation to the male followed by an abrupt runaway, regardless of whether the female remained in the side with the male or exited through the partition.
Locomotion. Females (n = 20) were primed with E and P, injected with one of two doses of PT-141 (0 or 200 μg/kg), and placed into a chamber (76 cm × 51 cm × 61 cm) filled with 1 cm of bedding for 15 min. All tests were videotaped. The open field was subsequently sectioned into a 3 × 3 grid on a video screen, and the number of grid crossings made by each female was determined. Each female was used as her own control, and the order of drug administration was randomized.
Conditioned place preference. Females (n = 20) were given a pretest to reveal their innate preference for one of the two distinctive sides of a conditioned place preference (CPP) apparatus (light versus dark). Females were then allowed to copulate in unilevel pacing chambers bisected by either a four-hole or one-hole Plexiglas divider for 30 min, after which they were placed into one distinctive side of the CPP apparatus for 15 min. Because females prefer copulation in the four-hole over the one-hole condition,∥ the four-hole condition was paired with the innately nonpreferred (light) side during training, whereas the one-hole condition was paired with the innately preferred (dark) side. It was expected that the female's preference for the four-hole condition would supercede her preference for the dark side of the chamber, and cause her to shift her preference and spend more time in the light side after conditioning. Conditioning to each side was done sequentially at 4-day intervals for a total of three pairings each. Females in one group received PT-141 (200 μg/kg) 5 min before copulation in the four-hole/light condition and saline before copulation in the one-hole/dark condition, whereas females in the other group received PT-141 and saline in the reverse conditions. CPP was tested 4 days after the final conditioning trial by placing each E+P-primed female into a start compartment equidistant between the two distinctive sides. Females were allowed to explore the CPP box for 10 min. Entry into either side was detected by photobeams, and the amount of time spent in each side was recorded between photobeam breaks by a computer.
Statistical Analyses. Data were subjected to one-way ANOVA to assess the overall effect of dose on each measure. All significant ANOVAs were followed by post hoc Newman–Keuls tests of significance between individual means. For all analyses, P < 0.05 determined significance.
Results
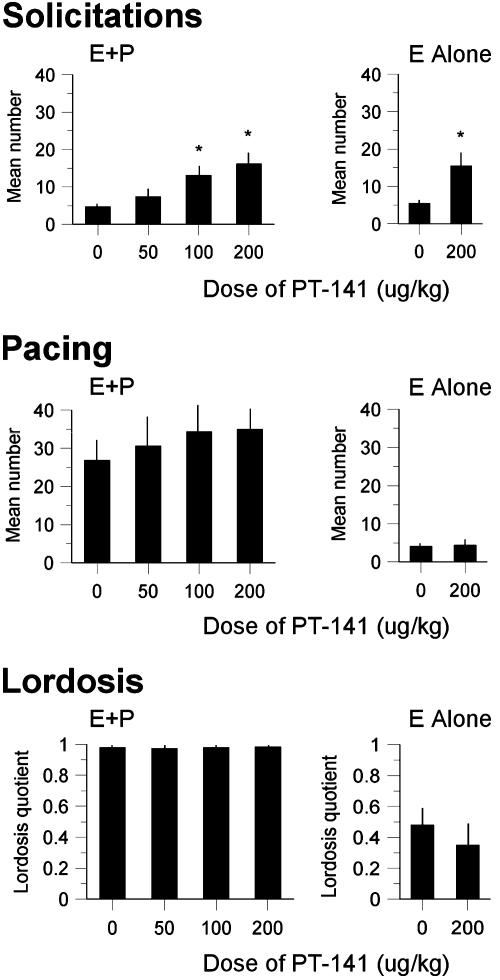
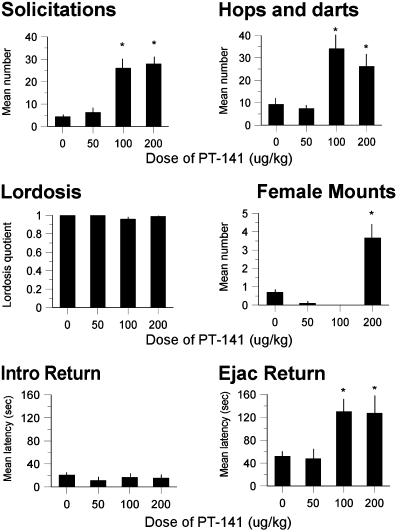
Treatment with PT-141 (100 or 200 μg/kg s.c.) significantly increased proceptive solicitations in females primed with E+Por E alone (E+O) in bilevel chambers (Fig. 2 Top), but did not affect pacing (Fig. 2 Middle), lordosis (Fig. 2 Bottom), or other measures of proceptivity such as hops and darts. The drug had even more pronounced effects in E+P-primed females that copulated in unilevel pacing chambers bisected by a four-hole divider (Fig. 3). Treatment with the same doses of PT-141 significantly increased solicitations (Fig. 3 Top Left) and hops and darts (Fig. 3 Top Right), but not lordosis (Fig. 3 Middle Left). Females in unilevel chambers make more use of hops and darts as proceptive behaviors after solicitation (14); therefore, we expected this measure to increase concurrently with solicitations in the unilevel chambers. Females treated with the highest dose of PT-141 also attempted to mount the males (Fig. 3 Middle Right). Female mounting of male rats is typically observed in E+P-primed females when they are paired with castrated or sexually sluggish males, and is considered a measure of proceptive sexual behavior.∥ The latency to run away and return to the male after mounts (data not shown) or intromissions (Fig. 3 Bottom Left) was not affected by PT-141. However, the latency to run away and return to the male after an ejaculation (Fig. 3 Bottom Right) was increased significantly by PT-141. Ejaculation typically results in an increased return latency (15, 16); thus, the effect of PT-141 appeared to enhance the impact of ejaculation.
Fig. 2.
Dose–response effects of PT-141 on sexual behavior in bilevel chambers. (Top) Effects on solicitations in females primed with estrogen and progesterone (E+P) or estrogen alone (E alone). For E+P-treated females, F3,35 = 3.73, P < 0.02. For females treated with E alone, F1,37 = 5.52, P < 0.03. Post hoc tests revealed that both the 100- and 200-μg/kg doses increased the number of solicitations significantly compared to saline-treated controls (P values < 0.05). (Middle) Effects on pacing in females primed with E+P or E alone. (Bottom) Effects on lordosis quotients in females primed with E+PorE alone. Data are means + SEM. *, P < 0.05 from control.
Fig. 3.
Dose–response effects of PT-141 on the sexual behavior of E+P-primed female rats in unilevel pacing chambers. (Top Left) Effects on solicitations (F3,34 = 6.62, P < 0.002). (Top Right) Effects on hops and darts (F3,34 = 4.12, P < 0.02). Post hoc tests revealed that both the 100- and 200-μg/kg doses increased solicitation and hops and darts significantly (P values < 0.05). (Middle Left) Effects on lordosis quotients. (Middle Right) Effects on female mounting of the male (FMM). F3,34 = 3.84, P < 0.02. Post hoc tests revealed a significant effect of the highest dose only (P < 0.05). (Bottom Left) Effects on the intromission return latency. (Bottom Right) Effects on the ejaculation return latency). F3,34 = 3.65, P < 0.02. Post hoc tests revealed a significant effect of the 100- and 200-μg/kg doses (P values < 0.05) Data are means ± SEM. *, P < 0.05 from control.
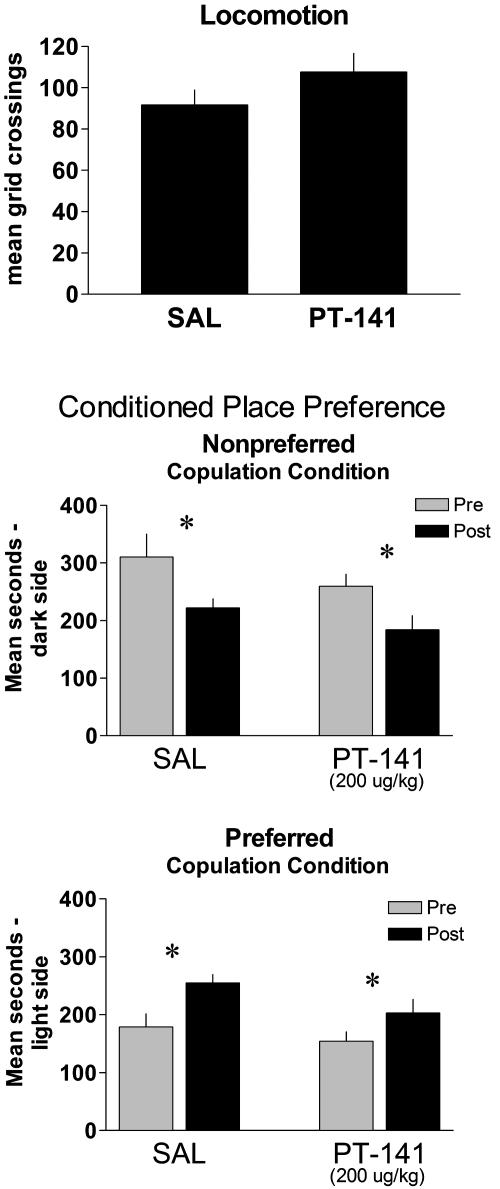
To examine whether the effects of PT-141 could have been caused by a general increase in locomotion, behavioral activity in fully primed females was examined in an open field. PT-141 did not affect general locomotion (Fig. 4 Upper), indicating that the increase in solicitations was not secondary to a general increase in behavioral activity. Finally, the ability of PT-141 to alter the perception of sexual reward induced by paced copulation was assessed by using CPP. In this paradigm, females display an innate preference for a dark compartment over a light compartment. Females find paced copulation rewarding (17), and previous studies have shown that pairing paced copulation with the light compartment and nonpaced copulation with the dark compartment result in a shift of preference to the light compartment in subsequent choice tests.∥ The rewarding condition used here was copulation in a unilevel chamber bisected by a Plexiglas divider that contained four small holes through which the female, but not the male, could cross. The less-rewarding (nonpreferred) condition was the same unilevel chamber bisected by a Plexiglas divider that contained a single small hole. PT-141 or saline was injected before copulation in the preferred, versus nonpreferred, condition in a counterbalanced fashion. PT-141 did not alter the normal development of CPP in either condition (Fig. 4 Lower), indicating that the drug does not enhance the sexual reward induced by a four-hole pacing condition, nor did it enhance sexual reward in a less-preferred one-hole pacing condition.
Fig. 4.
Effects of PT-141 or saline on locomotion and CPP. (Upper) Effects of saline or PT-141 (200 μg/kg) on the number of grid crossings in an open field during a 15-min test of locomotor activity. (Lower) Effects of PT-141 or saline on CPP induced by sequential paring of copulation in the one-hole versus four-hole condition. Data are means + SEM. *, P < 0.05 from pretest.
Discussion
These results show a selective pharmacological enhancement of appetitive sexual behavior in female rats primed with E and P or with E alone. Solicitation and hops and darts are appetitive precopulatory sexual behaviors that female rats use to arouse males (14). Females that solicit and pace their copulatory contact receive vigorous copulatory stimulation from males at a desired interval, thus increasing the likelihood of pregnancy (15). The ability of PT-141 to enhance solicitation in two distinctive testing environments indicates that the effect is selective and stable, and suggests that central melanocortin systems are part of the neurochemical network that evokes appetitive sexual behavior in female rats. PT-141 shows high affinity for melanocortin receptor subtypes 1, 3, and 4, with no significant binding to 30 other neuropeptide or monoamine receptors found in the CNS (12). At present, the specific brain regions in which melanocortins may exert this effect are unknown. Considering that PT-141 also binds with high affinity to the melanocortin type 1 receptor, we cannot rule out a peripheral mechanism of action, especially for the increased return latency after ejaculation. However, PT-141's selective binding to melanocortin type 3 and 4 receptors within the CNS make a central action at hypothalamic and/or limbic structures that control appetitive sexual behavior likely (18, 19). An increase in the activation of hypothalamic melanocortin systems induced by estrogen may also explain the peak in female-initiated sexual behaviors that occurs in many species, including humans, during ovulation (20).
Although the sexual behavior of rats is different from that of humans, the effects of pharmacological manipulations of appetitive and consummatory sexual behaviors are similar in male rats and men (21–23). This finding suggests that many aspects of the neurochemistry and neuroanatomy of male sexual responses are similar across species, and allows sexual responses of male rats to be used as models of human male sexual response. Behavioral concordance between female rats and women has received less attention. This is due, in part, to a concentration on the lordosis reflex as the defining characteristic of sexual behavior in female rats. Although lordosis is critical for vaginal intromission, and indicates that the female is receptive to such stimulation, it accounts for only one aspect of the behavioral pattern exhibited by females during copulation (14). Moreover, there is no human counterpart to lordosis as a measure of sexual receptivity. Other behaviors displayed by female rats during copulation are divided between solicitations and pacing, behaviors that allow females to initiate and regulate the timing of sexual contact. Indeed, hops and darts are also used by female rats to entice males to mount (14, 15, 23), and those behaviors were increased after PT-141 treatment. To the extent that appetitive measures of sexual activity in female rats are analogous to sexual desire in women (24), we conclude that PT-141 has the behavioral properties expected of a pharmacological aid in the treatment of sexual desire disorders.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: E, estrogen; P, progesterone; CPP, conditioned place preference.
Footnotes
Afonso, V. M. & Pfaus, J. G. (2003). Soc. Neurosci. Abstr. 29, 237.6 (abstr.).
References
- 1.Dornan, W. A. & Malsbury, C. W. (1989) Neurosci. Biobehav. Rev. 13, 1-15. [DOI] [PubMed] [Google Scholar]
- 2.Pfaus, J. G. & Everitt, B. J. (1995) in Psychopharmacology: The Fourth Generation of Progress, eds. Bloom, F. E. & Kupfer, D. J. (Raven, New York), pp. 743-758
- 3.Pfaus, J. G. & Gorzalka, B. B. (1987) Neurosci. Biobehav. Rev. 11, 1-34. [DOI] [PubMed] [Google Scholar]
- 4.Cragniolini, A., Scimonelli, T., Cellis, M. E. & Schioth, H. B. (2000) Peptides 34, 211-215. [DOI] [PubMed] [Google Scholar]
- 5.Raible, L. H. & Gorzalka, B. B. (1986) Peptides 7, 581-586. [DOI] [PubMed] [Google Scholar]
- 6.Thody, A. J. & Wilson, C. A. (1983) Physiol. Behav. 31, 67-72. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez, M. I., Celis, M. E., Hole, D. R. & Wilson, C.A. (1993) Neuroendocrinology 58, 218-226. [DOI] [PubMed] [Google Scholar]
- 8.Medina, F., Siddiqui, A., Scimonelli, T., Fenske, C., Wilson, C. A. & Celis, M. E. (1998) Peptides 19, 1309-1316. [DOI] [PubMed] [Google Scholar]
- 9.Wilson, C. A., Thody, A. J., Hole, D. R., Grierson, J. P. & Cellis, M. E. (1991) Neuroendocrinology 54, 14-22. [DOI] [PubMed] [Google Scholar]
- 10.Diamond, L. E., Earle, D. C., Rosen, R. C., Willett, M. S. & Molinoff, P. B. (2004) Int. J. Impot. Res. 16, 51-59. [DOI] [PubMed] [Google Scholar]
- 11.Rosen, R. C., Diamond, L. E., Earle, D. C., Shadiack, A. M. & Molinoff, P. B. (2004) Int. J. Impot. Res. 16, 135-142. [DOI] [PubMed] [Google Scholar]
- 12.Molinoff, P. B., Shadiack, A. M., Earle, D., Diamond, L. E. & Quon, C. Y. (2003) Ann. N.Y. Acad. Sci. 994, 96-102. [DOI] [PubMed] [Google Scholar]
- 13.Rosen, R. C. (2000) Curr. Psychiatry Rep. 2, 189-195. [DOI] [PubMed] [Google Scholar]
- 14.Pfaus, J. G., Smith, W. J. & Coopersmith, C. B. (1999) Horm. Behav. 35, 224-240. [DOI] [PubMed] [Google Scholar]
- 15.Erskine, M. S., Kornberg, E. & Cherry, J. A. (1989) Physiol. Behav. 45, 33-39. [DOI] [PubMed] [Google Scholar]
- 16.Yang, Y.-L. & Clemens, L. G. (1997) Physiol. Behav. 61, 889-894. [DOI] [PubMed] [Google Scholar]
- 17.Paredes, R. G. & Alonso, A. (1997) Behav. Neurosci. 111, 123-128. [DOI] [PubMed] [Google Scholar]
- 18.Lindblom, J., Schioth, H. B., Larsson, A., Wikberg, J. E. & Bergstrom, L. (1998) Brain Res. 810, 161-171. [DOI] [PubMed] [Google Scholar]
- 19.Mountjoy, K. G., Mortrud, M. T., Low, M. J., Simerly, R. B. & Cone, R. D. (1994) Mol. Endocrinol. 8, 1294-1308. [DOI] [PubMed] [Google Scholar]
- 20.Wallen, K. (1995) in Sexual Nature Sexual Culture, eds. Abramson, P. R. & Pinkerton, S. D. (Univ. of Chicago Press, Chicago), pp. 57-79.
- 21.Everitt, B. J. & Bancroft, J. (1991) Annu. Rev. Sex Res. 2, 77-118. [Google Scholar]
- 22.Pfaus, J. G. (1999) Annu. Rev. Sex Res. 10, 120-157. [PubMed] [Google Scholar]
- 23.Pfaus, J. G. (1996) Horm. Behav. 30, 187-200. [DOI] [PubMed] [Google Scholar]
- 24.Pfaus, J. G., Kippin, T. E. & Coria-Avila, G. (2003) Annu. Rev. Sex Res. 14, 1-63. [PubMed] [Google Scholar]