The role of K+ and Ca2+ in plant growth and development is complex and needs extensive physiological studies. Here, the effects of K+ and Ca2+ ions on growth and membrane potential in the presence of either auxin (IAA) or fusicoccin (FC) were studied in maize coleoptiles. The results presented in this article demonstrate that the inhibitory effect of Ca2+ on growth is significantly restored in the presence of K+. This effect is probably due to competitive inhibition of K+ channels by Ca2+ ions, although other possibilities should be taken into consideration.
Keywords: Auxin, calcium, coleoptile, elongation growth, fusicoccin, potassium, Zea mays
Abstract
The role of potassium (K+) and calcium (Ca2+) in the regulation of plant growth and development is complex and needs a diverse range of physiological studies. Both elements are essential for satisfactory crop production. Here, the effects of K+ and Ca2+ ions on endogenous growth and growth in the presence of either indole-3-acetic acid (IAA) or fusicoccin (FC) were studied in maize (Zea mays) coleoptiles. Membrane potentials of coleoptile parenchymal cells, incubated in media containing IAA, FC and different concentrations of K+ and Ca2+, were also determined. Growth experiments have shown that in the absence of K+ in the incubation medium, both endogenous and IAA- or FC-induced growth were significantly inhibited by 0.1 and 1 mM Ca2+, respectively, while in the presence of 1 mM K+ they were inhibited only by 1 mM Ca2+. At 10 mM K+, endogenous growth and growth induced by either IAA or FC did not depend on Ca2+ concentration. TEA-Cl, a potassium channel blocker, added 1 h before IAA or FC, caused a reduction of growth by 59 or 45 %, respectively. In contrast to TEA-Cl, verapamil, the Ca2+ channel blocker, did not affect IAA- and FC-induced growth. It was also found that in parenchymal cells of maize coleoptile segments, membrane potential (Em) was strongly affected by the medium K+, independently of Ca2+. However, lack of Ca2+ in the incubation medium significantly reduced the IAA- and FC-induced membrane potential hyperpolarization. TEA-Cl applied to the control medium in the same way as in growth experiments caused Em hyperpolarization synergistic with hyperpolarization produced by IAA or FC. Verapamil did not change either the Em of parenchymal cells incubated in the control medium or the IAA- and FC-induced membrane hyperpolarization. The data presented here have been discussed considering the role of K+ uptake channels in regulation of plant cell growth.
Introduction
Potassium (K+) is the most abundant cation in plant tissues, constituting up to 10 % of plant dry weight (Leigh and Wyn Jones 1984; Epstein and Bloom 2005). The cytosolic K+ concentration, ranging from 60 to 200 mM, plays a role in such well-characterized functions as electrical compensation of anions, control of cell membrane potential, enzyme activation and long-distance transport of sucrose and nitrate (reviewed in Ahmad and Maathuis 2014; Anschütz et al. 2014; Véry et al. 2014, Zörb et al. 2014). Potassium is also involved in turgor-driven processes such as cell elongation, phototropism, gravitropism and stomatal movement (reviewed in Becker and Hedrich 2002). It is currently well established that auxin-induced growth in maize coleoptile segments involves K+ uptake through voltage-dependent, inwardly rectifying K+ channels (ZMK1, Zea mays K+ channel 1), the activity of which contributes to water uptake and consequently cell expansion (reviewed in Hager 2003). It has been shown that apart from posttranslational, auxin-dependent up-regulation of the K+ uptake channels, auxin also regulates the expression of the maize K+ uptake channel gene ZMK1 (Philippar et al. 1999). Use of the patch-clamp technique on maize coleoptile protoplasts (Philippar et al. 1999; Thiel and Weise 1999) has confirmed some earlier studies which showed that auxin-induced growth strictly depends on external K+ supply (Claussen et al. 1997). The idea that the K+ uptake channels are crucial for auxin-induced growth comes also from experiments in which cell elongation and K+ conductance appeared to be sensitive to extracellular calcium (Ca2+) and tetraethylammonium (TEA), a K+ channel blocker (Thiel et al. 1996).
The divalent Ca2+ cation is an essential nutrient with diverse intra- and extracellular functions (Hofer and Brown 2003; Kudla et al. 2010). In plants, perception of most abiotic stresses results in the generation of calcium signals, which, in turn, elicit distinct Ca2+ concentration-dependent responses (McAinsh and Pittman 2009). Calcium, as one of the most important second messengers, is involved in a multitude of cellular processes, by which it exerts influence on nearly every aspect of plant growth and development (for review, see Vanneste and Friml 2009, 2013; Kudla et al. 2010; Schönknecht 2013).
To understand how auxin signals, particularly the ion transport-dependent ones, are transduced at a cellular level to elicit growth responses, we have performed experiments investigating the effects of K+ and Ca2+ on endogenous growth (growth without growth substances) and growth in the presence of either indole-3-acetic acid (IAA) or fusicoccin (FC) in maize coleoptile segments. In addition, membrane potential changes in parenchymal cells of coleoptile segments, incubated in the presence or absence of K+, Ca2+ and growth stimulators, were examined.
Methods
Plant material
Experiments were conducted on coleoptile segments obtained from 4-day-old seedlings grown in dark at 27 ± 1 °C. The 10-mm-long segments, with their first leaves removed, were cut from coleoptiles 3 mm below the tip. Conditions for growing the maize seedlings have been described previously (Karcz and Burdach 2002).
Growth measurements
Growth measurements were performed with an angular position transducer (TWK Electronic, Düsseldorf, Germany) as described previously by Karcz and Burdach (2002) and by Polak et al. (2011). In the experiments, five unabraded coleoptile segments were strung on a stainless steel needle and inserted vertically in an intensively aerated medium (5 mL for each coleoptile segment), the composition of which varied dependently on the variant of the experiment (0.0, 1.0, 10.0 mM KCl; 0.0, 0.1, 1.0 mM CaCl2; 0.1 mM NaCl). In the growth measurement studies, every single experiment (extension of a stack of five segments) performed in a medium with a fixed ion concentration was repeated at least eight times. Temperature of growth solutions was thermostatically controlled at 25 ± 1 °C (LW 102, Auritronic, Poland).
Growth was sampled for 10 h at regular 3-min intervals by the CX 721 converter (Elmetron, Poland). Growth substances, IAA or FC, were administered after 2 h of the experiment. All data are expressed as growth rate (µm s−1 cm−1) and coleoptile elongation (µm cm−1).
Electrophysiological measurements
Electrophysiological experiments were carried out on intact 10-mm-long maize coleoptile segments prepared as for growth experiments. The experimental technique previously described by Karcz and Burdach (2002), Kurtyka et al. (2011) and Polak et al. (2012) was used. Membrane potential (Em) was measured by recording the voltage between a 3 M KCl-filled glass micropipette inserted into the parenchymal cells and a reference electrode in the bathing medium. Before the electrophysiological experiments, maize coleoptile segments were preincubated for 110 min in an intensively aerated solution of the same composition as for growth measurements. After preincubation, a single segment was transferred into a perfusion electrophysiological chamber filled with a bathing medium of identical ion composition as the preincubation medium. Subsequently, a microelectrode was inserted into the parenchymal cell by means of a micromanipulator (Hugo Sachs Electronik, March-Hugstteten, Germany). After stabilization of the Em (<10 min), the bathing medium was exchanged with a solution of the same ion composition, containing, in addition, IAA or FC. Each single electrophysiological experiment means measurement of the Em in coleoptile parenchymal cell (one cell per one segment), repeated at least seven times. A peristaltic pump (Type Peri-Star PRO; World Precision Instruments, Sarasota, FL, USA) was used for changing the bathing medium in the chamber (usually 4-fold within <2 min). Micropipettes were pulled on a vertical pipette puller (Model L/M-3P-A; List-Medical, Germany) from borosilicate glass capillaries (Type 1B150F-3; World Precision Instruments). Tip diameter did not exceed 1 µm.
Chemicals
Indole-3-acetic acid (IAA) (Merck, Germany) was used as potassium salt, since it could be rapidly dissolved in water. Indole-3-acetic acid was used at 10 µM. Fusicoccin (Sigma, USA) was dissolved in ethanol and added to the incubation medium at a final concentration of 1 µM. The maximal ethanol concentration of 0.2 % did not affect the growth of coleoptile segments (data not shown). Tetraethylammonium chloride (Sigma) and verapamil (Sigma) were dissolved in deionized water and used at a final concentration of 30 mM and 50 µM, respectively. Stock solutions of TEA and verapamil were prepared in concentrations 100-fold greater than those used in experiments.
Data analysis
Data were analysed with Statistica software for Windows (StatSoft 2008, STATISTICA data analysis software system, version 8.0, http://www.statsoft.com, USA) at a significance level 0.05.
One-way ANOVA was used to test statistical differences in coleoptile growth among the considered groups (that is, various calcium concentrations with fixed potassium level and various channel blockers). Data were tested for normal distribution and variance homogeneity using Levene's test. Subsequently, the post hoc least significant difference (LSD) test was used for further analysis (P < 0.05). Student's t-test was used to estimate the significance of differences between membrane potential values recorded at 0 and 30 min of the measurements. The curves of membrane potential changes were fitted with least-squares linear regression and subsequently smoothed using Statistica software for Windows (Version 8.0).
Results
Effect of K+ and Ca2+ on endogenous growth
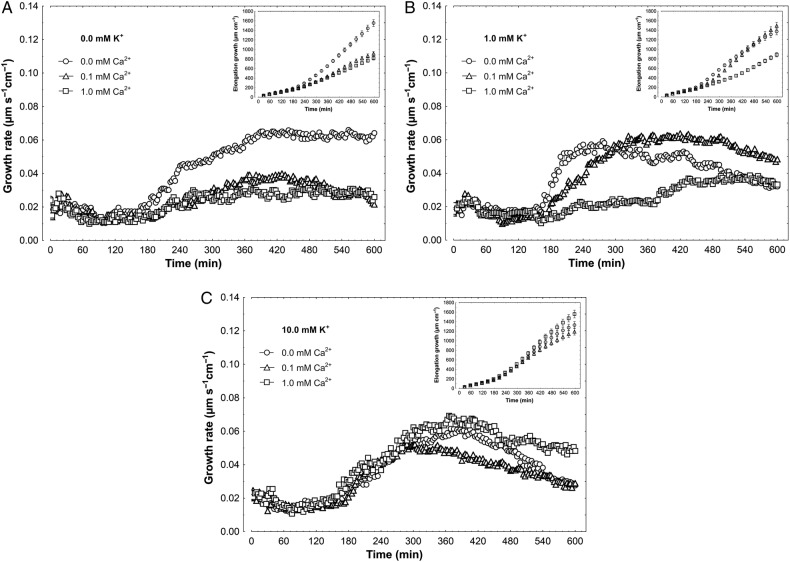
The effects of K+ (0.0, 1.0 and 10 mM) and Ca2+ (0.0, 0.1 and 1 mM) on the growth rate of maize coleoptile segments incubated in control medium (endogenous growth) are shown in Fig. 1. On the basis of growth rate responses, the total elongation growth (calculated as the sum of extensions from 3-min intervals) of coleoptile segments was also obtained (Fig. 1, insets). Taking these data into account, it should be stated that in media without K+, Ca2+ at 0.1 and 1 mM significantly (F2,23 = 15.21, P < 0.01) decreased endogenous growth of coleoptile segments by ∼50 %, as compared with growth in medium without Ca2+ and K+ (1554.4 ± 140.4 µm cm−1; mean ± SE, n = 8, Fig. 1A, inset). In the presence of 1 mM K+, endogenous growth of coleoptile segments was diminished only at 1 mM Ca2+ (F2,24 = 11.17, P < 0.01) (Fig. 1B). At 10 mM K+, endogenous growth of segments only slightly (especially over the first 6 h) depended on Ca2+ concentration (F2,24 = 3.47, P = 0.047) (Fig. 1C). Interestingly, in the absence of Ca2+ in the medium, elongation growth of coleoptile segments was comparable at all K+ concentrations studied (F2,25 = 1.25, P = 0.302) (Fig. 1).
Figure 1.
Effect of K+ and Ca2+ ions on the endogenous growth rate (growth without growth substances) of maize coleoptile segments. (A) Growth rate (µm s−1 cm−1) of coleoptile segments incubated in the medium with 0.0 mM K+ and Ca2+ at 0.0, 0.1 and 1.0 mM. (B) As in A but with 1.0 mM K+. (C) As in A and B but with 10 mM K+ in the medium. The growth rate of five coleoptile segments (10 mm in length) was recorded for 10 h in an intensively aerated incubation medium (5 mL segment−1) by means of an angular position transducer (TWK Electronic). Inset shows total elongation growth calculated as the sum of extensions measured in 3-min intervals for 10 h. All curves are mean values of at least eight independent measurements. Bars indicate ±SE.
Effect of K+ and Ca2+ on IAA- and FC-induced growth
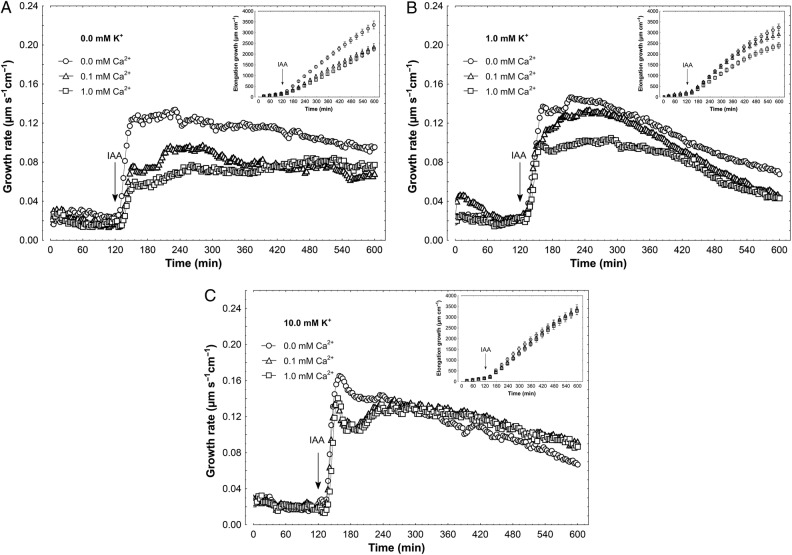
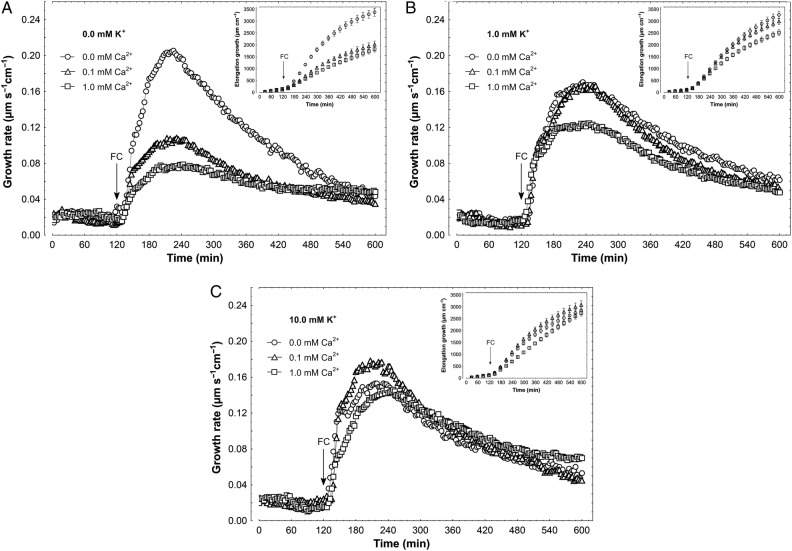
When IAA, at a final concentration of 10 µM, was added to the control medium, 2 h after the start of the experiment, a strong increase was observed in the growth rate (Fig. 2). The kinetics of IAA-induced growth could be divided into two phases (biphasic reaction) as previously described (Vanderhoef and Stahl 1975; Kazama and Katsumi 1976; Vanderhoef and Dute 1981; Philippar et al. 1999; Polak 2010); the first phase, very rapid, was followed by a long-lasting one, which began ∼30 min after auxin addition. In the absence of K+ in the incubation medium (Fig. 2A), Ca2+ at 0.1 and 1 mM significantly (F2,22 = 4.83, P = 0.018) diminished IAA-induced elongation growth by 31 %, as compared with IAA-induced growth in medium without Ca2+ and K+ (3357.8 ± 372.5 µm cm−1; mean ± SE, n = 9, Fig. 2A, inset). However, in the presence of 1 mM K+, IAA-induced elongation growth of coleoptile segments was significantly reduced (by 26 %) at 1 mM Ca2+ (F2,25 = 3.757, P = 0.037). When IAA was added to the medium containing 10 mM K+, auxin-induced growth did not depend on Ca2+ concentration (F2,21 = 0.031, P = 0.97) (Fig. 2C). Effects of K+ and Ca2+ were also studied for the growth of maize coleoptile segments incubated in the presence of FC (Fig. 3). This fungal toxin is known to enhance H+-ATPase activity, through phosphorylation of the penultimate Thr, as well as induce elongation growth (Marrè 1979; Kinoshita and Shimazaki 2001). Fusicoccin added to the incubation medium in the same way as IAA, at a final concentration of 1 µM, enhanced the endogenous growth of maize coleoptile segments to a level comparable with growth seen in the presence of IAA (Fig. 2). Independently of the ionic composition of the medium, FC added at 2 h of segment preincubation caused rapid growth with bell-shaped kinetics (Fig. 3). In the absence of K+ in incubation medium (Fig. 3A), Ca2+ at 0.1 and 1 mM significantly inhibited FC-induced elongation growth (F2,23 = 12.79, P < 0.01) by 46 % compared with growth in medium without Ca2+ and K+ (3378.3 ± 338.6 µm cm−1; mean ± SE, n = 8, Fig. 3A, inset). In medium with 1 mM K+ and Ca2,+ at both concentrations, inhibition of FC-induced growth was significant (F2,25 = 4.07, P = 0.029) but did not exceed 25 % (Fig. 3B). As in the case of IAA, FC-induced growth in a medium containing 10 mM K+ did not depend on Ca2+ concentration (F2,24 = 0.215, P = 0.807) (Fig. 3C).
Figure 2.
Effect of K+ and Ca2+ ions on the IAA-induced growth rate of maize coleoptile segments. (A) IAA-induced growth rate (µm s−1 cm−1) of coleoptile segments incubated in the medium with 0.0 mM K+ and Ca2+ at 0.0, 0.1 and 1.0 mM. (B) As in A but with 1.0 mM K+. (C) As in A and B but with 10 mM K+ in the medium. The growth rate of five coleoptile segments (10 mm in length) was recorded for 10 h in an intensively aerated incubation medium (5 mL segment−1) by means of an angular position transducer (TWK Electronic). Coleoptile segments were first preincubated for 2 h in a control medium, whereupon IAA was added at a final concentration of 10 µM. Inset shows total elongation growth, calculated as the sum of extensions measured in 3-min intervals for 10 h. All curves are mean values of at least eight independent measurements. Bars indicate ±SE.
Figure 3.
Effect of K+ and Ca2+ ions on the FC-induced growth rate of maize coleoptile segments. (A) FC-induced growth rate (µm s−1 cm−1) of coleoptile segments incubated in the medium with 0.0 mM K+ and Ca2+ at 0.0, 0.1 and 1.0 mM. (B) As in A but with 1.0 mM K+. (C) As in A and B but with 10 mM K+ in the medium. The growth rate of five coleoptile segments (10 mm in length) was recorded for 10 h in an intensively aerated incubation medium (5 mL segment−1) by means of an angular position transducer (TWK Electronic). Coleoptile segments were first preincubated for 2 h in a control medium, whereupon FC was added, at a final concentration of 1 µM. Inset shows total elongation growth calculated as the sum of extensions measured in 3-min intervals for 10 h. All curves are mean values of at least eight independent measurements. Bars indicate ±SE.
Effect of K+ and Ca2+ channel blockers on IAA- and FC-induced growth
Figure 4 shows the effects of K+ and Ca2+ channel blockers (TEA-Cl and verapamil, respectively) on IAA-induced growth of maize coleoptile segments incubated in the presence of 1 mM K+ and 0.1 mM Ca2+. The segments were first preincubated for 1 h before the blockers were added. At 2 h, IAA was applied to the incubation medium. Data in Fig. 4 indicate that TEA-Cl, added at a final concentration of 30 mM, significantly decreased the growth of IAA-incubated coleoptile segments (F1,17 = 46.61, P < 0.01) (2935.3 ± 324.6 µm cm−1; mean ± SE, n = 9) by 59 %. TEA-Cl added alone 1 h after start of the experiment diminished growth (F1,16 = 24.18, P < 0.01) by 40 % as compared with control (1494.7 ± 146.4 µm cm−1; mean ± SE, n = 9) (Fig. 4, inset). Addition of verapamil, at a final concentration of 50 µM, in the same time protocol as TEA-Cl, increased IAA-induced growth by 7 % (statistically not significant, F1,16 = 0.01, P = 0.76) (Fig. 4, inset). As presented in Fig. 5, FC added to incubation medium containing TEA-Cl enhanced the growth of coleoptile segments (F1,18 = 39.81, P < 0.01) to a level 45 % lower than FC alone (2998.6 ± 265.0 µm cm−1; mean ± SE, n = 10). Verapamil added in the same way as TEA-Cl did not change elongation growth in response to FC (F1,17 = 1.52, P = 0.23).
Figure 4.
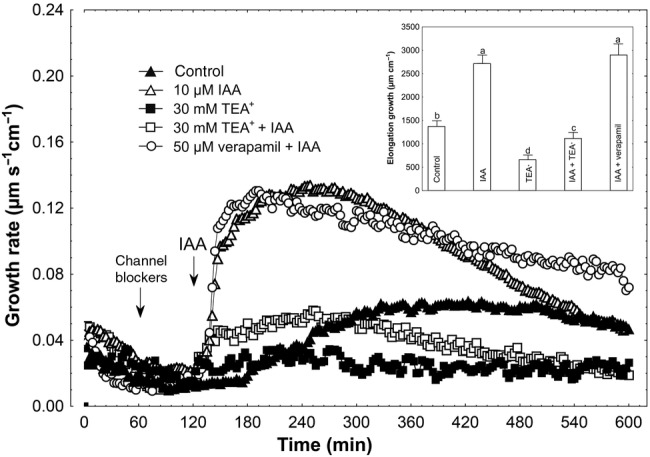
Effect of K+ and Ca2+ channel blockers (TEA-Cl and verapamil, respectively) on the IAA-induced growth rate (μm s−1 cm−1) of maize coleoptile segments. Coleoptile segments were first preincubated for over 1 h in a control medium, whereupon channel blockers were added. At 2 h, IAA was added to the incubation medium, at a final concentration of 10 µM. Inset on the right shows the total elongation growth, presented in a bar graph, calculated as the sum of extensions between 120 and 600 min of the experiment. Values are the mean of at least nine independent experiments. Bars indicate mean ± SE. Mean values followed by the same letter are not significantly different from each other according to the LSD test (P < 0.05).
Figure 5.
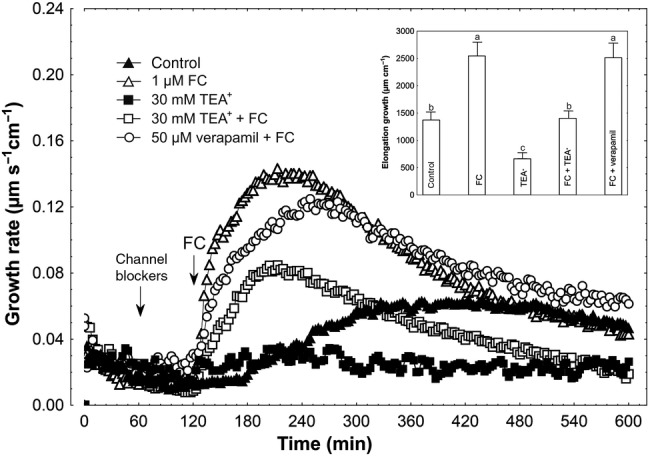
Effect of K+ and Ca2+ channel blockers (TEA-Cl and verapamil, respectively) on the FC-induced growth rate (μm s−1 cm−1) of maize coleoptile segments. Coleoptile segments were first preincubated for over 1 h in a control medium, whereupon channel blockers were added. At 2 h, FC was added to the incubation medium, at a final concentration of 1 µM. Inset on the right shows the total elongation growth, presented in a bar graph, calculated as the sum of extensions between 120 and 600 min of the experiment. Values are the mean of at least nine independent experiments. Bars indicate mean ± SE. Mean values followed by the same letter are not significantly different from each other according to the LSD test (P < 0.05).
Effect of K+ and Ca2+ on the IAA- and FC-induced Em changes
Results shown in Table 1 indicate that the membrane potential of parenchymal cells of maize coleoptile segments depended on K+ concentration, not on Ca2+ concentration (Table 1, column A). For example, a 10-fold increase in K+ concentration, independently of Ca2+ concentration, caused depolarization by ∼56 mV, which is near the value predicted from the Nernst equation. Addition of IAA to the incubation medium (Table 1, Fig. 6) caused transient depolarization of the Em, followed by a delayed hyperpolarization, during which membrane potential became more negative than the original potential. In the presence of IAA, membrane hyperpolarization was not statistically significant in media without Ca2+ (Table 1, Fig. 6). In contrast to IAA, FC caused an immediate hyperpolarization of the Em, attaining significantly higher values independently of K+ and Ca2+ concentrations. Similarly to IAA, the FC-induced membrane potential hyperpolarization was inhibited in the absence of Ca2+ (Table 1, Fig. 7) and showed a decrease with increasing K+ concentration.
Table 1.
Membrane potential (Em, mV) in parenchymal coleoptile cells. Data (mean ± SE) are mean values of at least seven independent experiments. Coleoptile segments were incubated in the indicated medium (the same as in growth experiments) for 110 min; afterwards a single segment was transferred into an electrophysiological chamber containing the same medium. Measurements of membrane potential (30 min) were carried out after insertion of a microelectrode into the cell and stabilization of the Em (<10 min) at 2 h (0 min, column A). Indole-3-acetic acid and FC were added after 2 h of segment incubation in the indicated medium; for the last 10 min before IAA or FC addition, coleoptile segments were incubated in the electrophysiological chamber. ns, Not statistically significant. *Statistically significant (P < 0.05).
| Treatment |
Membrane potential (mV) |
|||||||
|---|---|---|---|---|---|---|---|---|
| K+ | Ca2+ | A 0 min |
Transient depolarization | B 10 min |
C 20 min |
D 30 min |
ΔEm (D − A) (mV) | |
| (mM) |
||||||||
| IAA | 0.0 | 0.0 | −138.6 ± 7.1 | 1.4 | −138.2 ± 6.5 | −141.1 ± 7.6 | −141.2 ± 7.7 | −2.6ns |
| 0.1 | −136.5 ± 6.5 | 6.0 | −136.2 ± 7.3 | −151.5 ± 6.9 | −153.7 ± 6.8 | −17.2* | ||
| 1.0 | −134.1 ± 6.5 | 5.3 | −130.7 ± 6.1 | −140.8 ± 7.4 | −144.2 ± 7.0 | −10.1* | ||
| IAA | 1.0 | 0.0 | −124.5 ± 5.9 | 1.8 | −124.0 ± 6.2 | −126.5 ± 7.0 | −127.1 ± 6.8 | −2.6ns |
| 0.1 | −120.7 ± 6.1 | 5.5 | −118.3 ± 5.8 | −126.8 ± 6.7 | −129.3 ± 7.1 | −8.6* | ||
| 1.0 | −122.4 ± 6.3 | 7.3 | −116.3 ± 4.9 | −129.0 ± 7.2 | −132.4 ± 6.5 | −10.0* | ||
| IAA | 10 | 0.0 | −64.7 ± 5.0 | 1.5 | −64.5 ± 5.4 | −67.1 ± 3.9 | −67.4 ± 5.1 | −2.7ns |
| 0.1 | −65.6 ± 4.3 | 2.8 | −63.2 ± 5.0 | −69.6 ± 5.2 | −71.0 ± 4.7 | −5.4* | ||
| 1.0 | −64.8 ± 4.1 | 2.2 | −64.0 ± 4.7 | −70.3 ± 5.1 | −71.3 ± 4.3 | −6.5* | ||
| FC | 0.0 | 0.0 | −132.7 ± 7.1 | – | −139.0 ± 6.3 | −146.5 ± 6.9 | −146.6 ± 7.1 | −13.9* |
| 0.1 | −131.2 ± 6.6 | – | −140.8 ± 7.1 | −152.2 ± 7.3 | −155.8 ± 7.6 | −24.6* | ||
| 1.0 | −128.0 ± 7.2 | – | −142.1 ± 6.9 | −153.5 ± 7.9 | −155.4 ± 7.9 | −27.4* | ||
| FC | 1.0 | 0.0 | −120.1 ± 5.8 | – | −125.5 ± 6.2 | −128.1 ± 6.5 | −128.0 ± 6.1 | −7.9* |
| 0.1 | −118.7 ± 5.5 | – | −126.7 ± 6.6 | −135.8 ± 6.4 | −138.4 ± 7.2 | −19.7* | ||
| 1.0 | −121.3 ± 6.1 | – | −128.0 ± 6.4 | −135.0 ± 7.2 | −135.9 ± 6.3 | −14.6* | ||
| FC | 10 | 0.0 | −65.5 ± 4.2 | – | −66.9 ± 3.9 | −69.2 ± 5.1 | −69.5 ± 4.1 | −4.0ns |
| 0.1 | −68.4 ± 4.7 | – | −75.2 ± 4.6 | −78.5 ± 4.8 | −81.3 ± 5.3 | −12.9* | ||
| 1.0 | −64.6 ± 4.6 | – | −71.0 ± 5.1 | −75.7 ± 4.9 | −77.3 ± 4.8 | −12.7* | ||
Figure 6.
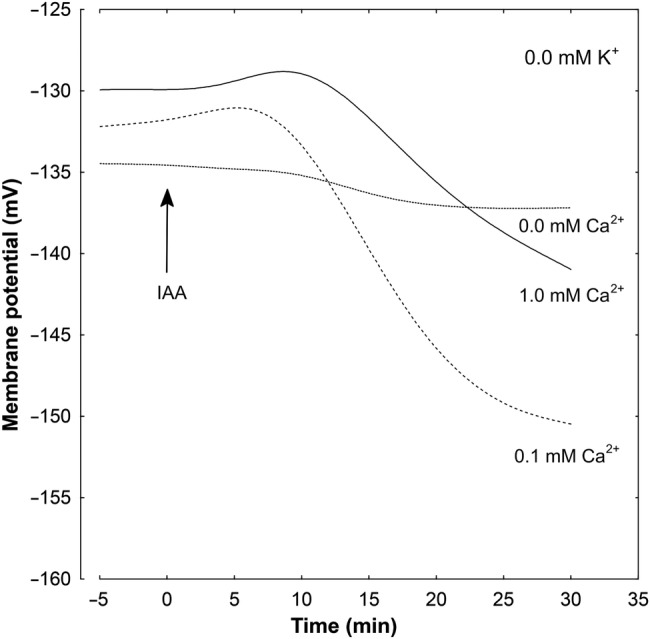
Effect of different concentrations of Ca2+ ions on the IAA-induced changes in the Em measured in maize coleoptile parenchymal cells in a medium without K+. Coleoptile segments were incubated in the indicated medium (the same as in growth experiments) for 110 min; afterwards a single segment was transferred into an electrophysiological chamber containing the same medium. Measurements of membrane potential (30 min) were carried out after insertion of a microelectrode into the cell (one cell per one segment) and stabilization of the Em (<10 min) at 2 h (0 min). Indole-3-acetic acid, at a final concentration of 10 µM, was added after 2 h of segment preincubation in the indicated medium; for the last 10 min, coleoptile segments were incubated in the electrophysiological chamber. Representative curves are shown. These curves were fitted with least-squares linear regression and subsequently smoothed using Statistica software for Windows (Version 8.0).
Figure 7.
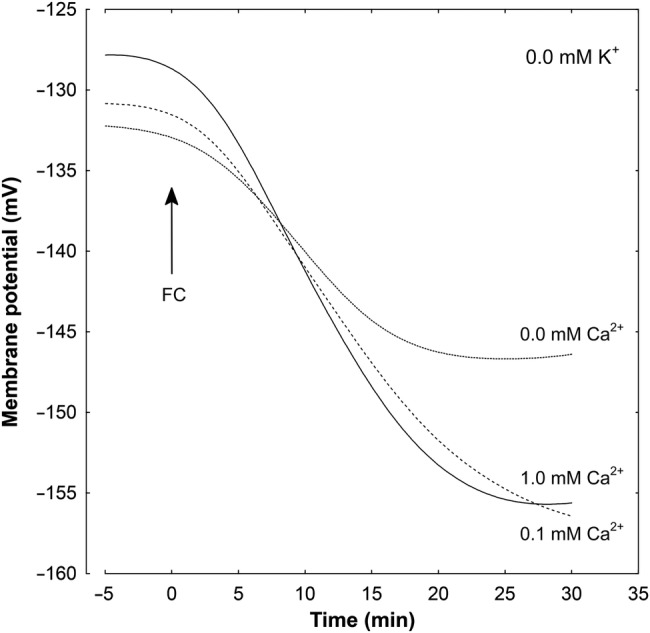
Effect of different concentrations of Ca2+ ions on the FC-induced changes in the Em measured in maize coleoptile parenchymal cells in a medium without K+. Coleoptile segments were incubated in the indicated medium (the same as in growth experiments) for 110 min; afterwards a single segment was transferred into an electrophysiological chamber containing the same medium. Measurements of membrane potential (30 min) were carried out after insertion of a microelectrode into the cell (one cell per one segment) and stabilization of the Em (<10 min) at 2 h (0 min). Fusicoccin, at a final concentration of 1 µM, was added after 2 h of segment preincubation in the indicated medium; for the last 10 min, coleoptile segments were incubated in the electrophysiological chamber. Representative curves are shown. These curves were fitted with least-squares linear regression and subsequently smoothed using Statistica software for Windows (Version 8.0).
Effect of TEA-Cl and verapamil on the IAA- and FC-induced Em changes
Figure 8 and Table 2 show the effects of TEA-Cl and verapamil on either IAA- or FC-induced Em changes in parenchymal cells of maize coleoptile segments incubated in medium with 1 mM K+ and 0.1 mM Ca2+. Indole-3-acetic acid, added at 2 h of segment incubation in medium with 1 mM K+ and 0.1 mM Ca2+ (for the last 10 min, coleoptile segments were incubated in the electrophysiological chamber), caused transient depolarization of the Em followed by a delayed membrane hyperpolarization during which membrane potential became by 9.2 mV more negative than the original potential (−121.0 ± 7.2 mV, mean ± SE, n = 13) (Fig. 8, Table 2). When TEA-Cl, at a final concentration of 30 mM, was added to the medium with 1 mM K+ and 0.1 mM Ca2+, after 1 h of segment incubation (for the last 10 min, coleoptile segments were incubated in the electrophysiological chamber), the Em of cells was hyperpolarized by 16.3 mV. In turn, addition of IAA at this hyperpolarized state of membrane caused additional hyperpolarization of the Em by 25.4 mV at 30 min. Verapamil, added to medium in the same way as TEA-Cl, did not affect the original Em (Fig. 8, Table 2). Administration of IAA in the presence of verapamil induced hyperpolarization by 4.3 mV higher than for auxin alone. Similar dependences were recorded in the presence of FC and both channel blockers (Fig. 8, Table 2). All substances used with FC were added to the incubation medium in the same time protocol as was IAA. Addition of FC produced immediate hyperpolarization of Em, where the Em was by 19.7 mV more negative than the original value (−121.6 ± 6.1 mV, mean ± SE, n = 9). At 30 mM TEA-Cl, FC-induced membrane hyperpolarization was by 30.3 mV more negative than the Em value after 1 h of segment incubation in medium with TEA-Cl. Verapamil did not basically change the original Em (Table 2, column A). Fusicoccin, added at 2 h to the incubation medium with verapamil, after 30 min, caused a 20.4 mV hyperpolarization (from −119.0 ± 4.5 to −139.4 ± 6.1 mV, Table 2).
Figure 8.
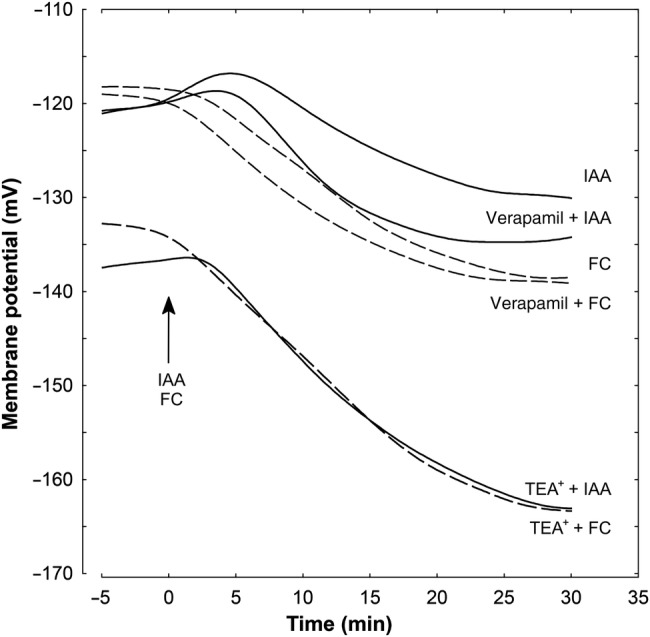
Effect of K+ and Ca2+ channel blockers (TEA-Cl and verapamil, respectively) on the IAA- and FC-induced changes in the Em measured in maize coleoptile parenchymal cells in a medium with 1 mM K+ and 0.1 mM Ca2+. Coleoptile segments were incubated in the indicated medium (the same as in growth experiments) for 60 min; afterwards the channel blockers were added. A total of 110 min after the start of preincubation, a single segment was transferred into an electrophysiological chamber containing the same medium (with channel blockers). Measurements of membrane potential (30 min) were carried out after insertion of a microelectrode into the cell (one cell per one segment) and stabilization of the Em (<10 min) at 2 h (0 min). Indole-3-acetic acid or FC, at a final concentration of 10 and 1 µM, respectively, were added after 2 h of segment preincubation in the indicated medium; for the last 10 min, coleoptile segments were incubated in the electrophysiological chamber. Representative curves are shown. These curves were fitted with least-squares linear regression and subsequently smoothed using Statistica software for Windows (Version 8.0).
Table 2.
Membrane potential (Em, mV) in parenchymal coleoptile cells. Data (mean ± SE) are mean values of at least seven independent experiments. Coleoptile segments were incubated in the indicated medium (the same as in growth experiments) for 110 min; afterwards a single segment was transferred into an electrophysiological chamber containing the same medium. Measurements of membrane potential (30 min) were carried out after insertion of a microelectrode into the cell and stabilization of the Em (<10 min) at 2 h (0 min, column A). Indole-3-acetic acid or FC were added after 2 h of segment preincubation in medium with 1 mM KCl and 0.1 mM CaCl2; for the last 10 min, coleoptile segments were incubated in the electrophysiological chamber. TEA-Cl or verapamil was added after 1 h of segment preincubation. All values in last column are statistically significant (P < 0.05).
| Treatment |
Membrane potential (mV) |
||||||
|---|---|---|---|---|---|---|---|
| A 0 min |
Transient depolarization | B 10 min |
C 20 min |
D 30 min |
ΔEm (D − A) (mV) | ||
| IAA | Control | −121.0 ± 7.2 | 5.2 | −120.6 ± 5.9 | −128.1 ± 7.1 | −130.2 ± 7.5 | 9.2 |
| TEA+ | −137.3 ± 6.9 | 2.6 | −147.3 ± 6.5 | −158.1 ± 6.9 | −162.7 ± 7.7 | 25.4 | |
| Verapamil | −120.7 ± 6.5 | 3.6 | −126.5 ± 5.9 | −134.1 ± 7.1 | −134.2 ± 7.3 | 13.5 | |
| FC | Control | −121.6 ± 6.1 | – | −126.7 ± 5.9 | −135.7 ± 6.4 | −141.3 ± 6.6 | 19.7 |
| TEA+ | −133.0 ± 6.6 | – | −146.6 ± 7.1 | −159.4 ± 7.3 | −163.3 ± 7.5 | 30.3 | |
| Verapamil | −119.0 ± 4.5 | – | −131.0 ± 5.5 | −137.3 ± 5.8 | −139.4 ± 6.1 | 20.4 | |
Discussion
The aim of this work was to determine the role of K+ and calcium ions in auxin- and FC-induced growth and Em changes in maize (Zea mays) coleoptile cells. It is now well established that the PM H+-ATPase, which generates the electrochemical gradient of H+ providing the driving force for a broad range of secondary transporters, plays a key role in the regulation of plant growth and development. According to the so-called ‘acid growth theory’, auxin activates the PM H+-ATPase, which acidifies the apoplasm and causes activation of enzymes involved in cell wall loosening (for review, see Rayle and Cleland 1992; Lüthen et al. 1999; Hager 2003). At least in maize coleoptile segments, auxin-induced medium acidification is mediated by the activity and/or amount of PM H+-ATPase (Hager et al. 1991; Frias et al. 1996). Interestingly, a similar scenario to that of the auxin-induced activation of PM H+-ATPase has been also reported for K+ uptake channels (ZMK1), as mentioned in the Introduction. Fusicoccin, a phytotoxic terpenoid produced by the fungus Fusicoccum amygdali (Ballio et al. 1964), mimics the effects of auxin in many respects (Marrè 1979), although its mode of action at the molecular level differs from that of auxin. It has been well documented that FC binds to the H+-ATPase/14-3-3 complex and stabilizes it, thus causing an increase in proton pump activity (Jahn et al. 1997; Baunsgaard et al. 1998; Fuglsang et al. 1999; Oecking and Hagemann 1999, Würtele et al. 2003). The data presented herein, showing that both IAA and FC result in acceleration of elongation growth as compared with endogenous growth (Figs 1–3) and that both substances cause hyperpolarization of the PM, that in case of IAA is preceded by a transient depolarization of the Em, are in good agreement with results obtained by other investigators (Cleland et al. 1977; Kutschera and Schopfer 1985a, b; Felle et al. 1986; Senn and Goldsmith 1988; Keller and Van Volkenburgh 1996; Karcz and Burdach 2002, 2007; Kurtyka et al. 2011; Rudnicka et al. 2014).
Growth experiments performed in media lacking K+ and Ca2+ revealed strong endogenous growth and growth in the presence of either auxin (IAA) or FC. This K+-independent growth was significantly diminished by 0.1 and 1.0 mM Ca2+ (Figs 1–3). When 1 mM K+ was included in the incubation medium, both IAA- and FC-induced growth of coleoptile segments were strongly restored, particularly at the lower Ca2+ concentration. However, in contrast to growth stimulated by IAA and FC, endogenous growth of coleoptile segments was still inhibited at 1 mM Ca2+. In medium containing 10 mM K+, endogenous growth and both IAA- and FC-induced growth depended weakly on Ca2+ and were comparable to those recorded in a medium without K+ and Ca2+. Both observations concerning growth, namely, its restoration at 1 mM K+ and its weak dependency on Ca2+ at 10 mM K+, may suggest that K+ channels are competitively inhibited by Ca2+, as previously proposed by Thiel et al. (1996). Another possibility, at least in the case of auxin action, is that it induces a rapid and transient increase in cytosolic Ca2+ concentration (Felle 1988; Gehring et al. 1990; Shishova and Lindberg 2004, 2010; Monshausen et al. 2011; Kirpichnikova et al. 2014) which, in turn, could inhibit PM H+-ATPase activity (Kinoshita et al. 1995; Polevoi et al. 1996; Brault et al. 2004; Fuglsang et al. 2007). Our results with TEA showed that when this K+-channel blocker was applied 1 h before addition of either IAA or FC, a 59 or 45 % growth reduction was observed, respectively (Figs 4 and 5). The lower inhibition of FC-induced growth by TEA is probably due to the fact that FC is unable to induce ZMK1 expression (Philippar et al. 1999). Inhibition of IAA- and FC-induced growth of maize coleoptile segments by TEA, used at the same concentration as in our experiments, was also shown previously by Claussen et al. (1997) and Tode and Lüthen (2001) and also recently by Burdach et al. (2014). Moreover, the authors found that TEA inhibits IAA-induced proton extrusion. In contrast to the K+-channel blocker, application of a Ca2+-channel blocker (verapamil) practically did not affect IAA- and FC-induced growth of maize coleoptile segments (Figs 4 and 5).
The above-mentioned results are generally in agreement with data obtained by Claussen et al. (1997) and Tode and Lüthen (2001), who showed that IAA- and FC-induced growth may occur in a K+-free medium lacking calcium. To explain the K+-independent growth of maize coleoptile segments incubated in a medium lacking K+ and Ca2+, Claussen et al. (1997) and Tode and Lüthen (2001) proposed a hypothesis assuming that the apoplastic Donnan space, being a huge reservoir for K+, can potentially supply this ion in amounts sufficient to maintain growth over a long time. If K+ in the Donnan space was replaced by Ca2+, the IAA- and FC-induced growth was abolished, indicating that in both cases K+ uptake was necessary for growth (Claussen et al. 1997; Tode and Lüthen 2001). Interestingly, IAA- and FC-pretreated coleoptile segments, which did not grow in the absence of K+ ions, displayed rapid growth if KCl was added to the medium (Claussen et al. 1997; Tode and Lüthen 2001). It is possible that the ZMK1 channel, as an orthologue of the Arabidopsis AKT1 channel (Geiger et al. 2009), may mediate the uptake of K+ under K+-limiting conditions. It is noteworthy that stimulation of coleoptile elongation growth in the presence of K+ and growth inhibition under the influence of Ca2+ were discovered in the 1950s (Cooil and Bonner 1957; Tagawa and Bonner 1957; Cleland and Rayle 1977).
Electrophysiological experiments showed that in parenchymal cells of coleoptile segments, membrane potential was strongly related to the medium K+ (Table 1, column A). This dependence did not change when calcium was included in the medium, suggesting that Ca2+ at 0.1 and 1.0 mM had no effect on K+ conductance. The above-described results are in line with data obtained in patch-clamp experiments by Thiel et al. (1996), who reported inhibition of K+ uptake channels at a higher (10 mM) concentration of Ca2+ in maize coleoptile protoplasts. Addition of IAA or FC to the incubation medium caused hyperpolarization of the Em, attaining higher values in the presence of FC (Table 1, Figs 6 and 7). There is no doubt that plasma membrane hyperpolarization in the presence of IAA or FC is a consequence of stimulated proton extrusion through the PM H+-ATPase (Rück et al. 1993; Hedrich et al. 1995; Hager 2003). In a medium lacking Ca2+, IAA- and FC-induced membrane hyperpolarization was strongly inhibited (Table 1, column ΔEm), although either IAA- or FC-induced growth was strongly stimulated (Figs 2 and 3). It is possible that under such conditions the stimulating effect of IAA and FC on K+ uptake and then on elongation growth does not require any additional activation of K+ uptake channels; they are probably already active enough.
Tetraethylammonium chloride applied after 1 h of segment incubation in the control medium produced hyperpolarization of the Em, which became by 16.3 mV more negative than the original membrane potential in control medium (−121.0 ± 7.2 mV) (Table 2). Indole-3-acetic acid added at 2 h to the medium containing TEA induced additional membrane hyperpolarization by 25.4 mV (Table 2), suggesting that both processes are synergistic. A similar effect was observed for FC, which hyperpolarized the Em synergically with TEA (Table 2). In contrast to the K+-channel blocker, verapamil administered in the same time protocol as TEA did not change the Em in parenchymal coleoptile cells incubated in the control medium (Table 2, column A). Indole-3-acetic acid or FC added to the medium with verapamil induced hyperpolarization of the Em comparable to the one observed in the presence of IAA or FC alone.
Conclusions
The results presented in this article demonstrate that the inhibitory effect of Ca2+ on endogenous growth and both IAA- and FC-induced growth is significantly restored in the presence of K+. This effect is probably due to the competitive inhibition of K+ channels by Ca2+ ions, although other possibilities could be taken into consideration.
Sources of Funding
Founding was provided by University of Silesia.
Contributions by the Authors
All authors have made a substantial contribution to the manuscript and the research presented.
Conflict of Interest Statement
None declared.
Acknowledgements
The authors thank the staff of AoB PLANTS and the anonymous reviewers for useful comments that have improved the manuscript.
Literature Cited
- Ahmad I, Maathuis FJM. 2014. Cellular and tissue distribution of potassium: physiological relevance, mechanisms and regulation. Journal of Plant Physiology 171:708–714. 10.1016/j.jplph.2013.10.016 [DOI] [PubMed] [Google Scholar]
- Anschütz U, Becker D, Shabala S. 2014. Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. Journal of Plant Physiology 171:670–687. 10.1016/j.jplph.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Ballio A, Chain EB, De Leo P, Erlanger BF, Mauri M, Tonolo A. 1964. Fusicoccin: a new wilting toxin produced by Fusicoccum amygdali Del. Nature 203:297 10.1038/203297a0 [DOI] [Google Scholar]
- Baunsgaard L, Fuglsang AT, Jahn T, Korthout HAAJ, De Boer AH, Palmgren MG. 1998. The 14-3-3 proteins associate with the plant plasma membrane H(+)-ATPase to generate a fusicoccin binding complex and a fusicoccin responsive system. Plant Journal 13:661–671. 10.1046/j.1365-313X.1998.00083.x [DOI] [PubMed] [Google Scholar]
- Becker D, Hedrich R. 2002. Channelling auxin action: modulation of ion transport by indole-3-acetic acid. Plant Molecular Biology 49:349–356. 10.1023/A:1015211231864 [DOI] [PubMed] [Google Scholar]
- Brault M, Amiar Z, Pennarun AM, Monestiez M, Zhang Z, Cornel D, Dellis O, Knight H, Bouteau F, Rona JP. 2004. Plasma membrane depolarization induced by abscisic acid in Arabidopsis suspension cells involves reduction of proton pumping in addition to anion channel activation, which are both Ca2+ dependent. Plant Physiology 135:231–243. 10.1104/pp.104.039255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdach Z, Kurtyka R, Siemieniuk A, Karcz W. 2014. Role of chloride ions in the promotion of auxin-induced growth of maize coleoptile segments. Annals of Botany 114:1023–1034. 10.1093/aob/mcu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussen M, Lüthen H, Blatt M, Böttger M. 1997. Auxin-induced growth and its linkage to potassium channels. Planta 201:227–234. 10.1007/BF01007708 [DOI] [Google Scholar]
- Cleland RE, Rayle DL. 1977. Reevaluation of the effect of calcium ions on auxin-induced elongation. Plant Physiology 60:709–712. 10.1104/pp.60.5.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland RE, Prins HBA, Harper JR, Higinbotham N. 1977. Rapid hormone-induced hyperpolarization of the oat coleoptile transmembrane potential. Plant Physiology 59:395–397. 10.1104/pp.59.3.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooil BJ, Bonner J. 1957. The nature of growth inhibition by calcium in the Avena coleoptile. Planta 48:696–723. 10.1007/BF01911596 [DOI] [Google Scholar]
- Epstein E, Bloom AJ. 2005. Mineral nutrition of plants: principles and perspectives. 2nd edn Sunderland, MA: Sinauer Associates Inc. [Google Scholar]
- Felle H. 1988. Auxin causes oscillations of cytosolic free calcium and pH in Zea mays coleoptiles. Planta 174:495–499. 10.1007/BF00634478 [DOI] [PubMed] [Google Scholar]
- Felle H, Brummer B, Bertl A, Parish RW. 1986. Indole-3-acetic acid and fusicoccin cause cytosolic acidification of corn coleoptile cells. Proceedings of the National Academy of Sciences of the USA 83:8992–8995. 10.1073/pnas.83.23.8992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias I, Caldeira MT, Perez-Castineira JR, Navarro-Avino JP, Culianez-Macia FA, Kuppinger O, Stransky H, Pages M, Hager A, Serrano R. 1996. A major isoform of the maize plasma membrane H(+)-ATPase: characterization and induction by auxin in coleoptiles. The Plant Cell 8:1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglsang AT, Visconti S, Drumm K, Jahn T, Stensballe A, Mattei B, Jensen ON, Aducci P, Palmgren MG. 1999. Binding of 14-3-3 protein to the plasma membrane H+-ATPase AHA2 involves the three C-terminal residues Tyr946-Thr-Val and requires phosphorylation of Thr947. Journal of Biological Chemistry 274:36774–36780. 10.1074/jbc.274.51.36774 [DOI] [PubMed] [Google Scholar]
- Fuglsang AT, Guo Y, Cuin TA, Qiu Q, Song C, Kristiansen KA, Bych K, Schulz A, Shabala S, Schumaker KS, Palmgren MG, Zhu JK. 2007. Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. The Plant Cell 19:1617–1634. 10.1105/tpc.105.035626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring CA, Irving HR, Parish RW. 1990. Effects of auxin and abscisic acid on cytosolic calcium and pH in plant cells. Proceedings of the National Academy of Sciences of the USA 87:9645–9649. 10.1073/pnas.87.24.9645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Becker D, Vosloh D, Gambale F, Palme K, Rehers M, Anschuetz U, Dreyer I, Kudla J, Hedrich R. 2009. Heteromeric AtKC1-AKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. Journal of Biological Chemistry 284:21288–21295. 10.1074/jbc.M109.017574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A. 2003. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. Journal of Plant Research 116:483–505. 10.1007/s10265-003-0110-x [DOI] [PubMed] [Google Scholar]
- Hager A, Debus G, Edel HG, Stransky H, Serrano R. 1991. Auxin induces exocytosis and the rapid synthesis of a high-turnover pool of plasma-membrane H+-ATPase. Planta 185:527–537. 10.1007/BF00202963 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Bregante M, Dreyer I, Gambale F. 1995. The voltage-dependent potassium-uptake channel of corn coleoptiles has permeation properties different from other K+ channels. Planta 197:193–199. 10.1007/BF00239956 [DOI] [Google Scholar]
- Hofer AM, Brown EM. 2003. Extracellular calcium sensing and signalling. Nature Reviews Molecular Cell Biology 4:530–538. 10.1038/nrm1154 [DOI] [PubMed] [Google Scholar]
- Jahn T, Fuglsang AT, Olsson A, Brüntrup IM, Collinge DB, Volkmann D, Sommarin M, Palmgren MG, Larsson C. 1997. The 14-3-3 protein interacts directly with the C-terminal region of the plant plasma membrane H(+)-ATPase. The Plant Cell 9:1805–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcz W, Burdach Z. 2002. A comparison of the effects of IAA and 4-Cl-IAA on growth, proton secretion and membrane potential in maize coleoptile segments. Journal of Experimental Botany 53:1089–1098. 10.1093/jexbot/53.371.1089 [DOI] [PubMed] [Google Scholar]
- Karcz W, Burdach Z. 2007. Effect of temperature on growth, proton extrusion and membrane potential in maize (Zea mays L.) coleoptile segments. Plant Growth Regulation 52:141–150. 10.1007/s10725-007-9184-0 [DOI] [Google Scholar]
- Kazama H, Katsumi M. 1976. Biphasic response of cucumber hypocotyl sections to auxin. Plant and Cell Physiology 17:467–473. [Google Scholar]
- Keller CP, Van Volkenburgh E. 1996. Osmoregulation by oat coleoptile protoplasts (effect of auxin). Plant Physiology 110:1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki KI. 2001. Analysis of the phosphorylation level in guard-cell plasma membrane H+-ATPase in response to fusicoccin. Plant and Cell Physiology 42:424–432. 10.1093/pcp/pce055 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Nishimura M, Shimazaki KI. 1995. Cytosolic concentration of Ca2+ regulates the plasma membrane H+-ATPase in guard cells of fava bean. The Plant Cell 7:1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpichnikova AA, Rudashevskaya EL, Yemelyanov VV, Shishova MF. 2014. Ca2+-transport through plasma membrane as a test of auxin sensitivity. Plants 3:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J, Batistič O, Hashimoto K. 2010. Calcium signals: the lead currency of plant information processing. The Plant Cell 22:541–563. 10.1105/tpc.109.072686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtyka R, Kita A, Karcz W. 2011. Fusicoccin counteracts the toxic effect of cadmium on the growth of maize coleoptile segments. Archives of Environmental Contamination and Toxicology 61:568–577. 10.1007/s00244-011-9662-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera U, Schopfer P. 1985a. Evidence against the acid-growth theory of auxin action. Planta 163:483–493. 10.1007/BF00392705 [DOI] [PubMed] [Google Scholar]
- Kutschera U, Schopfer P. 1985b. Evidence for the acid-growth theory of fusicoccin action. Planta 163:494–499. 10.1007/BF00392706 [DOI] [PubMed] [Google Scholar]
- Leigh RA, Wyn Jones RG. 1984. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytologist 97:1–13. 10.1111/j.1469-8137.1984.tb04103.x [DOI] [Google Scholar]
- Lüthen H, Claussen M, Böttger M. 1999. Growth: progress in auxin research. Progress in Botany 60:315–340. 10.1007/978-3-642-59940-8_12 [DOI] [Google Scholar]
- Marrè E. 1979. Fusicoccin: a tool in plant physiology. Annual Review of Plant Physiology 30:273–288. 10.1146/annurev.pp.30.060179.001421 [DOI] [Google Scholar]
- McAinsh MR, Pittman JK. 2009. Shaping the calcium signature. New Phytologist 181:275–294. 10.1111/j.1469-8137.2008.02682.x [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Miller ND, Murphy AS, Gilroy S. 2011. Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. The Plant Journal 65:309–318. 10.1111/j.1365-313X.2010.04423.x [DOI] [PubMed] [Google Scholar]
- Oecking C, Hagemann K. 1999. Association of 14-3-3 proteins with the C-terminal autoinhibitory domain of the plant plasma-membrane H+-ATPase generates a fusicoccin-binding complex. Planta 207:480–482. 10.1007/s004250050507 [DOI] [Google Scholar]
- Philippar K, Fuchs I, Lüthen H, Hoth S, Bauer CS, Haga K, Thiel G, Ljung K, Sandberg G, Böttger M, Becker D, Hedrich R. 1999. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proceedings of the National Academy of Sciences of the USA 96:12186–12191. 10.1073/pnas.96.21.12186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak M. 2010. The interdependencies between growth, medium pH and membrane potential in maize coleoptile segments incubated in the presence of auxin (IAA), fusicoccin (FC) and allicin. PhD Thesis, University of Silesia, Katowice, Poland. [Google Scholar]
- Polak M, Tukaj Z, Karcz W. 2011. Effect of temperature on the dose-response curves for auxin-induced elongation growth in maize coleoptile segments. Acta Physiologiae Plantarum 33:437–442. 10.1007/s11738-010-0563-1 [DOI] [Google Scholar]
- Polak M, Zaborska W, Tukaj Z, Karcz W. 2012. Effect of thiosulphinates contained in garlic extract on growth, proton fluxes and membrane potential in maize (Zea mays L.) coleoptile segments. Acta Physiologiae Plantarum 34:41–52. 10.1007/s11738-011-0803-z [DOI] [Google Scholar]
- Polevoi VV, Sinyutina NF, Salamatova TS, Inge-Vechtomova NI, Tankelyun OV, Sharova EI, Shishova MF. 1996. Mechanism of auxin action: second messengers. In: Smith AR, Berry AW, Harpham NVJ, Moshkov IE, Novikova GV, Kulaeva ON, Hall MA, eds. Plant hormone signal perception and transduction. The Netherlands: Springer, 223–231. [Google Scholar]
- Rayle DL, Cleland RE. 1992. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiology 99:1271–1274. 10.1104/pp.99.4.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rück A, Palme K, Venis MA, Napier RM, Felle HH. 1993. Patch-clamp analysis establishes a role for an auxin binding protein in the auxin stimulation of plasma membrane current in Zea mays protoplasts. The Plant Journal 4:41–46. 10.1046/j.1365-313X.1993.04010041.x [DOI] [Google Scholar]
- Rudnicka M, Polak M, Karcz W. 2014. Cellular responses to naphthoquinones: juglone as a case study. Plant Growth Regulation 72:239–248. 10.1007/s10725-013-9855-y [DOI] [Google Scholar]
- Schönknecht G. 2013. Calcium signals from the vacuole. Plants 2:589–614. 10.3390/plants2040589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn AP, Goldsmith MHM. 1988. Regulation of electrogenic proton pumping by auxin and fusicoccin as related to the growth of Avena coleoptiles. Plant Physiology 88:131–138. 10.1104/pp.88.1.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishova M, Lindberg S. 2004. Auxin induces an increase of Ca2+ concentration in the cytosol of wheat leaf protoplasts. Journal of Plant Physiology 161:937–945. 10.1016/j.jplph.2003.12.005 [DOI] [PubMed] [Google Scholar]
- Shishova M, Lindberg S. 2010. A new perspective on auxin perception. Journal of Plant Physiology 167:417–422. 10.1016/j.jplph.2009.12.014 [DOI] [PubMed] [Google Scholar]
- Tagawa T, Bonner J. 1957. Mechanical properties of the Avena coleoptile as related to auxin and to ionic interactions. Plant Physiology 32:207–212. 10.1104/pp.32.3.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel G, Weise R. 1999. Auxin augments conductance of K+ inward rectifier in maize coleoptile protoplasts. Planta 208:38–45. 10.1007/s004250050532 [DOI] [Google Scholar]
- Thiel G, Brüdern A, Gradmann D. 1996. Small inward rectifying K+ channels in coleoptiles: inhibition by external Ca2+ and function in cell elongation. Journal of Membrane Biology 149:9–20. 10.1007/s002329900002 [DOI] [PubMed] [Google Scholar]
- Tode K, Lüthen H. 2001. Fusicoccin- and IAA-induced elongation growth share the same pattern of K+ dependence. Journal of Experimental Botany 52:251–255. 10.1093/jexbot/52.355.251 [DOI] [PubMed] [Google Scholar]
- Vanderhoef LN, Dute RR. 1981. Auxin-regulated wall loosening and sustained growth in elongation. Plant Physiology 67:146–149. 10.1104/pp.67.1.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef LN, Stahl CA. 1975. Separation of two responses to auxin by means of cytokinin inhibition. Proceedings of the National Academy of Sciences of the USA 72:1822–1825. 10.1073/pnas.72.5.1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Friml J. 2009. Auxin: a trigger for change in plant development. Cell 136:1005–1016. 10.1016/j.cell.2009.03.001 [DOI] [PubMed] [Google Scholar]
- Vanneste S, Friml J. 2013. Calcium: the missing link in auxin action. Plants 2:650–675. 10.3390/plants2040650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry AA, Nieves-Cordones M, Daly M, Khan I, Fizames C, Sentenac H. 2014. Molecular biology of K+ transport across the plant cell membrane: what do we learn from comparison between plant species? Journal of Plant Physiology 171:748–769. 10.1016/j.jplph.2014.01.011 [DOI] [PubMed] [Google Scholar]
- Würtele M, Jelich-Ottmann C, Wittinghofer A, Oecking C. 2003. Structural view of a fungal toxin acting on a 14-3-3 regulatory complex. The EMBO Journal 22:987–994. 10.1093/emboj/cdg104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zörb C, Senbayram M, Peiter E. 2014. Potassium in agriculture—status and perspectives. Journal of Plant Physiology 171:656–669. 10.1016/j.jplph.2013.08.008 [DOI] [PubMed] [Google Scholar]