Abstract
Thyroid storm is a rare, but critical, illness that can lead to multiorgan failure and carries a high death rate. The following case series describes two adult men with Graves’ disease who presented in thyroid storm and either failed or could not tolerate conventional medical management. However, both patients responded well to plasmapheresis, which resulted in clinical and biochemical stabilisation of their disease processes. The treatment option of plasmapheresis should be considered as a stabilising measure, especially when patients have failed or cannot tolerate conventional therapy. Plasmapheresis leads to amelioration of symptoms and a significant decline in thyroid hormone levels, providing a window to treat definitively with thyroidectomy.
Background
Thyroid storm is a rare, severe and life-threatening exacerbation of thyrotoxicosis, characterised by dysfunction of the thermoregulatory, central nervous, gastrointestinal-hepatic and cardiovascular systems.1–3 Historically, medical management has focused on supportive measures and medications that act to halt the synthesis, release and peripheral effects of thyroid hormone. However, antithyroidal therapy is sometimes limited due to rare and serious side effects or failure to control disease progression, leaving few treatment alternatives. Several case reports dating back to the 1970s suggest the effectiveness of plasmapheresis as one of those alternatives. However, there is currently no clear consensus recommendation for or against its use. We report two cases of thyroid storm with multiorgan dysfunction, where traditional therapy was either contraindicated or ineffective and plasmapheresis successfully stabilised both patients prior to definitive surgical intervention.
Case presentation
Case 1: A 27-year-old African American man with a history of Graves’ disease and systolic heart failure (ejection fraction of 40% from 2 months prior) presented with a 3-day history of worsening palpitations, dyspnea, nausea and vomiting. He denied heat intolerance, chest pain, visual complaints and alcohol or drug abuse. He had not taken his prescribed methimazole and propanolol for 2 months prior to presentation.
Physical exam revealed a thin man who was moderately agitated but alert and oriented to person, place and time. The heart rate was 280 beats/min on cardiac monitoring, blood pressure 130/80 mm Hg, temperature 97.1°F, respiratory rate 24 breaths/min and SaO2 was 100% on 2 litres of oxygen per nasal cannula. He had bilateral proptosis and lid lag. The thyroid gland was firm, symmetric and diffusely enlarged at 80 g with an audible bruit. Cardiac auscultation revealed tachycardia with a normal S1 and S2, audible S3 and a 3/6 holosystolic murmur over the left sternal border. Jugular venous distension to the angle of the jaw was observed. There were bibasilar crackles over the lungs. The abdomen was soft, non-tender and non-distended, with hepatomegaly (liver span 18 cm in the right mid-clavicular line). Neurological exam showed 3+ reflexes in bilateral upper and lower extremities, an obvious tremor of the hands, bilateral upper-extremity and lower-extremity proximal weakness and intact cranial nerves. He had 1+ bilateral lower-extremity oedema.
Case 2: A second man with a history of Graves’ disease presented with palpitations, dyspnea and 3 weeks of worsening lower-extremity oedema that had progressed to include his scrotum. He denied heat intolerance, chest pain, fever, weakness and alcohol or drug abuse. He reported non-adherence to his prescribed methimazole and propanolol during the preceding 2 months.
Physical exam revealed a thin and anxious man who was alert and oriented to person, place and time. His heart rate was 165 beats/min on the cardiac monitor and irregular, blood pressure 133/80 mm Hg, temperature 98.2°F, respiratory rate 20 breaths/min and SaO2 100% on 4 litres of oxygen per nasal cannula. He exhibited bilateral proptosis and lid lag. The thyroid gland was firm, symmetric and diffusely enlarged at 70 g. Cardiac exam revealed tachycardia with normal S1 and S2 with no murmur, an audible S3 and jugular venous distension to the angle of the jaw. He had bibasilar crackles upon chest auscultation. The abdomen was soft, non-tender and non-distended. He had 2+ pitting oedema in his lower extremities extending to the scrotum. Neurological exam revealed a fine tremor of the hands, bilateral upper- and lower-extremity proximal weakness with a power grade of 4/5, deep tendon reflexes 3+ in upper extremities and intact cranial nerves.
Investigations
Case 1: On presentation, the ECG showed a wide complex ventricular tachycardia with a rate of 277 beats/min. Laboratory evaluation showed severe thyrotoxicosis, lactic acidosis, hypoglycaemia, acute kidney injury and abnormal liver function tests (see table 1). The patient was diagnosed with thyroid storm based on clinical presentation and a Burtch-Wartofsky score of 80.1 Cardiac enzymes were not elevated. Chest x-ray showed a right-sided pleural effusion with cardiomegaly and prominent pulmonary arteries. Transthoracic echocardiogram showed a left ventricular ejection fraction of less than 20% with severe global hypokinesis.
Table 1.
Laboratory assessment during the course of the patient's illness (patient A)
| Test | Day 1 | Day 1 (17:00) | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 |
|---|---|---|---|---|---|---|---|
| TSH (0.35–5.5 µIU/ml) | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ||
| Free T4 0.89–1.76 ng/dl) | 9.69 | 6.48 | 2.43 | 2.24 | 1.76 | ||
| Free T3 (2.3–4.2 pg/ml) | 11.27 | 6.45 | 4.09 | 3.37 | |||
| Total T3 (60–181 ng/dl) | 815 | 101 | |||||
| TSI (0–139%) | 730 | 384 | |||||
| ALT (30–65 U/l) | 103 | 1795 | 2724 | 2252 | 1729 | 656 | 304 |
| AST (15–37 U/l) | 141 | 4040 | 5760 | 3990 | 1544 | 396 | 117 |
| Lactic acid (0.4–2.0 mmol/l) | 4.7 | 10.8 | 7.0 | 2.1 | |||
| Creatinine (0.6–1.3 mg/dl) | 0.9 | 1.5 | 1.3 | 0.9 | 0.4 | 0.6 | 0.5 |
| Glucose (74–106 mg/dl) | 67 | 64 | 109 | 115 | 80 | 63 | 81 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; T3, tri-iodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone; TSI, thyroid-stimulating immunoglobulin.
Case 2: ECG demonstrated atrial fibrillation with rapid ventricular response with heart rate of 165 beats/min, and chest x-ray revealed bilateral pleural effusions. Laboratory testing confirmed thyrotoxicosis, as evidenced by an undetectable TSH and significantly elevated levels of both free T4 and total T3 (see table 2). Transthoracic echocardiogram revealed left ventricular ejection fraction of 50% with severe right and left atrial dilation and a pericardial effusion.
Table 2.
Laboratory assessment during the course of the patient's illness (patient B)
| Test | Day 1 | Day 2 | Day 3 | Day 3 (16:05) | Day 3 (19:00) | Day 4 | Day 4 (14:00) | Day 5 (00:00) | Day 5 (04:00) | Day 6 | Day 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TSH (0.35–5.5 µIU/ml) | <0.01 | 0.03 | |||||||||
| Free T4 (0.89–1.76 ng/dl) | 7.47 | 8.2 | 9.66 | 10.9 | 8.57 | 7.53 | 5.02 | 5.67 | 7.03 | 3.10 | 1.98 |
| Total T3 (60–81 ng/dl) | 475 | ||||||||||
| TSI (0–139%) | 551 | 393 | 535 | 617 | 786 | ||||||
| ALT (30–65 U/l) | 42 | 129 | 177 | 172 | 107 | 74 | |||||
| AST (15–37 U/l) | 25 | 130 | 200 | 110 | 41 | 15 | |||||
| Creatinine (0.6–1.3 mg/dl) | 0.9 | 2.2 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; T3, tri-iodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone; TSI, thyroid-stimulating immunoglobulin.
Treatment
Case 1: On presentation, esmolol intravenous infusion was started at 3 mg/min and the arrhythmia converted to atrial flutter with ventricular rate in the 120 s. He was transferred to the medical intensive care unit, where he required intermittent non-invasive positive pressure ventilation.
Treatment was initiated with propylthiouracil (PTU) 300 mg orally every 6 h, supersaturated potassium iodide (SSKI) five drops orally every 6 h (which were started after the PTU), hydrocortisone 100 mg intravenously every 8 h, and ongoing esmolol infusion (requiring up to 250 mcg/kg/min). Bicarbonate was infused for the lactic acidosis. The patient remained afebrile during the hospitalisation; however, empiric cefepime and vancomycin were administered and discontinued on hospital day 4 after blood cultures were negative.
As transaminases continued to rise (see table 1), PTU was replaced with methimazole (MMI) 30 mg orally every 8 h. Thyroid hormone levels and transaminases then decreased but remained dangerously elevated. On hospital day 3, MMI was replaced with lithium 300 mg orally every 12 h.4 Atrial flutter with tachycardia persisted and the patient developed disseminated intravascular coagulation.
In view of overall deteriorating status and inability to continue MMI due to worsening liver dysfunction, a decision was made to start plasmapheresis. Throughout hospital days 4 and 5, he received two plasmapheresis treatments, each with 2.5 litres of combined fresh frozen plasma (FFP) and albumin.
Case 2: The patient was admitted to the general medicine service with a diagnosis of thyrotoxicosis. He began oral methimazole 20 mg daily and propanolol 40 mg twice daily, in addition to empiric cefepime and vancomycin. However, less than 2 h following admission, the temperature increased to 100.5°F, and he developed chest pain with worsening dyspnea. He was found unresponsive and pulseless. After cardiopulmonary resuscitation, he regained pulses but developed ventricular fibrillation, which was successfully treated with electrical cardioversion. He regained consciousness, was placed on non-invasive ventilatory support and was transferred to the critical care unit.
He was diagnosed with thyroid storm (Burch-Wartofsky score of 70)1 and placed on methimazole 20 mg every 8 h, esmolol continuous intravenous infusion at 200 mcg/kg/min, hydrocortisone 50 mg every 8 h and SSKI five drops every 8 h. Methimazole was titrated to 30 mg every 8 h, but thyroid hormone and transaminase levels increased further with no improvement in the tachyarrhythmia. The temperature rose and peaked at 104.6°F. He developed acute kidney injury and became neurologically unresponsive off sedation.
On hospital day 3, the first plasmapheresis treatment was administered using FFP and albumin. Two more plasmapheresis treatments were completed on hospital days 4 and 5, respectively.
Outcome and follow-up
Case 1: After completing two plasmapheresis treatments, a remarkable decline was seen in levels of free T4, free T3, thyroid-stimulating immunoglobulin (TSI) and transaminases (table 1). The atrial flutter rate declined to ranges of 90s–100s, and intravenous esmolol was transitioned to oral propanolol. The patient became more alert, was weaned off mechanical ventilation, and began tolerating oral feeds. Treatment with oral lithium, propanolol and hydrocortisone was continued. After stabilisation to a clinically and biochemically euthyroid state, he underwent definitive treatment with total thyroidectomy.
Case 2: After receiving the first plasmapheresis treatment, free T4 levels and TSI levels immediately and significantly declined (table 2). Free T4, TSI and transaminases continued to trend downwards (table 2) while he received his second and third plasmapheresis treatments. The patient became clinically and biochemically euthyroid afterwards and continued treatments with methimazole, SSKI and steroids. He then underwent definitive treatment with a total thyroidectomy.
Discussion
Thyroid storm is an extreme, life-threatening manifestation of thyrotoxicosis. The death rate is high, between 20% and 30%.5 The point at which severe thyrotoxicosis becomes thyroid storm is controversial, and to some degree, subjective.6 Diagnosis of thyroid storm is clinical, based on a suggestive history as well as the presence of hyperthermia, acute mental status changes and cardiovascular and gastrointestinal dysfunction. To help standardise the diagnosis, Burch and Wartofsky developed a scoring system (see supplementary table 1) that quantifies the likelihood of thyroid storm by assessing the degree of dysfunction in the aforementioned organ systems.1 A score of 45 or more is highly suggestive of thyroid storm; our patients scored 80 and 70, respectively.
Both patients A and B were non-adherent with antithyroid therapy, and this led to their severe presentations. This is a well-recognised risk factor for thyroid storm. Multiple aspects of patient A's presentation of thyroid storm are rare manifestations. Hypoglycaemia and lactic acidosis have only been reported in three other cases.2 3 7 The other rare features are normothermia2 7 and unusually severe multiorgan dysfunction.3 7
Hypoglycaemia is not usually a defining characteristic of thyroid storm. Patient A was hypoglycaemic in spite of receiving high-dose intravenous steroids. In his case, hypoglycaemia may have been the result of liver dysfunction from low cardiac output leading to shock liver and impaired gluconeogenesis, use of PTU and/or MMI, renal failure and decreased adrenal reserve (as the body cannot synthesise adequate cortisol to meet the increased metabolic needs of thyroid storm).6 8 Adrenal insufficiency also occurs in greater frequency in patients with Graves’ disease.9 Also, we cannot rule out the possibility of culture-negative sepsis as a cause of hypoglycaemia.8 Other groups have noted the presence of thyroid storm with hypoglycaemia occurring with relative glucagon deficiency9 and more than 2 weeks of starvation.10
Additionally, patient A also had a profound lactic acidosis. Multiple factors may have contributed including increased demands of the hypermetabolic state (ie, when the basal metabolic rate exceeds delivery of oxygen to tissues), hypoglycaemia, low-oxygen delivery to tissues via a low flow state due to congestive heart failure, increased work of breathing, possible sepsis and inadequate disposal of lactic acid because of hepatic failure.2 3 11
The other unusual feature of patient A is the severity of the multiorgan failure at presentation. The levels of aspartate aminotransferase and alanine aminotransferase were each greater than 1000 U/l. While most patients with thyroid storm have mild elevations of transaminases, this degree of severity is rare.3 11 Tachycardia, myocardial contractility and peripheral vasodilatation associated with hyperthyroidism, if left untreated, can lead to dilated cardiomyopathy and heart failure.2 12 The acute kidney injury is likely the result of decreased cardiac output.
Furthermore, patient A's presentation of thyroid storm did not manifest hyperthermia. As suggested by a large case series,13 normothermic thyroid storm is rare. It is believed that the febrile response is either due to abnormal thermoregulation in the central nervous system or elevation in thermogenesis and the basal metabolic rate beyond the body's ability to dispel heat.14 Though blood cultures were negative, patient A may still have been septic (culture negative sepsis). It is unclear as to why he did not manifest fever.
Patient B had a more typical presentation of thyroid storm, which rapidly progressed to multiorgan dysfunction. He showed virtually no response to traditional therapy. While potential drug toxicity led to the use of plasmapheresis in patient A, patient B required plasmapheresis because of actual lack of response to conventional treatment.
The exact mechanisms that precipitate thyroid storm remain incompletely understood, but the abrupt release of hormones may play an important role. It has been shown that the total T4 and T3 concentrations in patients in crisis are not significantly higher than those of severely thyrotoxic patients not in crisis.15 There is no arbitrary cut-off for T4 or T3 levels to distinguish thyroid storm from severe thyrotoxicosis. However, a report by Brooks and Waldstein16 revealed a significant elevation in free T4 concentrations in a series of five thyroid storm patients compared with a larger group of patients with simple thyrotoxicosis despite the presence of similar levels of total T4. One explanation is decreased affinity of serum thyroxine binding globulin (TBG) for T4; thus decreased hormone-binding capacity (associated with a variety of illnesses) may increase concentrations of free, hormonally active moieties in thyroid storm. In one case report17 modest elevations in total T4 and normal levels of total T3 were accompanied by marked elevations in free T4 and free T3 in a patient who developed thyroid storm. In fact, elevations in percent free, dialysable T4 and T3 have been well described in patients with non-thyroidal illnesses,18 possibly explaining the array of scenarios which allow for precipitation of thyroid storm. Another theory is a possible increase in target cell-β-adrenergic receptor density or modification in postreceptor signalling pathways, leading to increased sensitivity to β-adrenergic stimulation in thyroid storm.
Therapeutic plasma exchange (TPE) has been used in a variety of illnesses to remove harmful plasma constituents rapidly or to decrease the concentrations of antibodies, immune complexes and toxins.19 Historically, the use of therapeutic plasmapheresis has been utilised for treatment of hepatic coma,20 21 rheumatoid arthritis22 and myasthenia gravis.23 During plasma exchange, the patient's plasma is extracted from the components of the blood, and instead of this plasma, a colloid replacement solution (in our cases, FFP and albumin) is infused back to the patient.24 During this procedure, TBG, with bound thyroid hormones, is removed with the plasma. Then the colloid replacement (eg, albumin) provides new binding sites for circulating free thyroid hormone.24 25 Although albumin binds thyroid hormone less avidly than TBG, it provides a much larger capacity for low-affinity binding that may contribute to lower free thyroid hormone levels. TPE is a reasonably safe procedure, with a recommendation of grade IIc and category III in the latest American Society for Apheresis document.26 The overall incidence of adverse effects of this technique, which are largely reversible, is approximately 5%. Notable side effects include transfusion reaction, citrate-related nausea and vomiting, vasovagal or hypotensive reactions, respiratory distress and tetany or seizure. Death is rare and usually due to an underlying disease.27
Treatment of thyrotoxic crisis by use of plasmapheresis was first described by Ashkar et al in 1970,28 in which they described three cases of thyroid storm that did not respond to conventional treatment. With imminent death unavoidable, plasmapheresis was initiated, and each of the three cases improved rapidly and dramatically. In 1973, Herrmann et al29 reported two cases in which plasmapheresis was used effectively with a reduction in circulating thyroid hormone. Since then, multiple case reports25 30–32 have demonstrated the effective use of plasmapheresis or charcoal plasmapheresis in thyroid storm, leading to rapid clinical response and rapid normalisation of circulating thyroid hormone levels. It can also be used in amiodarone-induced thyrotoxicosis.33 However, at least one case series31 reported two cases in which there was no improvement in thyroid storm with plasmapheresis.
In 2009, Ezer et al24 published the largest plasmapheresis case series to date which included 11 patients with severe thyrotoxicosis (but not in thyroid storm) scheduled for surgery, and the use of TPE to decrease hormone levels in the preoperative period enabling patients to undergo surgery more safely. All patients demonstrated improvement in thyrotoxic manifestations (tachycardia, atrial fibrillation, tremor, palpitations and sweating) along with declining free T3 and free T4 levels after TPE, further supporting its effectiveness. Most recently, Koball et al32 reported a case where conventional TPE failed, but a single pass albumin dialysis led to profound improvement, thought to be secondary to the larger quantity of thyroid hormone removal. Additionally, the role of plasma exchange in thyroid storm has recently been reviewed by Muller et al,33 who describe patients with amiodarone-induced thyroid disease, and suggest that TPE be performed daily until clinical improvement is noted with recommendations to monitor FT3 and FT4 and after each session.
Alternative therapies for thyroid storm also include lithium carbonate, cholestyramine and potassium perchlorate.6 Lithium carbonate blocks the release of thyroid hormone from the gland and indirectly inhibits new hormone synthesis.4 34 This therapy was minimally effective in patient A, but was continued (with regular monitoring of lithium levels) given the lack of options. Cholestyramine is an anion exchange resin that helps decrease the reabsorption of thyroid hormone by interfering with the entero-hepatic circulation; however, its effect is minimal, and its efficacy is only proven in combination with MMI or PTU.35–37 Potassium perchlorate functions as a competitive inhibitor of iodine transport.6 It is used in combination with methimazole in amiodarone-induced thyrotoxicosis.38 Though it was historically used to treat thyrotoxicosis, it has fallen out of favour due to risk of developing aplastic anaemia and nephrotic syndrome,6 and is not currently available in the USA.
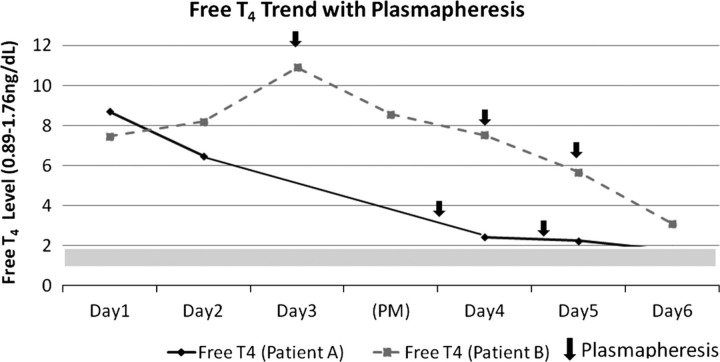
There exists no clear consensus regarding the use of plasmapheresis in thyroid storm. Recently published guidelines on management of thyrotoxicosis make no mention or indication of plasma exchange for treatment of thyroid storm, suggesting the need for further investigation. However, in extreme cases when conventional measures have failed, plasmapheresis is a reasonably safe option to decrease circulating thyroid hormone levels and should be considered as a stabilising measure. In both of our case reports, our patients demonstrated dramatic clinical improvement and decline in circulating hormone levels attributed to the use of plasmapheresis (figure 1). Both patients survived until more definitive surgical intervention with total thyroidectomy was performed.
Figure 1.
Free T4 trend with plasmapheresis.
Learning points
Thyroid storm is a rare, but critical illness that can lead to multiorgan failure and carries a high death rate.
When conventional medical management of thyroid storm either fails or is not feasible, plasmapheresis may be considered as a treatment option.
During plasmapheresis, thyroid-binding globulin and bound thyroid hormones are removed with the plasma. Then the colloid replacement (albumin) provides new binding sites for circulating free thyroid hormone.
Plasmapheresis leads to amelioration of symptoms and a significant decline in thyroid hormone levels, providing a window to treat definitively with thyroidectomy.
Footnotes
An additional supplementary table is published online only. To view this file please visit the journal online (http://dx.doi.org/10.1136/bcr-2012-006696)
Competing interests: None.
Patient consent: Obtained.
References
- 1.Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am 1993;22:263–77. [PubMed] [Google Scholar]
- 2.Jiang YZ, Hutchinson KA, Bartelloni P, et al. Thyroid storm presenting as multiple organ dysfunction syndrome. Chest 2000;118:877–9. [DOI] [PubMed] [Google Scholar]
- 3.Chong HW, See KC, Phua J. Thyroid storm with multiorgan failure. Thyroid 2010;20:333–6. [DOI] [PubMed] [Google Scholar]
- 4.Kearney T, Dang C. Diabetic and endocrine emergencies. Postgrad Med J 2007;83:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tietgens ST, Leinung MC. Thyroid storm. Med Clin North Am 1995;79:169–84. [DOI] [PubMed] [Google Scholar]
- 6.Nayak B, Burman K. Thyrotoxicosis and thyroid storm. Endocrinol Metab Clin North Am 2006;35:663–86, vii. [DOI] [PubMed] [Google Scholar]
- 7.Izumi K, Kondo S, Okada T. A case of atypical thyroid storm with hypoglycemia and lactic acidosis. Endocr J 2009;56:747–52. [DOI] [PubMed] [Google Scholar]
- 8.Service FJ. Hypoglycemic disorders. N Engl J Med 1995;332:1144–52. [DOI] [PubMed] [Google Scholar]
- 9.Homma M, Shimizu S, Ogata M, et al. Hypoglycemic coma masquerading thyrotoxic storm. Intern Med 1999;38:871–4. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi C, Sasaki H, Kosuge K, et al. Severe starvation hypoglycemia and congestive heart failure induced by thyroid crisis, with accidentally induced severe liver dysfunction and disseminated intravascular coagulation. Intern Med 2005;44:234–9. [DOI] [PubMed] [Google Scholar]
- 11.Choudhary AM, Roberts I. Thyroid storm presenting with liver failure. J Clin Gastroenterol 1999;29:318–21. [DOI] [PubMed] [Google Scholar]
- 12.Umpierrez GE, Challapalli S, Patterson C. Congestive heart failure due to reversible cardiomyopathy in patients with hyperthyroidism. Am J Med Sci 1995;310:99–102. [DOI] [PubMed] [Google Scholar]
- 13.Mazzaferri EL, Skillman TG. Thyroid storm. A review of 22 episodes with special emphasis on the use of guanethidine. Arch Intern Med 1969;124:684–90. [DOI] [PubMed] [Google Scholar]
- 14.Mc AJ, Rawson RW, Means JH, et al. Thyrotoxic crisis; an analysis of the thirty-six cases at the Massachusetts General Hospital during the past twenty-five years. J Am Med Assoc 1947;134:868–74. [DOI] [PubMed] [Google Scholar]
- 15.Brooks M. Serum triiodothyronine concentration in thyroid storm. J Clin Endocrinol Metabol 1975;40:339. [DOI] [PubMed] [Google Scholar]
- 16.Brooks MH, Waldstein SS. Free thyroxine concentrations in thyroid storm. Ann Intern Med 1980;93:694–7. [DOI] [PubMed] [Google Scholar]
- 17.Colebunders R, Bourdoux P, Bekaert J, et al. Determination of free thyroid hormones and their binding proteins in a patient with severe hyperthyroidism (thyroid storm?) and thyroid encephalopathy. J Endocrinol Invest 1984;7:379–81. [DOI] [PubMed] [Google Scholar]
- 18.Chopra IJ, Hershman JM, Pardridge WM, et al. Thyroid function in nonthyroidal illnesses. Ann Intern Med 1983;98:946–57. [DOI] [PubMed] [Google Scholar]
- 19.Szczepiorkowski ZM, Bandarenko N, Kim HC, et al. Guidelines on the use of therapeutic apheresis in clinical practice: evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Apher 2007;22:106–75. [DOI] [PubMed] [Google Scholar]
- 20.Lepore MJ, McKenna PJ, Martinez DB, et al. Fulminant hepatitis with coma successfully treated by plasmapheresis and hyperimmune Australia-Antibody-rich plasma. Am J Gastroenterol 1972;58:381–9. [PubMed] [Google Scholar]
- 21.Lepore MJ, Martel AJ. Plasmapheresis with plasma exchange in hepatic coma. Methods and results in five patients with acute fulminant hepatic necrosis. Ann Intern Med 1970;72:165–74. [DOI] [PubMed] [Google Scholar]
- 22.Lepore L, Agosti E, Pitacco F, et al. Therapy with plasmapheresis and lymphoplasmapheresis combined with immunosuppressive agents in 2 cases of intractable juvenile rheumatoid arthritis. Pediatr Med Chir 1987;9:321–4. [PubMed] [Google Scholar]
- 23.Pinching AJ, Peters DK. Remission of myasthenia gravis following plasma-exchange. Lancet 1976;2:1373–6. [DOI] [PubMed] [Google Scholar]
- 24.Ezer A, Caliskan K, Parlakgumus A, et al. Preoperative therapeutic plasma exchange in patients with thyrotoxicosis. J Clin Apher 2009;24:111–14. [DOI] [PubMed] [Google Scholar]
- 25.Vyas AA, Vyas P, Fillipon NL, et al. Successful treatment of thyroid storm with plasmapheresis in a patient with methimazole-induced agranulocytosis. Endocr Pract 2010;16:673–6. [DOI] [PubMed] [Google Scholar]
- 26.Szczepiorkowski ZM, Winters JL, Bandarenko N, et al. Guidelines on the use of therapeutic apheresis in clinical practice—evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Apher 2010;25:83–177. [DOI] [PubMed] [Google Scholar]
- 27.McLeod BC, Sniecinski I, Ciavarella D, et al. Frequency of immediate adverse effects associated with therapeutic apheresis. Transfusion 1999;39:282–8. [DOI] [PubMed] [Google Scholar]
- 28.Ashkar FSKR, Katims RB, Smoak WM, III, et al. Thyroid storm treatment with blood exchange and plasmapheresis. JAMA 1970;214:1275–9. [PubMed] [Google Scholar]
- 29.Herrmann J, Hilger P, Kruskemper HL. Plasmapheresis in the treatment of thyrotoxic crisis (measurement of half-concentration times for free and total T3 and T4). Acta Endocrinol Suppl (Copenh) 1973;173:22. [PubMed] [Google Scholar]
- 30.Petry J, Van Schil PE, Abrams P, et al. Plasmapheresis as effective treatment for thyrotoxic storm after sleeve pneumonectomy. Ann Thorac Surg 2004;77:1839–41. [DOI] [PubMed] [Google Scholar]
- 31.Ligtenberg J, Tulleken J, Zijlstra J. Plasmapheresis in thyrotoxicosis. Ann Intern Med 1999;131:71–2. [DOI] [PubMed] [Google Scholar]
- 32.Koball S, Hickstein H, Gloger M, et al. Treatment of thyrotoxic crisis with plasmapheresis and single pass albumin dialysis: a case report. Artif Organs 2010;34:E55–8. [DOI] [PubMed] [Google Scholar]
- 33.Muller C, Perrin P, Faller B, et al. Role of plasma exchange in the thyroid storm. Ther Apher Dial 2011;15:522–31. [DOI] [PubMed] [Google Scholar]
- 34.Spaulding SW, Burrow GN, Bermudez F, et al. The inhibitory effect of lithium on thyroid hormone release in both euthyroid and thyrotoxic patients. J Clin Endocrinol Metab 1972;35:905–11. [DOI] [PubMed] [Google Scholar]
- 35.Solomon BL, Wartofsky L, Burman KD. Adjunctive cholestyramine therapy for thyrotoxicosis. Clin Endocrinol (Oxf) 1993;38:39–43. [DOI] [PubMed] [Google Scholar]
- 36.Mercado M, Mendoza-Zubieta V, Bautista-Osorio R, et al. Treatment of hyperthyroidism with a combination of methimazole and cholestyramine. J Clin Endocrinol Metab 1996;81:3191–3. [DOI] [PubMed] [Google Scholar]
- 37.Tsai WC, Pei D, Wang TF, et al. The effect of combination therapy with propylthiouracil and cholestyramine in the treatment of Graves’ hyperthyroidism. Clin Endocrinol (Oxf) 2005;62:521–4. [DOI] [PubMed] [Google Scholar]
- 38.Erdogan MF, Gulec S, Tutar E, et al. A stepwise approach to the treatment of amiodarone-induced thyrotoxicosis. Thyroid 2003;13:205–9. [DOI] [PubMed] [Google Scholar]