Abstract
A 70-year-old woman developed ventricular fibrillation subsequent to initiation of phentermine therapy. She was hospitalised and experienced recurrent ventricular fibrillation. During cardiac catheterisation, she was found to have a right coronary artery vasospasm, which resolved with intravenous nitroglycerin. Her phentermine was discontinued and the patient remained symptom free at last follow-up.
Background
Between 1980 and 2008, the worldwide prevalence of obesity doubled.1 Today, the prevalence of obesity, defined as body mass index (BMI) >30, is 35.7% in the USA and 24.2% in the UK.2 3 Pharmacological approaches offer an attractive but modest addition to diet and exercise in patients attempting to combat this epidemic and its associated complications, which has lead to an increase in the use of antiobesity medications. Unfortunately, patients often overlook and disregard the serious side effects associated with weight-loss medications due to their desire to lose weight. The most frequently prescribed weight-loss drug is phentermine, commonly sold under the trade name Adipex-P (Gate Pharmaceuticals, North Wales, PA, USA) or Lonamin (Rhone-Poulenc Rorer Canada Inc, St Laurent, Quebec, Canada).4 5
Case presentation
A 70-year-old woman with a BMI of 25.1 and well-controlled type II diabetes developed loss of consciousness and documented ventricular fibrillation 3 days after beginning phentermine therapy. She was resuscitated in the field and hospitalised. The patient had a history of chest pain, but no other cardiac history. Other medications included metformin, omeprazole and calcium plus vitamin D. There was no significant family history of cardiovascular disease.
On admission, she was haemodynamically stable and in normal sinus rhythm. Her admission ECG revealed sinus rhythm of 80/min and poor R-wave progression in the anterior leads. A transthoracic echocardiogram demonstrated an ejection fraction of 55—60%, with normal wall motion and no valvular abnormalities. Her hospital course was complicated by recurrent ventricular fibrillation. Prior to the second episode of ventricular fibrillation arrest, her ECG revealed sinus bradycardia at 50/min with a PR interval of 296 ms and acute inferior ST elevation. Repeat transthoracic echocardiogram showed an ejection fraction of 45—50% with inferior akinesis and moderate acute mitral regurgitation.
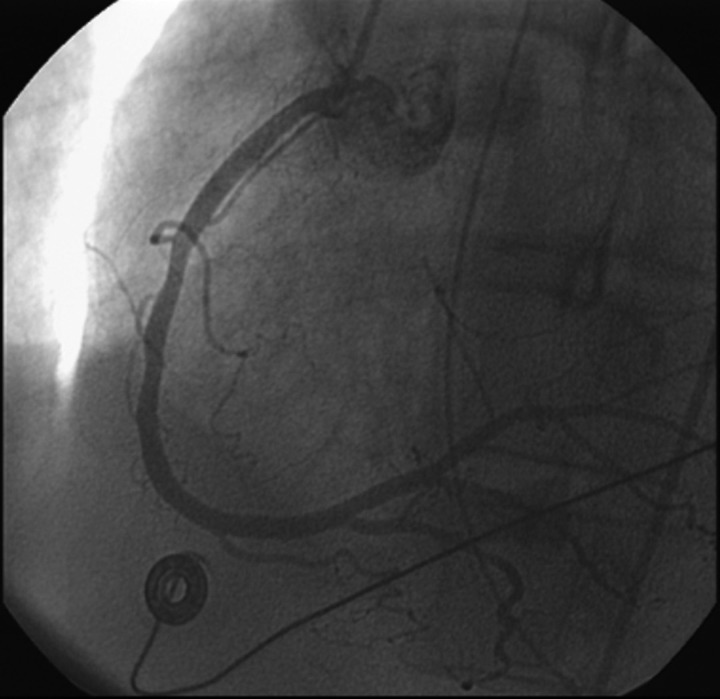
Blood work showed a peak creatine kinase of 1511 U/l, a peak creatinine kinase-MB fraction of 36.1 U/l and a troponin I level of 30.82 ng/ml. Other laboratory data were unremarkable. She underwent emergent cardiac catheterisation, and a right coronary artery vasospasm was found without thrombus or atheromatous plaque (figure 1). This resolved with intravenous nitroglycerin. Her phentermine was discontinued during the hospitalisation.
Figure 1.
Resolution of RCA spasm after injection of intracoronary nitroglycerin.
Outcome and follow-up
The patient was symptom free at last clinical follow-up.
Discussion
Phentermine, an amphetamine derivative, works by promoting both noradrenergic and dopaminergic central nervous system transmission through enhancing the release and inhibiting the uptake of norepinephrine, as well as increasing the release of dopamine. The mechanism of weight loss is unknown, but is thought to be associated with activation of the central and sympathetic nervous systems, which leads to a decrease in appetite and an increase in basal energy expenditure. Since Food and Drug Administration approval of phentermine hydrochloride in 1973, there have been sporadic case reports of ischaemic stroke, myocardial infarction, ventricular arrhythmias, pulmonary hypertension and cardiac arrest associated with its use.6–9 The exact mechanism of cardiac toxicity is speculative, but is thought to involve phentermine's release of bound endogenous norepinephrine, leading to increased stimulation of peripheral adrenergic receptors, which effects myocardial contractility and automaticity of the conduction system.10 11
Phentermine was part of the combination drug ‘Fen-Phen,’ which also included fenfluroxamine, and was a popular weight-loss agent in the 1990s. It was withdrawn from the market in 1997 because of the development of valvular heart disease. Later reports found similar valvular heart disease development in patients taking fenfluroxamine alone, whereas phentermine monotherapy was not found to be associated with this development.12 Case reports of arrhythmias from the use of amphetamine and its derivatives are rare; however, it is likely that there is under-reporting of these serious adverse effects. Further epidemiological studies are needed in order to assess the magnitude of risk for life-threatening arrhythmias associated with phentermine, especially since its use is increasing. Physicians should be more vigilant of possible cardiovascular adverse effects of phentermine and other similar appetite suppressants and should report any adverse effects to drug-monitoring agencies.
Learning points.
Awareness of potential side effects of weight-loss medications, including possible cardiac toxicity.
Identification of appropriate weight-loss strategies in patients and determining if medication therapy is indicated.
Importance of reporting side effects associated with weight-loss medications to drug-monitoring agencies.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Obesity Facts and Figures (internet). WHO/Europe; 2012. http://www.euro.who.int/en/what-we-do/health-topics/noncommunicable-diseases/obesity/facts-and-figures (accessed 17 Mar 2012).
- 2.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010;303:235–41. [DOI] [PubMed] [Google Scholar]
- 3.Obesity: in statistics (internet). BBC News (updated 02 Jan 2008). http://news.bbc.co.uk/2/hi/health/7151813.stm (accessed 17 Mar 2012).
- 4.Stafford RS, Radley DC. National trends in antiobesity medication use. Arch Intern Med 2003;163:1046–50. [DOI] [PubMed] [Google Scholar]
- 5.Hendricks EJ, Rothman RB, Greenway FL. How physician obesity specialists use drugs to treat obesity. Obesity 2009;17:1730–5.. [DOI] [PubMed] [Google Scholar]
- 6.Makaryus JN, Makaryus AN. Cardiac arrest in the setting of diet pill consumption. AJEM 2008;26:732 e731–3. [DOI] [PubMed] [Google Scholar]
- 7.Kokkinos J, Levine S. Possible association of ischemic stroke with phentermine. Stroke 1993;24:310–13. [DOI] [PubMed] [Google Scholar]
- 8.Azarisman SM, Magdi YA, Noorfaizan S, et al. Myocardial infarction induced by appetite suppressants in Malaysia. N Engl J Med 2007;357:1873–4. [DOI] [PubMed] [Google Scholar]
- 9.Hung YM, Chang JC. Weight-reducing regimen associated with polymorphic ventricular tachycardia. Am J Emerg Med 2006;24:714–16. [DOI] [PubMed] [Google Scholar]
- 10.Laurence BL, Lazo JS, Parker K, et al. Goodman and Gilman's the pharmacological basis of therapeutics. New York, NY, USA: McGraw-Hill Professional Publishing, 2005:254. [Google Scholar]
- 11.Rothman RB, Baumann MH, Dersch CM, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they dopamine and serotonin. Synapse 2001;39:32–41. [DOI] [PubMed] [Google Scholar]
- 12.Shekelle PG, Morton SC, Maglione Met al. Pharmacological and surgical treatment of obesity. Evid Rep Technol Assess 2004;103:1–6. [PMC free article] [PubMed] [Google Scholar]