Abstract
A 23-year-old man with poorly controlled insulin-dependent diabetes mellitus presented to casualty with community-acquired pneumonia and diabetic ketoacidosis. Shortly after admission he deteriorated and developed cardiac failure, pulmonary oedema and further decreased level of consciousness. He was sedated and ventilated for 3 weeks in the intensive care unit. On waking from sedation he was found to be tetraplegic. MRI scan showed gross oedema of the cervical spinal cord with area suspicious of infarction. We describe a rare cause of spinal cord injury and discuss the proposed hypotheses.
Background
Cerebral and spinal cord oedema and infarction are rare complications of diabetic ketoacidosis (DKA). This is the second reported case of spinal cord oedema and suspected infarction and the first with findings of isolated spinal cord damage without evidence of cerebral oedema. Generally, the prognosis after these complications is very poor; however, our patient survived but developed permanent tetraplegia.
Case presentation
A 23-year-old man with poorly controlled insulin-dependent diabetes mellitus (IDDM) since the age of 4, presented to casualty with drowsiness following symptoms of nausea and vomiting for 48 h. He was found to have DKA with blood glucose levels of 86 mmol/l and a pH level of 6.9. He was also severely dehydrated with sodium of 127 mmol/l, potassium of 6.3 mmol/l, urea was 32.2 mmol/l and creatinine was 480 μmol/l. Suspected cause was a community-acquired pneumonia and inflammatory markers were raised with white cell count (WCC) of 30.5×109/ l and C reactive protein of 59 mg/l. Despite initial treatment with insulin and fluid resuscitation he developed further reduced level of consciousness with Glasgow Coma Scale 10/15, heart failure and pulmonary oedema and was admitted to the intensive care unit for mechanical ventilation and fluid balance monitoring.
Three weeks later when the patient was awake from sedation, he was found to be tetraplegic with a complete flaccid paralysis below the C3 level, American Spinal Injuries Association Level-A (ASIA-A), areflexic and with no sensation of touch or pain. There was no involvement of the cranial nerves.
Investigations
Urgent MRI of the brain did not show any cerebral oedema or focal infarction but the MRI of the cervical spine showed an expanded cervical spinal cord from C2 to C7 level occupying the whole spinal canal with gross oedema (figure 1). On T2-weighted images there was a bright signal seen within the spinal cord from mid C2 level downwards (figure 2). Following gadolinium there was patchy enhancement within this region (figures 3 and 4). MRI of the whole spine revealed T2 bright signal and after review of the images by two neuroradiologists it was concluded that these appearances were most likely due to ischaemic changes and infarction. There was no evidence of infection or intraspinal haemorrhage on the MRI, but due to the history of diabetes an epidural collection or abscess at the level of the cervical spine could not be excluded.
Figure 1.
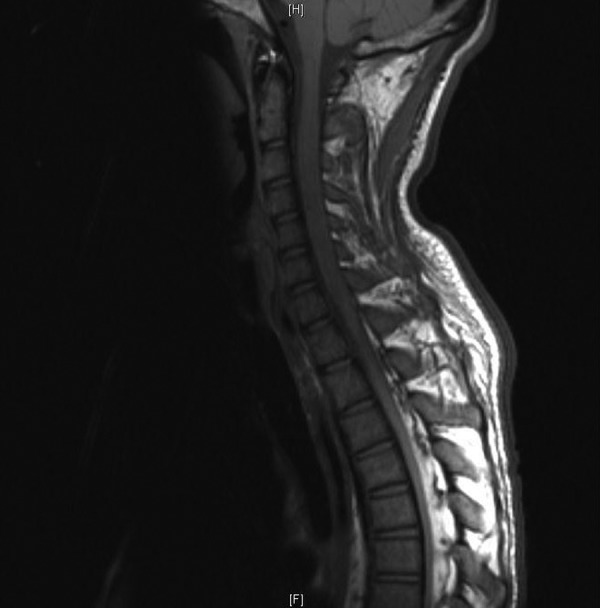
MRI-T1-weighted image, cervical spine, sagittal view, spinal cord expansion from C2 to C7.
Figure 2.
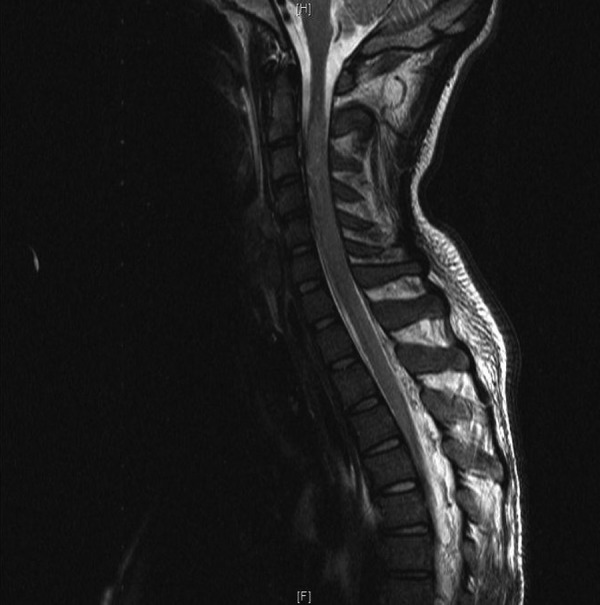
MRI-T2-weighted image, cervical spine, sagittal view, T2-bright signal areas within spinal cord suspicious of ischaemic origin.
Figure 3.
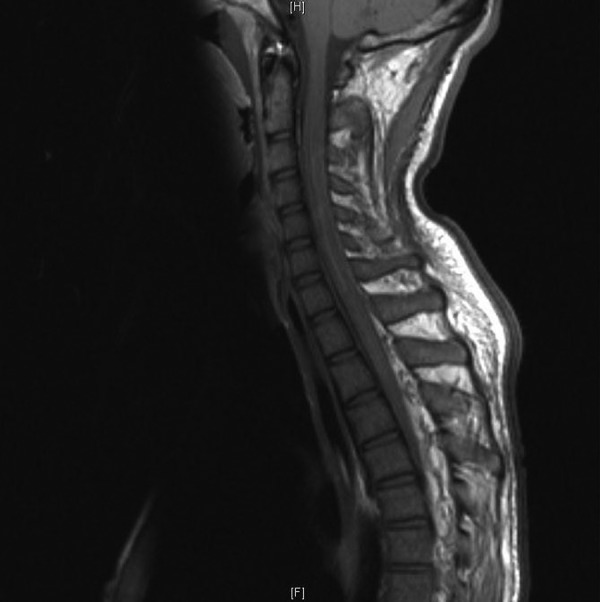
MRI-T1-weighted image, postgadolinium, cervical spine, sagittal view and patchy enhancements with gadolinium.
Figure 4.
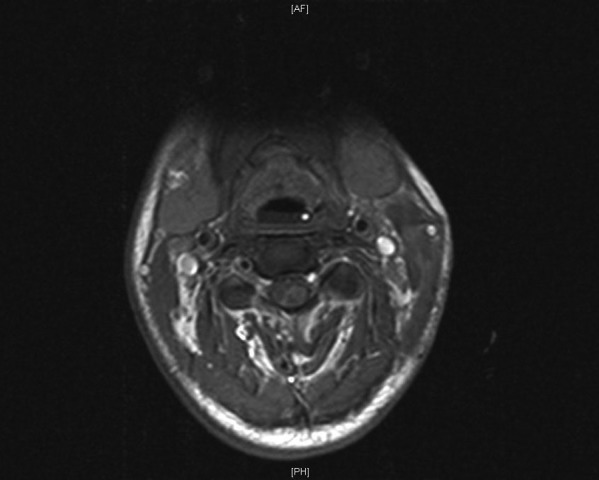
MRI-T1-weighted image postgadolinium, axial view and patchy enhancements within the spinal cord.
Differential diagnosis
Simultaneously, screening for myelitis and autoantibodies was negative. Blood cultures performed on admission were negative. Endocarditis and myocarditis were also excluded. Lumbar puncture was performed to exclude Guillain-Barre syndrome and cerebrospinal fluid (CSF) analysis showed normal WCC, glucose 5.2 mmol/l and raised protein 6.05 g/l. Culture of the CSF showed no growth.
Treatment
The patient underwent an Emergency Decompressive Cervical Laminectomy from C2 to C7. Intraoperatively the dura was found to be white and tense and there was no sign of epidural abscess or haematoma.
Electromyogram and Nerve Conduction Study conducted shortly afterwards suggested the possibility of Acute Motor-Sensory Axonal Neuropathy (AMSAN) syndrome but immunoglobulin treatment did not improve any function. Muscle biopsy only showed severe atrophy and no vasculitis.
Two months postadmission the patient was weaned off assisted breathing and was transferred to the North West Spinal Injuries Unit for rehabilitation. His last neurological examination showed C6 complete tetraplegia (ASIA-A) with best muscle power 3/5 in his right wrist. There was no sensation from the C4 level downwards and also no perianal sensation with an absent anal tone and bulbocavernous reflex. He also developed spasticity for which he required oral baclofen.
Outcome and follow-up
An MRI about 2 months after the first one showed evidence of patchy T2 bright signal in the cervical spinal cord extending to the upper dorsal cord. In these MRI series the dorsal spinal cord appeared to be extremely atrophic with no enhancement after gadolinium contrast.
The patient completed his rehabilitation programme and was discharged to a care home. He was able to use a manual chair but still required 24 h care and also a long-term catheter for drainage of his bladder. Although the patients’ neurological status is now stable he is occasionally re-admitted to hospital with urinary tract infections or chest infections that complicate his diabetes.
Discussion
We present a case of a patient with poorly controlled IDDM who developed rare complications of DKA. During our literature search, we found only one other published case report, by Dixon et al1 where a patient developed pulmonary and cerebral oedema and also multiple CNS infarctions including the spinal cord. We also found reports of cerebral oedema in children with DKA but without spinal cord oedema.2 3
There are no existing theories in the literature to explain spinal cord oedema associated with DKA and this is due to the rarity of this presentation. The existing theories are limited to cerebral oedema and are mostly in the paediatric diabetic population. However, the pathophysiology behind cerebral oedema and infarction is not clear and there are a few existing theories in the literature, such as: systemic hypotension and hypoxia, rapid changes in serum osmolarity, disseminated intravascular coagulation and thrombosis secondary to dehydration, haemoconcentration and hyperviscosity.1 3 4
The first possible mechanism of cerebral oedema was suggested by Dillon et al.5 It described hypoxia and ischaemia as possible mechanisms due to reduced blood volume secondary to dehydration which was aggravated by a low PaCO2. This caused acidosis and hyperventilation and led to vasoconstriction resulting in cerebral ischaemia, hypoxia and increased capillary permeability. Other proposed causes of ischaemia have been the increased blood viscocity and electrolyte abnormalities.5 6
A second possible mechanism suggested in the literature was an increased hyperosmolar state due to a longstanding hyperglycaemia and rapid fluid administration for the management of DKA. This theory states that due to the disease state, there is prolonged hyperosmolarity in the serum, and the brain cells protect their volume status by producing intracellular osmoles via production of metabolic products thought to be primarily taurine and myoinositol. These osmoles dissipate from the intracellular space slowly after serum osmolality begins to decrease, taking hours to days to return to normal. Owing to this slow dissipation, when serum osmolarity decreases rapidly, there is a relative hyperosmolarity in brain cell which favours a shift of fluid into the brain cells causing cerebral oedema.6 7
The working hypothesis in our case was that the cardiac failure and pulmonary oedema were secondary to rapid fluid resuscitation for the management of the patients DKA. It is possible that the fluid resuscitation caused a rapid decrease of the serum osmolarity which therefore favoured fluid shift into the CNS cells resulting in oedema and infarction of the spinal cord, as explained in the second suggested mechanism above.
It is unclear in this case as to at what stage the spinal cord oedema appeared as the patient was sedated and ventilated for 3 weeks. We cannot exclude the possibility of cerebral oedema during the first 24 h of his admission as a scan of the brain was not performed. However, the MRI of the patients’ brain, carried out at the same time as the MRI of the spine 3 weeks later, did not suggest any cerebral abnormality. The drowsiness the patient presented with on admission in casualty was considered to be due to the severe DKA and dehydration with uraemia. The patient appeared to have acute kidney injury on admission, which was considered to be prerenal as his renal functions returned to normal with rehydration and fluid balance monitoring. Owing to his drowsiness a full neurological examination was not performed on admission.
The other cause for tetraplegia that was considered in this case was a coexisting AMSAN syndrome, but there was no record of antiganglioside serology.8 He was treated with immunoglobulins but the limited function he regained about 2 months later could also be attributed to a resolution of spinal cord oedema. In summary, the MRI conducted at 2 and 9 months after the onset showed atrophic changes in the spinal cord, supporting the hypothesis that a spinal cord infarct might have taken place in the period that the patient was ventilated.
Learning points.
Central nervous system oedema and infarction, including the spinal cord, are rare complications of diabetic ketoacidosis and need to be considered as possible causes of tetraplegia.
Cardiac Failure and Pulmonary oedema are rare but possible complications in critically ill patients with diabetic ketoacidosis, therefore this needs to be frequently assessed during fluid resuscitation.
Magnetic Resonance Imaging of the Central Nervous System in complicated cases with new diagnosis of tetraplegia assists in forming a differential diagnosis and therefore a definitive management plan.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Dixon A N, Jude E B, Banerjee A K C, et al. Simultaneous pulmonary and cerebral oedema, and multiple CNS infarctions as complications of diabetic ketoacidosis: a case report. Diabet Med 2006;23:571–3. [DOI] [PubMed] [Google Scholar]
- 2.Young M C. Simultaneous acute cerebral and pulmonary edema complicating diabetic ketoacidosis. Diabetes Care 1995;18:1288–90. [DOI] [PubMed] [Google Scholar]
- 3.Timperley W R, Preston F E, Ward J D. Cerebral intravascular coagulation in diabetic ketoacidosis. Lancet 1974;952–6. [DOI] [PubMed] [Google Scholar]
- 4.Roe T F, Crawford T O, Huff K R, et al. Brain infarction in children with diabetic ketoacidosis. J Diabetes Complications 1996;10:100–8. [DOI] [PubMed] [Google Scholar]
- 5.Dillon ES, Riggs HS, Dyer WW. Cerebral lesions in uncomplicated fatal diabetic acidosis. Am J Sci 1936;192:360–5. [Google Scholar]
- 6.Levin D L. Cerebral edema in diabetic ketoacidosis. Pediatr Crit Care Med 2008;9:320–9. [DOI] [PubMed] [Google Scholar]
- 7.Glaser N S, Wootton-Gorges S L, Marcin J P, et al. Mechanism of cerebral oedema in children with diabetic ketoacidosis. J Paediatr 2004;145:164–71. [DOI] [PubMed] [Google Scholar]
- 8.Yuki N, Kuwabara S, Koga M, et al. Acute motor axonal neuropathy and acute motor-sensory axonal neuropathy share a common immunological profile. J Neurolog Sci 1999;168:121–6. [DOI] [PubMed] [Google Scholar]


