Abstract
Specialization is typically inferred at population and species level but in the last decade many authors highlighted this trait at the individual level, finding that generalist populations can be composed by both generalist and specialist individual. Despite hundreds of reported cases of individual specialization there is a complete lack of information on inter-individual diet variation in specialist species. We studied the diet of the Italian endemic Spectacled Salamander (Salamandrina perspicillata), in a temperate forest ecosystem, to disclose the realised trophic niche, prey selection strategy in function of phenotypic variation and inter-individual diet variation. Our results showed that Salamandrina is highly specialized on Collembola and the more specialized individuals are the better performing ones. Analyses of inter-individual diet variation showed that a subset of animals exhibited a broader trophic niche, adopting different foraging strategies. Our findings reflects the optimal foraging theory both at population and individual level, since animals in better physiological conditions are able to exploit the most profitable prey, suggesting that the two coexisting strategies are not equivalent. At last this species, feeding on decomposers of litter detritus, could play a key role determining litter retention rate, nutrient cycle and carbon sequestration.
Specialization is a widespread topic in ecology and evolutionary biology. Even if definitions of what should be called specialization and its boundaries have been debated by researchers for a long time1,2,3, there is a certain tendency in defining as specialist a species that utilizes a narrow range of resources. Similarly to the different definitions of ecological specialization, the set of resources on which a species can specialize is equally varied: indeed ecological specialization is inferred, among others, on habitat, space, shelter and food. Furthermore ecological specialization can be studied at different scales in space and time. As a matter of fact ecological specialization is reported at different biological levels3: population, species, community and, recently, specialization has been also studied at the individual level4. Nevertheless, despite discrepancies in the definitions of specialization and the coexistence of different and opposite views in these topics, many authors agree that specialist species are potentially more vulnerable to habitat fragmentation than generalists5,6,7 and that they are more temporally variable and more prone to extinction. Furthermore, for the aforementioned reasons, detecting patterns of specialization in itself may be a helpful tool for understanding and advising populations’ declines and to address proper conservation measures.
Specialization is almost always inferred at species or population level, and only recently a new approach to the study of specialization emerged: the study of inter-individual diet variation4. From this point of view the trophic niche of a population is seen as the result of the sum of the trophic niche of all individuals. As a matter of fact, the trophic niche of every single individual may be very different, at the point that a generalist population may be composed by individuals with different degrees of specialization, that feed on different prey types and adopt different trophic strategies. Intra-population niche variation is usually inferred as a result of different processes related to the individual characteristics4,8: i) sexual dimorphism and sexual differences in resource use, ii) ontogenetic diet shift, and iii) resource polymorphism. Evidences of individual specialization are mainly based on dietary data, but evidences for habitat selection and other niche axes are also documented, ranging from Plants to Mammals, through Crustaceans, Gastropods, Fishes, Amphibians and Birds8,9. Despite the fact that individual specialization has been observed, in many cases ante-litteram, in nearly 200 species regarding the use of some resource9, there is a complete lack of evidence for inter-individual resource variation in species or populations that are true specialists. The concept of “individual specialization” seems to exclude per se the possibility of studying inter-individual diet variation in populations where the emerging trophic strategy leads to specialization: furthermore an “individual specialist” is defined “as an individual whose niche is substantially narrower than its population’s niche for reasons not attributable to its sex, age or discrete morphological group”8.
In this study we analyze the diet variation of the Italian endemic Spectacled Salamander (Salamandrina perspicillata Savi, 1821) which, on the basis of previous studies10,11, seems to be a specialist predator on Collembola, at least in autumn. The aims of this study are threefold: first we investigate more in detail the diet of Salamandrina perspicillata to verify if the specialization observed in previous studies is a species-specific trait, as well as its seasonal trend. Second we evaluated the prey selection strategy, relating the realized trophic niche to trophic availability, and its relationships with phenotypic variation. Third, we evaluate, for the first time, the inter-individual level of diet variation in a specialist predator and its seasonal variation, turning the classic question “Are there, within a generalist population, any specialized individuals?” into the less common “Are there, within a specialist population, any generalist individuals that adopt a different foraging strategy?”.
Results
Sampling salamanders and their prey
During our surveys we captured and processed 187 Salamanders (120 and 67 in Autumn and Spring, respectively, for a total of 117 males, 70 females) from two neighboring populations and all of them provided stomach contents, from which we obtained 3912 invertebrates representing 17 taxa in Autumn and 18 in Spring. All the individuals processed in this study are reproductive adults; their body size lies within a narrow range [40.40 (3.07) – Mean body length (SD)], and juveniles were not included in the analyses. Environmental sampling produced a total number of 3824 invertebrates (1488 and 2336 in Autumn and Spring, respectively) representing 26 taxa in Autumn and 21 in Spring. Since our interest is to describe the trophic strategy at species-level we verified, through multivariate analysis of similarity (ANOSIM), i) that the invertebrate community composition was similar between sites during both seasons (Autumn; R = −0.04; p = 0.691 – Spring; R = −0.05; p = 0.801), ii) that the two populations had similar diet composition (Autumn; R = −0.003; p = 0.654 – Spring; R = −0.029; p = 0.830) and then we merged the data to analyze prey selection. The complete dataset both for stomach contents and for environmental sampling are shown in Table 1.
Table 1. Prey seasonal variation in the diet of Salamandrina perspicillata and in the environment traps at the study site.
|
Environment |
Diet |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
Autumn |
Spring |
Autumn |
Spring |
|||||
| Prey data in the environment and in the diet of Salamandrina perspicillata | n | % | n | % | n | % | n | % | |
| Arachnida | Acarina | 355 | 23.86 | 622 | 26.63 | 58 | 3.69 | 165 | 7.05 |
| Araneae | 57 | 3.83 | 17 | 0.73 | 194 | 12.36 | 92 | 3.93 | |
| Opiliones | 22 | 1.48 | 5 | 0.21 | 14 | 0.89 | 6 | 0.26 | |
| Pseudoscorpiones | 16 | 1.08 | 5 | 0.21 | 21 | 1.34 | 20 | 0.85 | |
| Miriapoda | Symphila | 1 | 0.07 | 5 | 0.21 | 0 | 0 | 0 | 0 |
| Diplopoda | 16 | 1.08 | 32 | 1.37 | 5 | 0.32 | 0 | 0 | |
| Chilopoda | 17 | 1.14 | 12 | 0.51 | 4 | 0.25 | 1 | 0.04 | |
| Hexapoda | Protura | 7 | 0.47 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diplura | 0 | 0 | 3 | 0.13 | 0 | 0 | 1 | 0.04 | |
| Collembola | 411 | 27.62 | 788 | 33.73 | 1122 | 71.46 | 1899 | 81.08 | |
| Coleoptera Larvae | 40 | 2.69 | 26 | 1.11 | 14 | 0.89 | 4 | 0.17 | |
| Coleoptera Adults | 42 | 2.82 | 81 | 3.47 | 13 | 0.83 | 24 | 1.02 | |
| Diptera Larvae | 6 | 0.40 | 71 | 3.04 | 27 | 1.72 | 3 | 0.13 | |
| Diptera Adults | 233 | 15.66 | 459 | 19.65 | 25 | 1.59 | 60 | 2.56 | |
| Lepidoptera Larvae | 5 | 0.34 | 22 | 0.94 | 0 | 0 | 7 | 0.3 | |
| Lepidoptera Adults | 28 | 1.88 | 5 | 0.21 | 1 | 0.06 | 0 | 0 | |
| Hymenoptera | 47 | 3.16 | 36 | 1.54 | 27 | 1.72 | 8 | 0.34 | |
| Formicidae | 88 | 5.91 | 18 | 0.77 | 1 | 0.06 | 4 | 0.17 | |
| Embioptera | 2 | 0.13 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Dermaptera | 1 | 0.07 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hemiptera | 50 | 3.36 | 46 | 1.97 | 28 | 1.78 | 23 | 0.98 | |
| Orthoptera | 2 | 0.13 | 0 | 0 | 0 | 0 | 1 | 0.04 | |
| Trichoptera | 1 | 0.07 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mollusca | Gasteropoda | 20 | 1.34 | 17 | 0.73 | 3 | 0.19 | 1 | 0.04 |
| Crustacea | Isopoda | 4 | 0.27 | 27 | 1.16 | 13 | 0.83 | 23 | 0.98 |
| Anellida | Lumbricidae | 14 | 0.94 | 39 | 1.67 | 0 | 0 | 0 | 0 |
| Nematomorpha | Gordioida | 3 | 0.20 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 1488 | 100 | 2336 | 100 | 1570 | 100 | 2342 | 100 | |
Is Salamandrina a specialist predator?
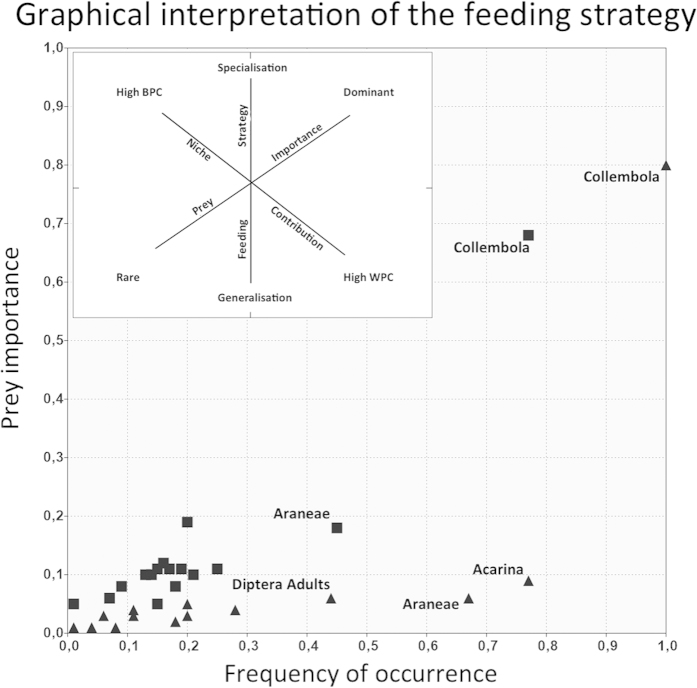
With regard to the trophic niche width of Salamandrina, the diversity in the diet composition was significantly higher (t-test: p < 0.001) in Autumn (Shannon-Weaver index = 1.16 – Evenness = 0.40) than in Spring (S-W index = 0.83 – Evenness = 0.28), and the diversity, for the invertebrate community, significantly differed between seasons (t-test: p < 0.001) and was higher in Autumn (S-W index = 2.23 – Evenness = 0.68) than in Spring (S-W index = 1.86 – Evenness = 0.61). There was no significant evidence of different prey use between sexes (ANOSIM: Autumn; R = −0.06; p = 0.711 – Spring; R = −0.07; p = 0.842). The graphical representation of the feeding strategy (Fig. 1) confirmed that Salamandrina, in both seasons, behaves as a specialist predator on Collembola. Furthermore, the graphical analysis suggests that Salamandrina has a narrow trophic niche, and Collembola were the bulk of the realized trophic niche, along with Araneae and Acarina, while these contributed to a lesser extent. Even if Salamandrina appears to be specialized on Collembola in both seasons, the intensity of specialization is even higher in Spring than in Autumn: indeed all salamanders fed on Collembola in Spring.
Figure 1. Costello’s modified graphical interpretation of the foraging strategy (Amundsen et al., 1992).
Autumn data are represented by squares, while spring data are represented by triangles. Labels of prey categories with both values of Prey importance and Frequency of occurrence lower than 0.30 are not shown.
How does Salamandrina select prey?
Salamandrina positively selects only few prey categories, within a relatively large array of available taxa (Fig. 2). In both seasons, Collembola and Araneae are positively selected, while all other prey categories are clearly used less than expected based on their availability (e.g. Acarina). In Autumn, Salamandrina showed a positive selection for more prey categories (Collembola, Coleoptera larvae, Araneae) compared to Spring (Aranea, Collembola).
Figure 2.
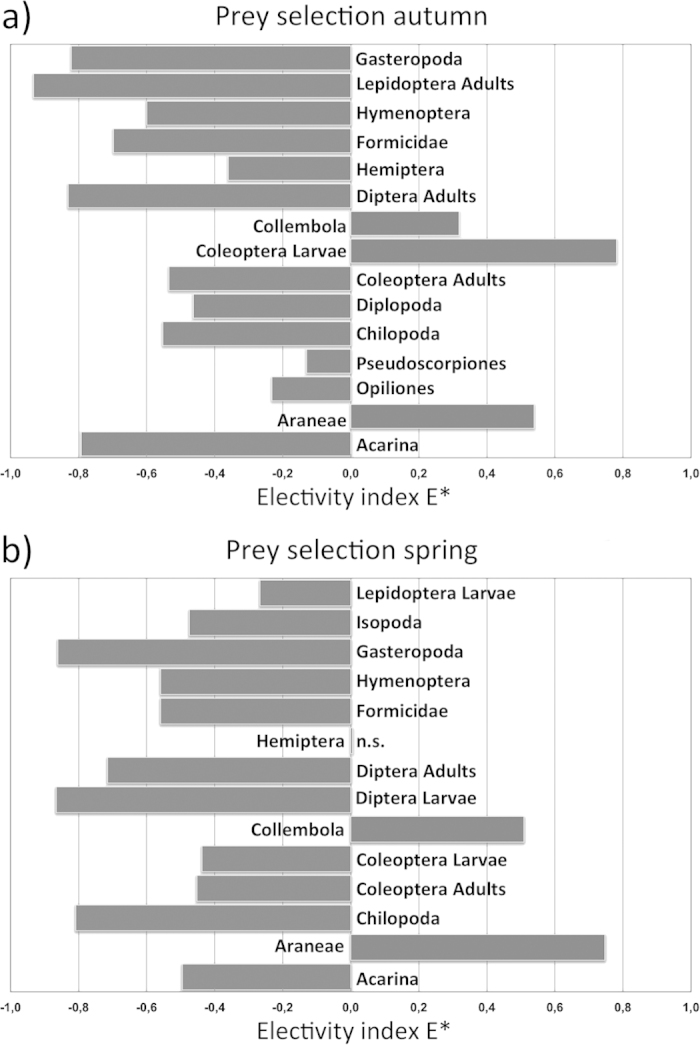
Vanderploeg and Scavia’s (1979) electivity index E* for the most representative prey categories in autumn (a) and spring (b). n.s. = not significant values.
Resource selection probability functions (RSPF) were applied to the three main selected prey types: Collembola, Araneae and Acarina. Collembola accounted for the highest estimated probability of use [0.83 (0.21) – Mean probability of use (SD)], while the best model included season, snout-vent length (SVL), and body condition index (SMI). Probability of use was higher in Spring than in Autumn [13.04 (13.10; 14.64) – Beta’s estimates (95% Confidence Intervals)] and was positively influenced by SVL [0.46 (0.31; 0.62)] and SMI [0.52 (0.39; 0.72)]. The estimated probability of use for Araneae [0.01 (0.002) – Mean probability of use (SD)] and Acarina [0.07 (0.023) – Mean probability of use (SD)] was remarkably lower than that estimated for Collembola. Acarina probability of use resulted to be influenced by season [0.73 (0.40; 1.21)] but negatively affected by SVL [−0.16 (−0.9; 0.03)]. Similarly, Araneae’s probability of use was influenced by season [−0.80 (−1.11; −0.59)], but with an opposite trend in comparison to Collembola and Acarina. Furthermore the use of Araneae resulted to be negatively affected by SMI [−0.19 (−0.26; −0.10)] and index of foraging intensity, FORI [0.42 (−0.56; −0.28)].
Is there an inter-individual diet variation?
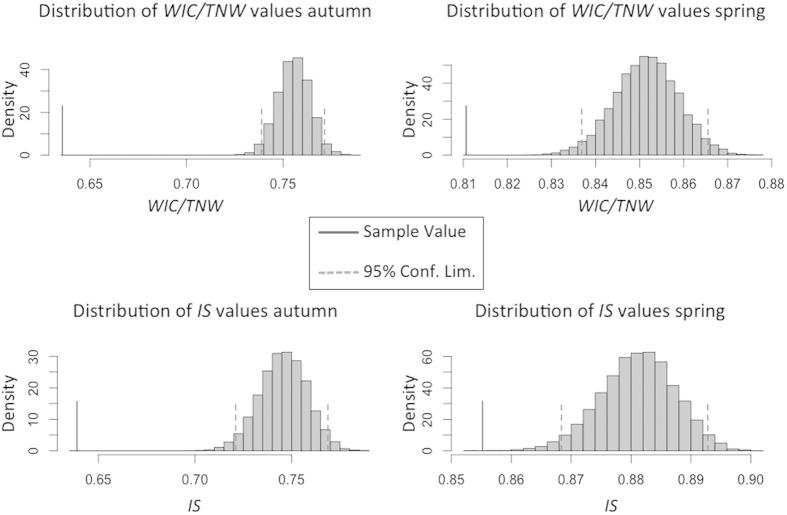
The Total Niche Width (TNW) of the population in Autumn is wider than in Spring (TNW = 1.16—0.83; in Autumn and Spring, respectively). The Within Individual Component of the trophic niche (WIC) resulted similar between seasons (WIC = 0.74—0.67; in Autumn and Spring respectively), while the Between Individual Component (BIC) decreases from Autumn to Spring (BIC = 0.42—0.15; in Autumn and Spring, respectively). The value of the ratio WIC/TNW is lower in Autumn (0.63) than in Spring (0.81), and in both season it’s statistically significant (Monte Carlo resampling; p = 0.001) (Fig. 3). The population has been divided into two groups at the proportional similarity index (PSi) value 0.73 in autumn and 0.87 in spring; obtaining two sub-groups on the basis on the individual specialization (48 and 72 individuals in Autumn; 33 and 34 individuals in Spring). In Autumn individuals with low values of PSi had significantly lower values of SMI (Mann Withney; U = 1159, p < 0.001) while during Spring the two groups had similar values (Mann Withney; U = 438, P = 0.12). SVL did not differ significantly between groups in both seasons. The diet of groups characterized by low values of PSi had significantly higher diversity (Mann-Withney; U = 1255, p = 0.006 in Autumn; U = 260, p < 0.001 in Spring) and evenness (Mann-Withney; U = 1084, p < 0.001 in Autumn; U = 220, p < 0.001 in Spring). At the same time the “low PSi” groups accounted for a smaller FORI (Mann-Withney; U = 380, p < 0.001 in Autumn; U = 180, p < 0.001 in Spring) and showed a lower relative abundance of Collembola in their stomachs (Mann-Withney; U = 702, p < 0.001 in Autumn; U = 245, p = 0.002 in Spring) in both seasons. Complete data for the aforementioned analyses, including means and standard deviations of the two subgroups, are presented in Table 2.
Figure 3. Histograms of distributions of within-individual component/trophic niche width (WIC/TNW) ratio and individual specialization (IS) indices obtained by Monte Carlo resampling procedure, both for autumn and spring data.
Vertical broken lines show the 95% confidence limits of the simulated distribution, while the vertical solid line shows the actual index value for the original data.
Table 2. Comparison between groups in function of inter-individual diet variation. For definition of groups see text.
| Comparison between groups |
Autumn |
Spring |
||
|---|---|---|---|---|
| Group 1 Psi < 0, 73 Mean (S.D.) | Group 2 Psi < 0, 73 Mean (S.D.) | Group 1 Psi < 0, 87 Mean (S.D.) | Group 2 Psi < 0, 87 Mean (S.D.) | |
| Scaled mass index | 1.33 (0.21) | 1.46 (0.17) | n.s. | n.s. |
| Shannon-Weaver index | 0.80 (0.34) | 0.68 (0.25) | 0.77 (0.34) | 0.61 (0.05) |
| Evenness (J) | 0.65 (0.34) | 0.55 (0.17) | 0.58 (0.17) | 0.41 (0.11) |
| Foraging intensity | 6.4 (4.50) | 20.6 (13.2) | 23.4 (11.9) | 46.1 (18.9) |
| Relative abundance of Collembola | 0.45 (0.20) | 0.78 (0.08) | 0.74 (0.11) | 0.85 (0.05) |
Discussion
Prior to this study, Salamandrina was known to behave as a specialist predator on Collembola, especially in Autumn10,11, while during the transition from Autumn to Spring a shift towards a more generalist foraging strategy was described10. However in the present study, based on a larger sample size, Salamandrina behaves as a specialist predator in both seasons. Furthermore in Spring the realized trophic niche is narrower and the degree of specialization is higher than in Autumn. This pattern of variation, related to seasonal changes in trophic availability is well known and fully reflects the Optimal Foraging Theory12. In fact Optimal Foraging Theory states that individuals are capable to categorize prey items on the basis of their net energetic value (net gain of energy considering the cost of capture, handling and digesting) and always select those prey that guarantee the highest energy intake per time unit. When more profitable prey categories become scarce, an expansion of the trophic niche is expected, and other prey categories are also exploited, maximizing the energy intake per time unit.
The slightly contrasting results we obtained from available data10 may only be apparent. In the other studied site10 Salamandrina shares the environment with another terrestrial salamander, the plethodontid Speleomantes strinatii. As reported by those authors10 the dietary pattern showed in their study population, contrasting the Optimal Foraging Theory, may be strongly influenced by the syntopy of two (or more) species that have similar ecological and trophic requirements and niche partitioning may play a key role in their long-term coexistence13,14,15. Conversely, Salamandrina is the only salamander occurring in our study area and our results revealed its trophic habits when trophic competition does not occur.
Results from the Electivity Index E* show how Salamandrina selects only few prey categories from a highly diverse pool of resources available in the environment. Collembola, Araneae and Coleoptera larvae are selected during the Autumn, while in Spring only Araneae and Collembola are selected. Furthermore, in agreement with Optimal Foraging Theory, the fact that some prey categories, such as Acarina, that are particularly abundant in the environment, are avoided in both seasons should suggests that those prey are categorized by Salamandrina as a low profitable resource. Indeed, even if the size and energy content of Collembola and Acarina is similar16, Acarina have harder bodies and are only partially digested by salamanders11, providing a lower energy intake per volume unit.
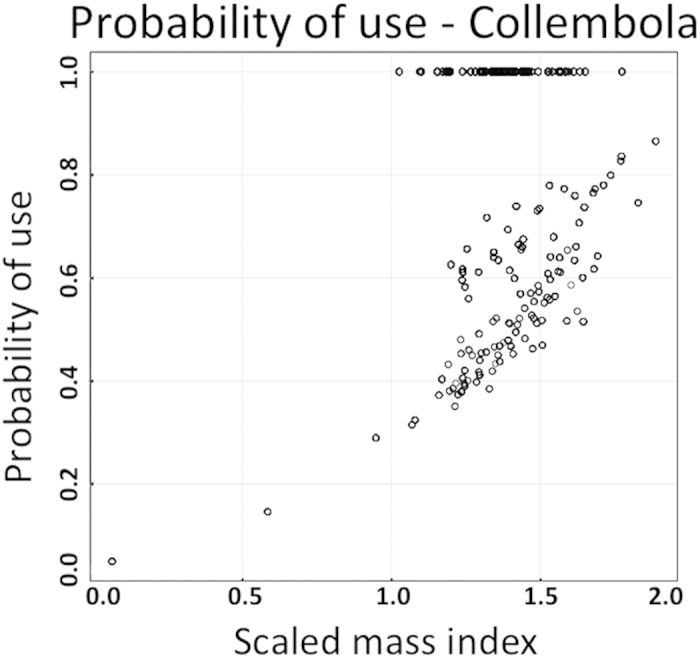
Collembola are little armored and therefore highly energetic17 but, at the same time, hard to catch because they evolved their furcula as an escape mechanism to avoid predators, being able to jump like “miniature kangaroos”18. Thus, our findings concerning the seasonality effect, which mainly deal with prey availability, and the phenotypic variation of salamanders, may be regarded as a balance between the energy content of a given food and the difficulty in obtaining it. Larger salamanders, and in a better physiological condition, have higher probabilities of consuming Collembola (Fig. 4), while smaller and under-performing individuals had high probabilities of selecting Acarina and Araneae.
Figure 4. Scatterplot of the probability of use of Collembola, obtained from resource selection probability functions (RSPF), in relation to body condition index (SMI).
Diet variation among individuals may arise from their differences in experience and foraging ability8,19,20. The observed inter-individual variation in prey selection could be explained by Optimal Foraging Theory, since phenotypic variation may play an important role in determining prey selection. Different individuals may have different abilities to detect or consume prey and those differences will lead to a different categorization of which one is the most profitable prey. Therefore, individuals with lower fitness, for genetic or environmental causes, are not able to exploit the most profitable prey category, for instance due to intraspecific competition when the preferred resource is scarce, and shift their selection on other prey categories.
Animal populations are commonly composed of both generalist and specialist individuals with the latter representing a small subset of the whole population20. We observed for the first time a different pattern, where generalists are a smaller subset within a population mainly composed by specialized individuals. Diet specialization in Salamandrina seems to be the rule, while inferior performing individuals shift their diet, in particular during the season with low trophic availability, toward a wider trophic niche that included low-energy but easy-to-catch prey. Indeed in other taxa, specialist individuals had higher foraging success21, a feature related to a higher fitness leading to a higher reproductive success21,22. Among amphibians diet specialization was known so far only for anurans23,24 and it is often linked with the evolution of aposematism25. Our study revealed that Salamandrina perspicillata is the only salamander in the world exhibiting a true diet specialization. Among Urodela diet specialization is known only for a single population of the Italian Crested Newt (Triturus carnifex, a generalist species26,27), living in a deep flooded karstic sinkhole, that in summer prey almost exclusively on the pre-imaginal stages of the small China-mark, Cataclysta lemnata28. Consequently our study and the previous one10, for populations separated by 520 km as the crow flies, reveal that diet specialization in Salamandrina is a species-level habit, highlighting the existence of the first urodelan in the world with a truly specialized diet. Specialization at individual level intrigued ecologist for decades but it has been studied only within generalist populations/species. We wanted to contribute to this debated topic by providing a different perspective, i.e. studying what emerged by analyzing the feeding habits of individuals in a specialized species. On the whole the population we studied consist by two subgroups of salamanders, the more performing one highly specialized on a very energetic but hard to catch prey type. The less performing animals instead shift their trophic strategy towards more easy-to-catch but less energetic taxa, in particular during the season with lower trophic availability. In our study case, specialization is related to a better physiological status, suggesting that the two strategies are not equivalent and that specialization consists in a ecological advantage.
Finally, our findings are also relevant for the conservation of this species and for the understanding of its ecologic role in forest ecosystems. Indeed the fact that a species with a narrow trophic niche could be able to shift on different prey categories when resources are scarce, though showing a decrease in fitness, could represent a highly adaptive trait in case of habitat fragmentation and other stressing situations that could affect the viability of Salamandrina populations. Lastly, the large amount of predation on Collembola suggests that Salamandrina could be able to regulate the abundance of these arthropods, that play a role in litter turnover in many forest ecosystem processes29, and that this top-down effect could have serious implications in litter retention rate and carbon capture30,31.
Methods
Study area and species
Fieldwork was carried out in Central Italy (41°44′50 N, 14°11′40′′ E), in two Apennine forest sites, 3 km apart, where Salamandrina perspicillata breeds. These sites, situated at about 900 m a.s.l., can be attributed to a Supra-Mediterranean mixed deciduous forest32 dominated by Beech (Fagus sylvatica) and Turkey Oak (Quercus cerris), with a limited occurrence of Hornbeam (Carpinus betulus), Maple (Acer spp.) and Silver Fir (Abies alba).
This salamander is endemic to central and northern Italy33 and inhabits mainly shady and damp areas but also Mediterranean habitats. Adults are terrestrial and females go to water only for spawning34. The foraging activity of Salamandrina is performed only during its terrestrial phase35. At the study site Salamandrina perspicillata is the only Urodelan observed.
Sampling of salamanders and their prey
We carried out sampling of salamanders within the early morning hours, or during light rain, when they are more active on the ground. Captured animals were processed in the field or in some cases transported to the lab for immediate treatment, in order to prevent prey digestion36,37. Total length (TL: distance from the tip of the snout to the tip of the tail) and snout-vent length (SVL: distance from the tip of the snout to the posterior margin of the cloacal slit) were measured with a caliper (precision 0.01 mm), while the body mass was measured using a digital scale (precision 0.01 g). Sex was determined by the observation of the cloaca walls38. Stomach flushing39 was performed always by the same person using a 5 ml syringe (one injection per salamander) connected to a flexible plastic tube, and stomach contents were preserved in 70% ethanol. All treated animals were returned to their capture site within few hours after capture and we did not observe mortality.
In order to investigate prey use and selection we collected samples of potential invertebrate prey at the study site in two seasons: Autumn (25–29 October 2012) and Spring (8–12 May 2013, at the end of spawning). To obtain representative data of prey availability we used three different sampling methods. Ground-dwelling invertebrates were sampled using ten pitfall traps, each one consisting of a 500 cm3 container, partially filled with a killing/preserving solution40. Pitfall traps were active for 14 days before salamanders’ sampling (between October 12 and 25 – between April 25 and May 8). Two soil cores of 1000 cm3 were obtained the first day of salamanders’ sampling and invertebrates were extracted using Berlese-Tullgren extractor41. Aerial invertebrates were sampled by ten sticky traps, active for six days before salamanders’ collection, and consisting of transparent acetate sheets (0.062 m2) covered with entomological glue and fixed to vegetation at 1.20 m from the soil. The positions of the traps were recorded in Autumn 2012 by flagging and, in Spring 2013, they were repositioned exactly in the same locations in order to reduce possible variations resulting from microhabitat differences.
Invertebrates obtained from environmental sampling and from stomach contents were sorted, identified, and counted using a dissecting microscope and taxonomic keys. Since invertebrates obtained with stomach flushing are partly digested, the achievable taxonomic rank is low (Order) and all invertebrates, both from stomach contents and from environmental sampling, have been determined at the Order level, annotating the life stage if ecologically relevant (e.g Lepidoptera larvae).
Data Analysis: Is Salamandrina a specialist predator?
Since the first goal of our study was to confirm that Salamandrina is a specialized predator on Collembola, we conducted the following analysis merging together the diet data from the two populations, after appropriate analyses (see Results). Prey diversity both in the realized trophic niche and in the environment was estimated using the Shannon-Weaver (S-W) index. Possible presence of ecological sex dimorphism in diet was tested by ANOSIM42. The observed feeding strategy was then assessed using Costello’s43 modified graphical analysis44, which plots prey frequency of occurrence [FO; frequency of occurrence of predators feeding on prey (i)] against prey specific abundance [Pi; relative abundance of prey item (i) calculated on the total items found only in those individuals that fed on this prey category]. This method, used in dietary studies both in terrestrial and aquatic amphibians10,11,28, gives useful interpretation of the realized trophic niche and allows to identify the emerging trophic strategy of the population (e.g. generalist vs specialist).
Data Analysis: How does Salamandrina select prey?
With regard to prey selection in the environment we compared the relative abundance of each prey category in the diet with the relative abundance of the same prey sampled in the environment. For this purpose we used the Vanderploeg and Scavia’s Relativized Electivity Index E*45, which is strongly supported46. This index ranges from −1 (negative selection) to 1 (positive selection), assuming a zero value for random feeding. E* was calculated only for prey categories with more than four individuals sampled in the environment. The threshold value above and below which E* was considered different from zero, was the 5th percentile of the absolute value of E*47. In order to determinate the effect of phenotypic variation, sex and seasonality on prey selection we used in logistic resource selection probability functions (RSPF) under a use-availability design48,49,50, obtaining the probability of use for each prey category, along with the effect of the covariates included in the models. The dataset for this analysis was obtained considering each prey item in the stomach of a salamander as a single predation event, and therefore we built a dataset composed by near 4000 observations of predation, along with continuous and categorical covariates, relative to individuals or season/site. Sex, seasonality, site, SVL and an index of foraging intensity (FORI – calculated as the number of ingested prey items) were used as covariates, along with the body condition index, which is basically derived from individual body mass and lenght and is considered as a good predictor of energy reserve and physiological status of the individual51. Among the many formulations of body condition indices, available in literature, we adopted the Scaled Mass Index (SMI) which is actually considered the best predictor of energy reserve52. Using the R package “ResourceSelection” we built several models for each prey category; starting from simplest models with only one covariate and adding more covariates in a stepwise approach. Models were then ranked by Akaike’s Information Criterion (AIC)53 taking into account that models with score differences >2 do not have the same empirical support and show substantial differences54, moreover the Hosmer-Lemshow goodness of fit test for logistic regression has been used to assess model fit55.
Data Analysis: Is there inter-individual diet variation?
In order to assess the presence of inter-individual diet variation, and to investigate its relationship with seasonality or phenotypic variation, we analyzed the realized trophic niche using the R package “RInSp”56. In both seasons the trophic niche width of the population (TNW) was calculated using the equations proposed by Roughgarden57 and modified for discrete data4, using the Shannon-Weaver index as a proxy for variance. TNW has been broken down in its two components: Between Individual Component (BIC) and Within Individual Component (WIC). The ratio WIC/TNW, that ranges from 0 to 1, is a measure of inter-individual diet variation: values near 1 indicate low inter-individual diet variation, while values near 0 indicate decreasing inter-individual overlap and higher individual specialization8. In order to assess statistical significance of WIC/TNW we generated, through Monte Carlo resampling, 999 simulated populations from the original dataset, each one of the generated populations having a number of individual equal to the number of the real population and assigning to each individual random diet items from the population’s resource distribution, yielding to a null model corresponding to a population composed by generalists individuals4. The index WIC/TNW is then recalculated for each resampled dataset: the proportion of resampled populations that had index values lower than the observed one corresponds to a non-parametric p-value of the observed WIC/TNW. Another measure of individual specialization is the proportional similarity index (Psi), that describes the overlap between an individual i’s diet and the diet of the population as a whole4,58. For individuals consuming prey in direct proportion to the population as a whole, PSi will be 1 and it will decrease in case of individual specialization. In addition, we calculated the IS index which is the average of the PSi values, that represents a general measure of the individual specialization at the population level4,59. Statistical significance for this index was calculated following the same simulation approach as for the WIC/TNW index. Furthermore, in both seasons we divided the dataset in two groups: individuals that shown significant inter-individual variation (Low PSi), and individuals consuming prey in direct proportion with the population (High PSi). The threshold value of PSi below which we considered the individuals as “individual specialist” was set according to the lower confidence interval limit of the null distribution derived from 999 Monte Carlo resamplings of the original data set. We looked for differences in SVL, SMI, Shannon-Weaver diversity index, FORI and relative abundance of Collembola calculated on each stomach for these two groups, using Mann-Whitney non-parametric test.
Ethic statement
All the experimental protocols were approved by the Italian Ministry of Environment with the authorisation number PNM-II-2012-0015691. All methods and experimental protocols were carried out in accordance with the guidelines provided by the Italian Ministry of Environment.
Additional Information
How to cite this article: Costa, A. et al. Generalisation within specialization: inter-individual diet variation in the only specialized salamander in the world. Sci. Rep. 5, 13260; doi: 10.1038/srep13260 (2015).
Acknowledgments
This study was supported by the Life project ManFor CBD LIFE09 ENV/IT/000078 (Managing Forests for multiple purposes: Carbon, Biodiversity and socio-economic well-being). All the experimental protocols were approved by the Italian Ministry of Environment with the authorisation number PNM-II-2012-0015691. All methods and experimental protocols were carried out in accordance with the guidelines provided by the Italian Ministry of Environment. We are grateful to Rosario Balestrieri, Giovanni Capobianco for their contribution in the field sampling and to Marco Basile for useful comments. Tiziana Altea and Domenico De Vincenzi (Ufficio Territoriale Biodiversità of National Forest Service of Italy of Castel di Sangro and of Isernia, respectively) greatly contributed providing logistics and laboratory facility close to the sampling site.
Footnotes
Author Contributions A.C., A.R. and S.S. originally formulated the idea, developed sampling design, conducted the fieldwork, sorted and determined invertebrate samples, performed statistical analysis and wrote the manuscript; M.P. contributed to the fieldwork and to the ms drafting; G.M. and B.D.C. contributed to provide the framework and design for the experiment and provided editorial advice.
References
- Futuyma D. J. & Moreno G. The evolution of ecological specialization. Annu. Rev. Ecol. Evol. Syst. 19, 207–233 (1988). [Google Scholar]
- Ferry–Graham L. A., Bolnick D. I. & Wainwright P. C. Using functional morphology to examine the ecology and evolution of specialization. Integr. Comp. Biol. 42, 265–277 (2002). [DOI] [PubMed] [Google Scholar]
- Devictor et al. Defining and measuring ecological specialization. J. Appl. Ecol. 47, 15–25 (2010). [Google Scholar]
- Bolnick D. I., Yang L. H., Fordyce J. A., Davis J. M. & Svanbäck R. Measuring individual–level resource specialization. Ecology 83, 2936–2941 (2002). [Google Scholar]
- McKinney M. Extinction vulnerability and selectivity: combining ecological and paleontological views. Ann. Rev. Ecol. Evol. Syst. 28, 495–516 (1997). [Google Scholar]
- Julliard R., Jiguet F. & Couvet D. Common birds facing global changes: what makes a species at risk? Glob. Chang. Biol. 10, 148–154 (2003). [Google Scholar]
- Devictor V., Julliard R. & Jiguet F. Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117, 507–514 (2008). [Google Scholar]
- Bolnick D. I., Svanbäck R., Fordyce J. A., Yang L. H. & Davis J. M. The Ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28 (2003). [DOI] [PubMed] [Google Scholar]
- Araújo M. S., Bolnick D. I. & Layman C. A. The ecological causes of individual specialization. Ecol. Lett. 14, 948–958 (2011). [DOI] [PubMed] [Google Scholar]
- Salvidio S. et al. Different season, different strategies: Feeding ecology of two syntopic forest-dwelling salamanders. Acta Oecol. 43, 42–50 (2012). [Google Scholar]
- Costa A. et al. What goes in does not come out: different non–lethal dietary methods give contradictory interpretation of prey selectivity in amphibians. Amphibia-Reptilia 35, 255–262 (2014). [Google Scholar]
- Stephens D. W. & Krebs J. R. Foraging Theory. Princeton University Press (1986). [Google Scholar]
- Schoener T. W. The evolution of bill size differences among sympatric congeneric species of birds. Evolution 19, 169–213 (1965). [Google Scholar]
- Huey R. B. & Pianka E. R. Ecological consequences of foraging mode. Ecology 62, 991–999 (1981). [Google Scholar]
- Alatalo R. V. & Moreno J. Body size, interspecific interactions, and use of foraging sites in tits (Paridae). Ecology 68, 1773–1777 (1987). [DOI] [PubMed] [Google Scholar]
- Naess S. J., Steigen A. L. & Solhøy T. in Fennoscandian Tundra Ecosystems (Ed Weigolaski F. E. ) Standing crop and calorific content in invertebrates from Hardangervidda, 151–159 (Springer-Verlag, 1975). [Google Scholar]
- Crovetto F., Romano A. & Salvidio S. A comparison of two non–lethal methods for dietary studies in terrestrial salamanders. Wildlife Res. 39, 266–270 (2012). [Google Scholar]
- Hopkin S. P. in Encyclopedia Of Soil Science (ed. Lal R. ) Collembola, 207–210 (Marcel Dekker, 2002). [Google Scholar]
- Svanbäck R. & Bolnick D. I. Intraspecific competition drives increased resource use diversity within a natural population. Proc. R. Soc. Lond. 274, 839–844 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires M. M. et al. The nested assembly of individual-resource networks. J. Anim. Ecol. 80, 896–903 (2011). [DOI] [PubMed] [Google Scholar]
- Terraube J., Guixé D. & Arroyo B. Diet composition and foraging success in generalist predators: Are specialist individuals better foragers? Basic Appl. Ecol. 15, 616–624 (2014). [Google Scholar]
- McDonald P. G., Olsen P. D. & Cockburn A. Weather dictates reproductive success and survival in the Australian brown falcon Falco berigora. J. Anim. Ecol. 73, 683–692 (2004). [Google Scholar]
- Simon M. P. & Toft C. A. Diet specialization in small vertebrates: mite-eating in frogs. Oikos 61, 263–278 (1991). [Google Scholar]
- Wells K. D. The Ecology And Behavior Of Amphibians. University of Chicago Press (2010). [Google Scholar]
- Santos J. C., Coloma L. A. & Cannatella D. C. Multiple, recurring origins of aposematism and diet specialization in poison frogs. Proc. Natl. Acad. Sci. USA 100, 12792–12797 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni S., Andreone F. & Tripepi S. In Fauna D’Italia XLII: Amphibia (eds Lanza B., Andreaone F., Bologna M. A., Corti C. & Razzetti E. ) Triturus carnifex (Laurenti, 1768), 265–272 (Edizioni Calderini, 2007). [Google Scholar]
- Vignoli L., Luiselli L. & Bologna M. A. Dietary patterns and overlap in an amphibian assemblage at a pond in Mediterranean central Italy. Vie Milieu 59, 47–57 (2009). [Google Scholar]
- Romano A., Salvidio S., Palozzi R. & Sbordoni V. Diet of the newt, Triturus carnifex (Laurenti, 1768), in a flooded karstic sinkhole (“Pozzo del Merro”, Central Italy). J. Cave Karst Stud. 74, 271–277 (2012). [Google Scholar]
- Rusek J. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers. Conserv. 7, 1207–1219 (1998). [Google Scholar]
- Davic R. D. & Welsh H. H. Jr On the ecological roles of Salamanders. Annu. Rev. Ecol. Evol. Syst. 35, 405–434 (2004). [Google Scholar]
- Best M. L. & Welsh H. H. Jr The trophic role of a forest salamander: impacts on invertebrates, leaf litter retention, and the humification process. Ecosphere 5, art16 (2014). [Google Scholar]
- Blondel J. & Aronson J. Biology And Wildlife Of The Mediterranean Region. Oxford University Press, Oxford, UK (1999). [Google Scholar]
- Romano A. et al. Distribution and morphological characterisation of the endemic Italian salamanders Salamandrina perspicillata (Savi, 1821) and S. terdigitata (Bonnaterre, 1789) (Caudata: Salamandridae). Ital. J. Zool. 76, 422–432 (2009). [Google Scholar]
- Lanza B. Anfibi e Rettili. Guide per il riconoscimento delle specie animali delle acque interne italiane 27. Consiglio Nazionale delle Ricerche (1983). [Google Scholar]
- Vanni S. Note sulla Salamandrina dagli occhiali (Salamandrina terdigitata Lacépède, 1788) in Toscana (Amphibia: Salamandridae). Atti Soc. Tosc. Sc. Nat. Mem. 87, 135–159 (1981). [Google Scholar]
- Caldwell J. P. The evolution of myrmecophagy and its correlates in poison frogs (Family Dendrobatidae). J. Zool. 240, 75–101 (1996). [Google Scholar]
- Sole M., Beckmann O., Pelz B., Kwet A. & Engels W. Stomach–flushing for diet analysis in anurans: An improved protocol evaluated in a case study in Araucaria forests, southern Brazil. St Stud. Neotrop. Fauna E. 40, 23–28 (2005). [Google Scholar]
- Romano A., Bruni G. & Paoletti C. Sexual dimorphism in the Italian endemic species Salamandrina perspicillata (Savi, 1821) and testing of a field method for sexing salamanders. Amphibia-Reptilia 30, 425–434 (2009). [Google Scholar]
- Fraser D. F. Coexistence of salamanders in the genus Plethodon: A variation of the Santa Rosalia theme. Ecology 55, 238–251 (1976). [Google Scholar]
- Woodcock B. A. in Insect Sampling In Forest Ecosystems (ed. Leather S. R. ) Pitfall trapping in ecological studies, 37–57 (Blackwell Science, 2005). [Google Scholar]
- Southwood T. R. E. & Henderson P. A. Ecological Methods, 3rd edn. Blackwell Science (2000). [Google Scholar]
- Clarke K. R. Non–parametric multivariate analysis of changes in community structure. Aust. J. Ecol. 18, 117–143 (1993). [Google Scholar]
- Costello M. J. Predator feeding strategy and prey importance: a new graphical analysis. J. Fish Biol. 36, 261–263 (1990). [Google Scholar]
- Amundsen P. A., Gabler H. M. & Staldvik F. J. A new approach to graphical analysis of feeding strategy from stomach contents data – modification of the Costello (1990) method. J. Fish Biol. 48, 607–614 (1996). [Google Scholar]
- Vanderploeg H. A. & Scavia D. Two electivity indices for feeding with special reference to zooplankton grazing. J. Fish Res. Board. Can. 36, 362–365 (1979). [Google Scholar]
- Lechowicz M. J. The sampling characteristics of electivity indices. Oecologia 52, 22–30 (1982). [DOI] [PubMed] [Google Scholar]
- Ramos–Jiliberto R., Valdovinos F. S., Arias J., Alcaraz C. & García–Bertho E. A network–based approach to the analysis of ontogenetic diet shifts: An example with an endangered, small–sized fish.Ecol. Complexity 8, 123–129 (2011). [Google Scholar]
- Manly B. F. J., McDonald L. L., Thomas D. L., McDonald T. L. & Erickson W. P. Resource selection by animals: statistical analysis and design for field studies. Kluwer (2002). [Google Scholar]
- Lele S. R. & Keim J. L. Weighted distributions and estimation of resource selection probability functions. Ecology 87, 3021–3028 (2006). [DOI] [PubMed] [Google Scholar]
- Lele S. R. A new method for estimation of resource selection probability function. J. Wildl. Manag. 73, 122–127 (2009). [Google Scholar]
- Jakob E. M., Marshall S. D. & Uetz G. W. Estimating fitness: a comparison of body condition indices. Oikos 77, 61–67 (1996). [Google Scholar]
- Peig J. & Green A. J. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883–1891 (2009). [Google Scholar]
- Akaike H. in Proceedings of the second international symposium information theory (Eds Petrov B. N., Cazakil F. ) Information theory and an extension of the maximum likelihood principle, 267–281 (Akademiai Kidao, Budapest, Hungary, 1973). [Google Scholar]
- Burnham K. P. & Anderson D. R. Model Selection And Multimodel Inference: A Practical Information-theoretic Approach. (Springer –Verlag, 2002). [Google Scholar]
- Hosmer D. W., Lemeshow S. & Sturdivant R. X. Applied Logistic Regression. John Wiley and Sons (2013). [Google Scholar]
- Zaccarelli N., Bolnick D. I. & Mancinelli G. RInSp: an r package for the analysis of individual specialization in resource use. Methods Ecol. Evol. 4, 1018–1023 (2013). [Google Scholar]
- Roughgarden J. Evolution of niche width. Am. Nat. 106, 683–718 (1972). [Google Scholar]
- Schoener T. W. The Anolis lizards of Bimini: resource partitioning in a complex fauna. Ecology 49, 704–726 (1968). [Google Scholar]
- Araújo M. S, dos Reis S. F., Giaretta A. A., Machado G. & Bolnick D. I. Intrapopulation diet variation in four frogs (Leptodactylidae) of the Brazilian Savannah. Copeia 4, 855–865 (2007). [Google Scholar]