Abstract
Metformin and aspirin have been studied extensively as cancer preventative and therapeutic agents. However, the underlying molecular mechanisms for the inhibitory effects of pancreatic cancer development remain undefined. To gain further insight into their biological function in pancreatic cancer, we conducted a transcriptomic analysis using RNA sequencing to assess the differential gene expression induced by metformin (5 mM) and aspirin (2 mM), alone or in combination, after treatment of PANC-1 cells for 48 hours. Compared to an untreated control, metformin down-regulated 58 genes and up-regulated 91 genes, aspirin down-regulated 12 genes only, while metformin plus aspirin down-regulated 656 genes and up-regulated 449 genes (fold-change > 2, P < 10−5). Of the top 10 genes (fold-change > 10, P < 10−10) regulated by metformin plus aspirin, PCDH18, CCL2, RASL11A, FAM111B and BMP5 were down-regulated ≥ 20-fold, while NGFR, NPTX1, C7orf57, MRPL23AS1 and UNC5B were up-regulated ≥ 10-fold. Ingenuity Pathway Analysis (IPA) revealed that the pathways, “cholesterol biosynthesis”, “cell cycle: G1/S checkpoint regulation”, and “axonal guidance signaling” were the most statistically significant pathways modulated by metformin plus aspirin. Although the results need further functional validation, these data provide the first evidence for the synergistic action between metformin and aspirin in modulating the transcriptional profile of pancreatic cancer cells.
Pancreatic cancer is the fourth leading cause of cancer-related death, which accounts for more than 39,000 annual cancer deaths in the United States. Moreover, the overall 5-year survival rate of pancreatic cancer is still less than 5% indicating poor prognosis1. Currently, surgery, chemotherapy, and radiotherapy are main treatment approaches for pancreatic cancer. However, the mortality rate remains very high because of the low rate of surgical patients and non-sensitivity to chemotherapy and radiotherapy. Therefore, to explore new strategies of prevention and treatment for pancreatic cancer is urgent and important. Metformin and aspirin are emerging as cancer prevention candidate drugs in recent years, and numerous experimental data have demonstrated that both metformin and aspirin have a direct anti-proliferative effect on pancreatic cancer cells in vitro and in vivo2. However, the underlying molecular mechanisms for the inhibitory actions of pancreatic cancer progression remain largely unknown.
Potential mechanisms of metformin’s anti-neoplastic effect include the activation of 5′ AMP-activated protein kinase (AMPK)3, inhibition of mTOR pathway4, and lowering hyperinsulinemia5, modulating inflammatory responses6, as well as selectively killing cancer stem cells7. Aspirin with potential effects on AMPK-mTOR signaling8,9 also modulates multiple inflammatory components, e.g. COX1/COX2, nuclear factor kappa B (NFкB)10. Additionally, both metformin and aspirin have been reported to inhibit signal transducer and activator of transcription 3 (STAT3)11,12. These findings indicate that both metformin and aspirin are multi-target drugs that exert the anti-cancer effect through simultaneously targeting various signaling pathways and molecules that are involved in tumor initiation and development. The crosstalk and interaction among different signaling pathways is critical for understanding the underlying anti-cancer mechanism of metformin and aspirin action. However, an overview of genes and pathways related to metformin and aspirin action remains relatively understudied. Therefore, it is important to obtain a comprehensive profile of genes and pathways that are regulated by metformin and aspirin, especially when they are used in combination.
Recently, high-throughput RNA sequencing (RNA-seq) is rapidly becoming an attractive alternative to hybridization-based microarrays for transcriptome profiling. This approach enables investigators to get a relatively unbiased and precise measurement of levels of transcripts and their isoforms than other methods13. One of the main goals of RNA-seq is to identify the differentially expressed genes in two or more conditions. Such genes are selected based on a combination of expression change threshold and score cutoff, which are usually based on P values generated by statistical modeling.
In order to depict a comprehensive picture of the mechanisms underlying the antineoplastic activity of metformin and aspirin, it is necessary to obtain some new insights, on a whole-transcriptome level, on the gene regulation by metformin and aspirin. In this study, using RNA-seq technology, we comprehensively analyzed the differential gene expression induced by metformin (5 mM) and aspirin (2 mM), alone or in combination, after treatment of pancreatic cancer cell line PANC-1 cells for 48 hours. We identified the top genes and the most related biological networks and pathways which are not or seldom reported previously, to be modulated by metformin and aspirin. Future function validation is warranted to verify our results in vitro and in vivo.
Results
Overview and validation of differentially expressed genes induced by metformin and aspirin
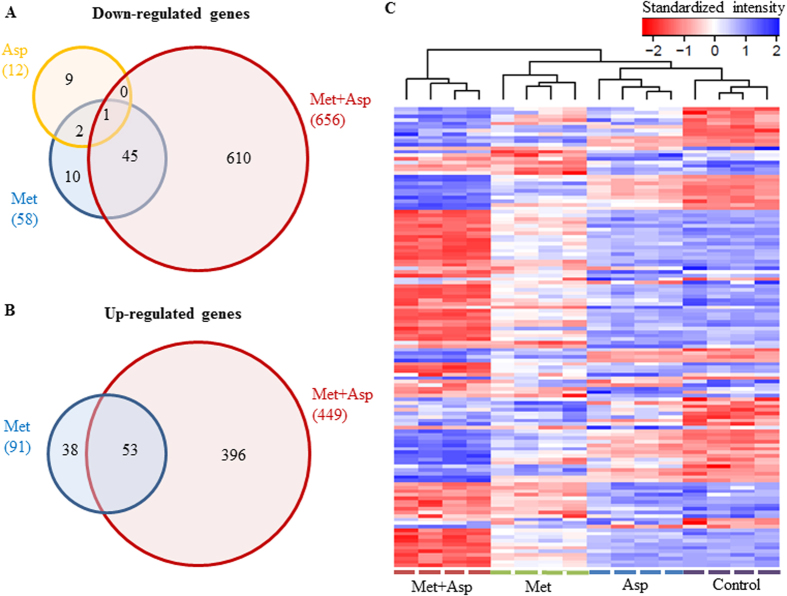
To determine the impact of metformin and aspirin on the whole transcriptome, we compared the global gene expression profiles of untreated PANC-1 cells to those treated by metformin, aspirin, or a combination. We detected and quantified 11,551 unique genes, and compared the gene expression profiles of all three drug-treated samples to the untreated sample. Differentially expressed genes were identified by arbitrarily using a 2.0-fold-change cut-off value (P value < 1 × 10−5) compared to the untreated PANC-1 cells. We identified 149 differentially expressed genes (58 down-regulated and 91 up-regulated) from the metformin treated cells and 12 genes (all down-regulated) from the aspirin treated cells. Moreover, we identified 1,105 genes (656 down-regulated and 449 up-regulated) that have significantly different expression levels in the metformin-aspirin-combination treated cells. Of the genes downregulated by metformin alone, 79.3% (46/58) were also down-regulated by the combination of metformin and aspirin (Fig. 1A), while 58.2% (53/91) of the up-regulated genes by metformin also appeared in the subset of up-regulated genes by the combination (Fig. 1B). However, the genes regulated by aspirin alone did not show much commonality with those by metformin alone or the combination of metformin and aspirin. Only 25.0% (3/12) and 8.3% (1/12) of the down-regulated genes by aspirin were also down-regulated in metformin treated cells and metformin-aspirin treated cells, respectively (Fig. 1A). As shown in Fig. 2C, the clustering of top differentially expressed genes showed clearly distinguishable patterns across four conditions.
Figure 1. Overview of genes regulated by metformin and aspirin.
(A,B) Venn diagrams comparing the down-regulated (A) and up-regulated (B) genes after treatments of metformin and aspirin, alone or the combination. (C) The clustering heat map of 16 samples based on the 150 top differentially expressed genes across 4 groups (untreated control, metformin, aspirin and metformin plus aspirin). Each column is labeled with different colors according to the sample type. Met, metformin; Asp, aspirin
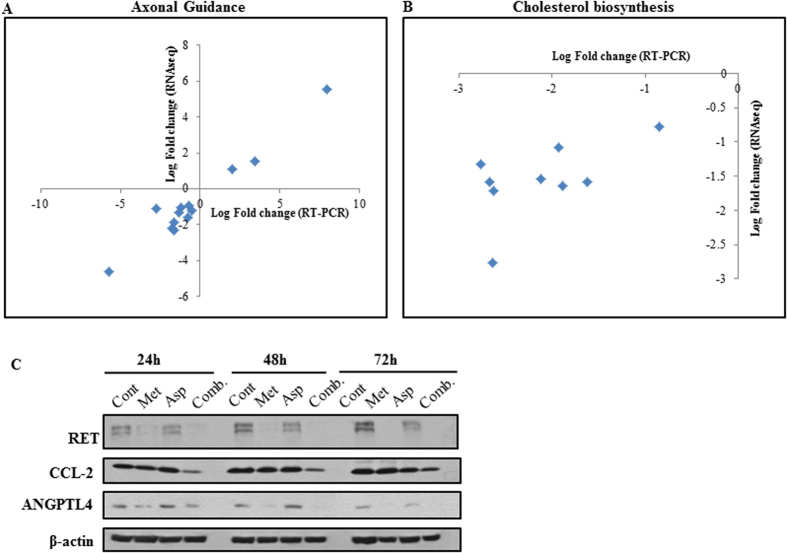
Figure 2. Validation of the mRNA and protein changes of the selected genes.
Nine genes in the “Superpathway of Cholesterol Biosynthesis” (A) and thirteen genes in the “Axonal Guidance Signaling” (B) were randomly selected and their mRNA levels were determined by qRT-PCR in PANC-1 cells untreated or treated with the combination of metformin (5 mM) and Aspirin (2 mM) for 48 h. The protein levels of the selected genes RET, CCL2, and ANGPTL4 were determined by Western blotting (C). PANC-1 cells were treated with metformin (5 mM) and aspirin (2 mM), alone or in combination for 24, 48 and 72 h, respectively. β-actin was used as a loading control. Cont, control; Met, metformin; Asp, aspirin; Comb, the combination of metformin and aspirin.
To validate the results from the RNA sequencing data, we randomly selected 10 genes found to be regulated by the combination of metformin and aspirin to determine their mRNA levels by using quantitative reverse transcription-PCR (qRT-PCR) (Supplementary Table S1). Our data showed that the results from qRT-PCR were consistent with those from the RNA-seq for all 10 selected genes, confirming that comparative RNA-seq analysis is a quantitative approach.
Top differentially expressed genes regulated by metformin and aspirin
Using a 2.0-fold-change cut-off value (P value < 10−5) relative to the transcriptome of the untreated PANC-1 cells, we identified genes that showed significant expression changes upon the treatment of metformin, aspirin or both. The top 10 down-regulated or up-regulated genes by metformin, aspirin or both were shown in Table 1 and Table 2, respectively. The full data set is available at the National Centre for Biotechnology Information Gene Expression Omnibus database (www.ncbi.nlm.nih.gov/geo/, accession number: SRP056109). Among the top down-regulated genes, ANGPTL4, FAM222A, RET were down-regulated to more than 4 folds upon metformin treatment (Table 1). DNAH10OS, LAMP3, PLIN4, DUSP15, HMOX1 are the top five down-regulated genes upon aspirin treatment (Table 1). PCDH18, CCL2, RASL11A, FAM111B, BMP5 were down-regulated to more than 20 folds by the combination of metformin and aspirin (Table 1). RET, CDH18 and PCDH18 down-regulated by metformin were also found to be down-regulated by the combination of metformin and aspirin (Table 1). Of the genes up-regulated by metformin alone, GRB7, GUCA1B, SGK2, PPP1R32 were up-regulated to more than 4 folds compared to the untreated control (Table 2). NGFR, NPTX1, C7orf57, MRPL23AS1, and UNC5B were up-regulated to more than 10 folds by the combination of metformin and aspirin (Table 2). However, we did not detect any genes that were significantly up-regulated by aspirin alone.
Table 1. Top 10 down-regulated genes in PANC-1 cells treated by metformin and aspirin.
| Gene ID | Fold Change | P-value | Function |
|---|---|---|---|
| Metformin | |||
| ANGPTL4 | 0.14 | 2.95E-25 | glucose homeostasis, lipid metabolism, insulin sensitivity, vascular growth, tumor cell invasion |
| FAM222A | 0.18 | 2.34E-19 | unknown |
| RET | 0.21 | 1.76E-22 | signaling transduction, cell growth and differentiation |
| SCDP1 | 0.31 | 1.76E-07 | pseudogene |
| CDH18 | 0.32 | 6.01E-11 | calcium-dependent cell-cell adhesion, synaptic adhesion, axon outgrowth and guidance |
| RCBTB2 | 0.33 | 3.04E-11 | guanine nucleotide exchange |
| FADS2 | 0.33 | 1.12E-42 | unsaturation of fatty acids |
| PCDH18 | 0.34 | 3.55E-13 | cell-cell connections in brains |
| FOXQ1 | 0.35 | 4.03E-07 | regulates epithelial-mesenchymal transition |
| NEU1 | 0.35 | 7.76E-17 | lysosomal enzyme |
| Aspirin | |||
| DNAH10OS | 0.33 | 1.22E-06 | unknown |
| LAMP3 | 0.35 | 4.58E-10 | dendritic cell function and adaptive immunity |
| PLIN4 | 0.36 | 1.50E-10 | lipid distribution and metabolism |
| DUSP15 | 0.40 | 2.39E-06 | oligodendrocyte differentiation |
| HMOX1 | 0.40 | 1.81E-07 | heme catabolism |
| FTLP3 | 0.41 | 1.90E-06 | pseudogene |
| PLIN2 | 0.42 | 2.06E-18 | lipid distribution and metabolism |
| APOC1 | 0.44 | 5.00E-11 | cholesterol metabolism, membrane remodeling, neuronal apoptosis and reorganization |
| SDSL | 0.46 | 9.25E-07 | low serine dehydratase and threonine dehydratase activity |
| SLC44A2 | 0.46 | 8.94E-18 | some choline transporter activity |
| Combination | |||
| PCDH18 | 0.01 | 1.03E-64 | cell-cell connections in brains |
| CCL2 | 0.02 | 3.96E-166 | macrophage infiltration |
| RASL11A | 0.03 | 9.34E-58 | Regulator of rDNA transcription |
| FAM111B | 0.04 | 7.69E-42 | unknown |
| BMP5 | 0.04 | 8.27E-35 | dendritic growth |
| SKIDA1 | 0.05 | 4.55E-39 | unknown |
| CDH18 | 0.07 | 3.10E-29 | calcium-dependent cell-cell adhesion, synaptic adhesion, axon outgrowth and guidance |
| AGR2 | 0.07 | 1.17E-26 | regulating cell proliferation, promoting cell growth |
| RET | 0.07 | 5.93E-49 | signaling transduction, cell growth and differentiation |
| RXFP4 | 0.08 | 4.66E-23 | high affinity receptor for INSL5, inhibit cAMP accumulation |
Table 2. Top 10 up-regulated genes in PANC-1 cells treated by metformin and aspirin.
| Gene ID | Fold Change | P-value | Function |
|---|---|---|---|
| Metformin | |||
| GRB7 | 6.48 | 1.41E-11 | integrin signaling pathway, cell migration |
| GUCA1B | 5.49 | 2.86E-18 | activates photoreceptor guanylate cyclases |
| SGK2 | 4.48 | 4.41E-12 | membrane transporters, cell growth, survival and proliferation |
| PPP1R32 | 4.19 | 7.49E-10 | unknown |
| WISP2 | 3.33 | 1.11E-11 | Downstream of WNT1 signaling pathway |
| JAK3 | 3.18 | 4.17E-11 | Stat3 pathway |
| TCP11L2 | 3.01 | 3.54E-11 | unknown |
| AMT | 3.00 | 4.49E-18 | glycine cleavage |
| NT5M | 2.93 | 7.16E-10 | Dephosphorylation |
| HES7 | 2.93 | 7.44E-09 | transcriptional repressor |
| Combination | |||
| NGFR | 47.48 | 1.69E-122 | cell survival and differentiation |
| NPTX1 | 20.17 | 1.80E-46 | activates photoreceptor guanylate cyclases |
| C7orf57 | 14.50 | 8.02E-41 | unknown |
| MRPL23AS1 | 12.39 | 4.49E-36 | unknown |
| UNC5B | 11.20 | 2.37E-51 | guidance of nerves/blood vessels, induce apoptosis |
| DUSP15 | 8.91 | 2.66E-60 | oligodendrocyte differentiation |
| HES7 | 8.30 | 1.80E-33 | transcriptional repressor |
| MTURN | 7.74 | 8.44E-24 | early neuronal development |
| SLC20A1 | 6.72 | 9.85E-36 | cellular metabolism, signal transduction, and nucleic acid and lipid synthesis, extracellular matrix and cartilage calcification as well as in vascular calcification |
| GPCPD1 | 6.42 | 6.24E-49 | unknown |
The main gene networks and canonical pathways modulated by metformin and aspirin
To investigate the possible biological function and pathways that were regulated by metformin and aspirin, we employed Ingenuity Pathway Analysis (IPA) software to do the “core analysis” for all the genes significantly up-regulated or down-regulated by metformin, aspirin or the combination. We first analyzed the gene networks that were most regulated by metformin, aspirin or the combination, respectively.
The top two gene networks that were regulated by metformin were: a) “Cardiovascular System Development and Function, Cellular Movement, Cellular Assembly and Organization” (score = 40); and b) “Cardiovascular System Development and Function, Cellular Movement, Skeletal and Muscular System Development and Function” (score = 38) (Supplementary Fig. S1). The first gene network was identified around Ras subfamily, TGF-β, FAK and ERK1. Ras has been found to be mutated in up to 90% of pancreatic cancers, and ERK1is part of Ras-ERK signaling pathway which is critical for pancreatic carcinogenesis. The second network was around PI3-K/Akt, interferon-α, pro-inflammatory cytokines, AMPK and Notch. PI3-K/Akt is an important tumor cell survival pathway, and interferon-α exhibits inhibitory effects on tumor cell growth.
The top gene network that was regulated by aspirin was “Cellular Compromise, Lipid Metabolism, Small Molecule Biochemistry” (score = 27) (Supplementary Fig. S2). The genes in this network were around TNF, HMOX1 and three perilipin family members, PLIN2, PLIN3 and PLIN4. TNF has been reported to modulate pancreatic cancer cell growth by affecting tumor-infiltrating macrophages, and HMOX1 protects against oxidative stress and modulates inflammation and angiogenesis.
The top two gene networks that were regulated by the combination of metformin and aspirin were: a) “Cell Cycle, Cell Morphology, Cellular Assembly and Organization” (score = 40), and b) “Cellular Assembly and Organization, Drug Metabolism, Small Molecule Biochemistry” (score = 35) (Supplementary Fig. S3). The first gene network was identified around Histone h3, which is involved in the structure of chromatin in eukaryotic cells, and RBBP4, which binds to histones and participates in histone acetylation and chromatin assembly. The second one was identified around VEGF, a main angiogenesis regulator that has been targeted for cancer therapy for years.
In addition, we used the IPA software to determine the canonical pathways modulated by the combination of metformin and aspirin (Table 3). “Superpathway of Cholesterol Biosynthesis”, “Cell Cycle: G1/S Checkpoint Regulation”, and “Axonal Guidance Signaling” are the top three most statistically significant canonical pathways regulated by the combination of metformin and aspirin. We randomly selected 9 genes in the “Superpathway of Cholesterol Biosynthesis” and 13 genes in the “Axonal Guidance Signaling” and determined their mRNA levels in PANC-1 cells untreated or treated by the combination of metformin and aspirin by qRT-PCR (Fig. 2A,B). The results further confirmed the down-regulation or up-regulation of these genes upon the treatment of metformin and aspirin. Furthermore, we selected to detect the protein levels of several genes which showed significant changes in the mRNA levels (Fig. 2C). The results showed that the protein levels of ANGPTL4 and RET decreased along with the downregulation of mRNA of these two genes. We only observed the down-regulation of CCL2 in the cells treated by the combination, which is also in accordance with the RNA-seq results.
Table 3. Top 5 canonical pathways of the genes regulated by metformin and aspirin.
| Ingenuity Canonical Pathways | Ratio | P-value | Target genes |
|---|---|---|---|
| Superpathway of Cholesterol Biosynthesis | 11/28 (0.393) | 2.75E-08 | MVD,FDPS,EBP,DHCR7,PMVK,ACAT2,DHCR24,MVK,LSS,TM7SF2,CYP51A1 |
| Cell Cycle: G1/S Checkpoint Regulation | 11/64 (0.172) | 2.00E-04 | CCND2*,TFDP1,HDAC11,SUV39H1,E2F1,TGFB3,TGFB2,CDKN2C,E2F2,HDAC5,CDC25A |
| Axonal Guidance Signaling | 37/433 (0.085) | 4.57E-04 | FYN,TUBA1B,ADAM17,MYL6,PDGFA,PIK3R1,UNC5B,PLCH2,VEGFA,EFNB2,GNG11,NGFR,WNT7B,ABLIM3,PRKAR1B,TUBA1C,PLXNB3,SEMA3F,UNC5C,PRKCA,BMP1,EPHA7,SEMA3E,TUBB4B,PLXND1,BMP5,PIK3R3,SEMA3A,TUBA1A,FZD4,TUBB6,LINGO1,NFATC2,PIK3CD,SEMA3C,BMP6,NRP1 |
| Chronic Myeloid Leukemia Signaling | 13/93 (0.140) | 4.57E-04 | PIK3R3,GAB2,TFDP1,HDAC11,SUV39H1,PIK3R1,E2F1,TGFB2,TGFB3,PIK3CD,IKBKE,E2F2,HDAC5 |
| Pancreatic Adenocarcinoma Signaling | 14/106 (0.132) | 5.01E-04 | PIK3R3,VEGFA,HMOX1,TFDP1,SUV39H1,PIK3R1,E2F1,TGFB2,TGFB3,PIK3CD,PLD6,JAK2,JAK3,E2F2 |
*Up-regulated genes are in bold type.
Discussion
In this study, we established, for the first time, a global transcriptome profile related to metformin, aspirin, and the combination of both in pancreatic cancer cells. We found that while metformin or aspirin alone only slightly changed the transcriptome profile of PANC-1 cells (149 and 12 genes, respectively), the combination of metformin and aspirin dramatically affected the transcription of 1,105 genes. We recently demonstrated that the combination of metformin and aspirin, at relatively low concentrations, significantly inhibited the cell viability in pancreatic cancer cell lines PANC-1 and BxPC314. These results indicate that the combination of metformin and aspirin exerts synergistic action on the transcriptome of PANC-1 cells and thus affects their proliferation and survival. It will be interesting to further examine the synergistic effects of the combination and the underlying mechanisms in the future.
To determine the optimum concentrations of metformin and aspirin used in this study, we first accessed the cell viability of pancreatic cancer cells upon the treatment of metformin and aspirin14. The combination of metformin and aspirin caused a synergistic inhibition of cell viability in both PANC-1 and BxPC-3 cells, mainly in low dosages of both drugs [e.g., PANC-1 cells: 5 mM metformin and 2 mM aspirin, and 10 mM metformin and 4 mM aspirin (P for the test of synergy = 0.034 and 0.007, respectively); BxPC-3 cells: 1 mM metformin and 0.25 mM aspirin, and 5 mM metformin and 0.5 mM aspirin (P for the test of synergy = 0.004 and 0.045, respectively)]. On the other hand, to our best knowledge, the plasma salicylate (the in vivo metabolite of aspirin) concentration in humans is 0.5–2.5 mM10,15, and the plasma concentration of metformin is about 16 μM16. Considering the results of cell viability and physiological concentration of metformin and aspirin that can be achieved in vivo, we chose 5 mM metformin and 2 mM aspirin as the concentration used in this study. Although the dose of metformin we had used is still higher that the physiologically achievable plasma concentration, metformin has been reported to accumulate in tissues at concentrations several folds higher than those in blood17,18. In addition, concentration of metformin in the mitochondrial matrix could achieve a concentration higher than 20 mM18 due to the positive charge of metformin. Taken together, it suggested that the concentrations we used in in vitro models might be attained during cancer treatment.
Of the top down-regulated genes in metformin treated cells, ANGPTL4 (angiopoietin-like 4) is the most dramatically down-regulated genes. ANGPTL4 has been reported as a tumor suppressor through inhibiting angiogenesis in gastric cancer19. Down-regulation of ANGPTL4 mRNA in hepatocellular carcinoma has been shown to be associated with advanced tumor stage, tumor recurrence, and poor postoperative20. Interestingly, metformin treatment was shown to attenuate the increased mRNA levels of ANGPTL4 in type I diabetic mice21. However, the precise function and role of ANGPTL4 in pancreatic cancer is still unclear.
It is worth noting that RET was dramatically down-regulated in both metformin treated cells (5 fold down-regulation vs. untreated cells) and metformin-aspirin-combination treated cells (14 fold down-regulation vs. untreated cells). RET, a single-pass transmembrane receptor tyrosine kinase (RTK), is transcriptionally regulated by DNA-binding factors that modulate basal transcription, such as SP1, SP3 and early growth response protein 1 (EGR1)22. Metformin was reported to decrease SP1/SP3 expression and their downstream targets, such as Bcl-2, survivin, and cyclin D1, in pancreatic cancer cells23. Recently, Nair et al. reported that the regulation of Sp transcription factors could be a novel mechanism by which metformin inhibits IGF-1R/mTOR and EGFR/K-Ras signaling24. Thus, it is possible that metformin regulates the transcription of RET through SP transcription factors. RET is expressed in 50–65% of pancreatic ductal carcinomas and is correlated to an advanced metastatic status22. Increased RET activity stimulates cell proliferation and transformation by promoting tumor-associated inflammation and recruiting primary immune cells to the tumor microenvironment22. Interestingly, STAT3 which has been shown to play an important role in pancreatic tumorigenesis, can also be activated by RET25. Metformin has been shown to inhibit STAT3 activity in various cancers2. However, the underlying mechanism by which metformin inhibits STAT3 is still unclear. Although further function validation studies are needed, the inhibition of RET by metformin provides a new possible explanation for the molecular mechanisms of the inhibition of STAT3 induced by metformin.
Additionally, we observed that CCL2 was dramatically down-regulated (50 fold down-regulation vs. untreated cells) in metformin-aspirin-combination treated cells. CCL2, an inflammatory chemokine, is closely connected with tumor associated macrophage (TAM) infiltration and cancer progression. CCL2 is overexpressed in various cancer types such as lung, breast and prostate cancer26. In pancreatic cancer, high CCL2 expression is associated with significantly decreased survival27. CCL2 secreted by tumor cells can recruit monocytes and TAMs. These tumor-infiltrating inflammatory cells form a tumor-protective microenvironment, thus enhancing tumor growth and metastasis26,28,29. Therefore, inhibition of CCL2 might be a new therapeutic approach for the prevention and treatment of pancreatic cancer. Given that both metformin and aspirin are used in combination with other chemotherapeutic drugs in ongoing clinical trials, it is of interest to explore the possibility of the use of metformin and aspirin as adjuvant cancer immunotherapy.
Of the genes that most dramatically up-regulated in metformin treated cells, SGK2 (serum/glucocorticoid regulated kinase 2) was reported to be important for cell proliferation and viability of HPV-positive cervical cancer cell lines30. GRB7 (growth factor receptor-bound protein 7) was found to be overexpressed in pancreatic tumor than normal pancreatic tissue and was associated with regional lymph node metastatic spread of pancreatic cancer31. However, the modulation of these two genes by metformin has not been previously investigated. WISP2 (WNT1 inducible signaling pathway protein 2) has been reported to be up-regulated in metformin-adapted MCF-7 cells32, which is consistent with our finding. Loss of WISP2 expression was associated with pancreatic cancer progression as WISP2 might prevent against epithelial to mesenchymal transition33. Further studies are warranted to investigate the role of metformin-induced WISP2 up-regulation in pancreatic cancer prevention and treatment.
Of the genes that most dramatically up-regulated in metformin-aspirin-combination treated cells, none of them has been previously reported to be regulated by metformin or aspirin. NGFR (nerve growth factor receptor) exerts a tumor-suppressing effect in bladder, stomach, liver, colorectal and prostate cancers while it promotes tumor progression in brain tumors and melanomas34. It has been reported that NGFR was overexpressed in pancreatic tumor compared to normal pancreatic tissue35,36. However, the exact role of NGFR in pancreatic cancer development is still unclear. NPTX1 (neuronal pentraxin I) has been shown to be silenced through methylation in 5′ CPG islands in pancreatic cancer cell lines37. Similar results were also obtained in colorectal tumors38. UNC5B has been identified as a tumor suppressor, which can induce apoptosis through p53-dependent manner in various cancer cells39. Reduced UNC5B expression is correlated with higher recurrence rate and poorer prognosis in both bladder and colorectal cancers40,41.
We observed only 12 genes were down-regulated, and none of the genes was up-regulated by aspirin. These minimal effects of aspirin on changes in gene expression might be due to the relatively low concentration which we used in the experiments. On the other hand, it is possible that the effects of aspirin on gene expression may be due to the post-translational modification of proteins, such as acetylation. It has been shown that aspirin causes the inactivation of cyclooxygenases (COX) through the acetylation on serine residues42,43. Aspirin could also acetylate p53 and induce the expression of p21 and Bax in breast cancer cells44. Interestingly, aspirin also acetylated multiple cellular proteins in colon cancer cells including histone H1b and histone H445, suggesting that aspirin may participate in the regulation of gene expression by modulating histone acetylation. In fact, aspirin was reported to induce the HDAC Sirtuin 1 through the production of hydrogen peroxide46, and promote the transcription of netrin-1 by enhancing histone acetylation47. These results suggest that the aspirin-induced changes of gene expression might be partially due to the shifting of equilibrium of the acetylation/deacetylation process. Additionally, aspirin is readily broken down in the body to salicylic acid, the main metabolite of aspirin. Mohammed et al. showed that salicylic acid induces greater caspase activity than aspirin in HT3 cervical cancer cell line48. It is possible that salicylic acid may have stronger effects than aspirin on modulation of gene expression. Further studies are warranted to compare the effects of aspirin and salicylic acid on the gene expression pattern in pancreatic cancer cells.
The canonical pathway analysis highlighted “Superpathway of Cholesterol Biosynthesis” is the most significantly regulated pathway in PANC-1 cells treated by the combination of metformin and aspirin. Cholesterol is a crucial component of cell membranes and the cholesterol levels are relatively high in cancer cells due to the needs of their high proliferation rate49,50. Also, cholesterol is essential for the assembly of lipid rafts, which regulate various signaling pathways intimately related to carcinogenesis and metastasis, such as Fas receptor, TRAIL, Akt and CD4451. In our study, 11 of the 28 molecules in “Superpathway of Cholesterol Biosynthesis” were down-regulated while other molecules did not show significant change. Of the 11 molecules, MVK, PMVK and MVD are involved in the formation of isopentenyl- pyrophosphate, which is the substrate of cholesterol synthesis; CYP51A1, DHCR7, DHCR24 and EBP are enzymes that catalyze the formation of cholesterol from lanosterol, which is the terminal process of cholesterol biosynthesis. The down-regulation of these genes indicates a possible reduction of the overall cholesterol synthesis in tumor cells. Metformin has been reported to inhibit fatty acid synthesis by decreasing the expression of acetyl CoA carboxylase, fatty acid synthase and citrate lyase, which is related to the blocking of tumorigenesis52,53. Recently, metformin was also shown to inhibit the cholesterol biosynthesis rate in macrophages54. Our results further support the inhibitive effect by metformin on cholesterol biosynthesis and provide more potential molecular targets of metformin in this pathway. Considering the importance of cholesterol synthesis in tumor development, the remodeling of the cholesterol metabolism by metformin and aspirin may be a novel mechanism of their antitumor activity.
Another canonical pathway worth noting is “Axonal Guidance Signaling”. Axonal guidance pathway plays an important role in neuronal extension and location during embryo development55. Recently, accumulating evidence indicates that axonal guidance pathway is also involved in tumor development and progression by regulating tumor cell migration, cell death and angiogenesis in various cancers, including pancreatic cancer56. UNC5A, UNC5B and UNC5C, which are the receptors of netrin 1, are all down-regulated in various cancers through promoter methylation56. However, the mechanism by which UNC5 proteins exert tumor suppressive effects is still unclear. According to our results, the expression of UNC5B in metformin-aspirin treated cells is up-regulated to 11 folds compared to untreated cells, indicating a possible role of UNC5B in the anti-cancer effect induced by the combination of metformin and aspirin. We also showed that four members of the class 3 semaphorin family were down-regulated by metformin and aspirin, including two pro-tumor semaphorins, SEMA3C and SEMA3E. Both SEMA3C and SEMA3E have been shown to be overexpressed in various cancers57. The overexpression of SEMA3C and SEMA3E in cancer cells correlates with multidrug resistance and increased metastatic capability58. Since axonal guidance pathway involves hundreds of genes, further investigations are needed to elucidate the effect of metformin and aspirin on this signaling pathway.
In summary, our study provided a quantitative gene expression profile of a pancreatic cancer cell line treated with metformin and aspirin, alone or in combination. We also analyzed the altered biological networks and pathways induced by metformin and aspirin. These findings provide new insight for the exploration of molecular mechanisms underlying the anti-cancer effect of metformin and aspirin in pancreatic cancer. However, future studies are needed to verify whether these altered gene expressions occur under physiologic conditions and contribute to the antineoplastic activity of metformin and aspirin in pancreatic cancer or other cancers.
Materials and Methods
Cell culture
Human pancreatic cancer cell line PANC-1 was purchased from the American Type Culture Collection (Manassas, VA, USA). PANC-1 cells were maintained in Dulbecco’s Modified Eagle’s Media (DMEM) (Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Sigma-Aldrich, St Louis, MO) at 37 °C and 5% CO2. PANC-1 cells were seeded in 6-well plates (5 × 105 cells per well). 24 hours later, cells were untreated or treated by 5 mM metformin, 2 mM aspirin or a combination of metformin (5 mM) and aspirin (2 mM) for 48 hours.
mRNA library preparation and sequencing
Total RNA was isolated from untreated and treated PANC-1 cells with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) followed by clean up with RNeasy Mini kit (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions. Quantity and quality of DNase treated RNA were determined using Agilent 2100 Bioanalyzer (RNA Nano 6000) and Nanodrop, respectively. A total of 16 RNA samples were sequenced [4 samples per condition × 4 conditions (untreated control, metformin, aspirin or a combination of metformin and aspirin)] using 500 ng of total RNA as input. Library preparation and RNA sequencing was carried out using the Illumina TruSeq™ RNA Sample Prep Kit v2 according to the manufacturer’s protocol (Illumina, San Diego, CA, USA). Samples were sequenced on the Illumina HiSeq 2500, 2 × 100 bp paired-end reads, to a minimum depth of 30 million reads per sample.
Computational analyses of RNA-seq data
A total of 240 million obtained reads of high quality clean tags were mapped and annotated human reference genome using Bioconductor package biomaRt (http://www.bioconductor.org)59. Mapped reads with mapping quality 10 or more were defined as uniquely mapped reads and used in the downstream analyses. Averagely, about 70.0% reads can be uniquely mapped to the annotated human genome. Ranking of genes by degree of differential expression was performed using the Bioconductor edgeR package version 2.14 (http://www.bioconductor.org) and in-house code developed in the R statistical language (http://www.r-project.org). Significant genes were arbitrarily identified by using the cutoff values of P ≤ 1 × 10−5 and fold change (FC) in mean expression of FC ≥ |2|.
Biological network and pathway analysis
Biological networks and pathways related to metformin, aspirin and the combination were analyzed with Ingenuity Pathway Analysis (IPA) software (Qiagen, CA, USA). The lists of all genes identified in gene expression analysis were uploaded into the IPA software. For the analysis of networks and pathways, the cutoff values are set as P ≤ 1 × 10−5 and FC ≥ |2|.
Validation of RNA-seq results by qRT-PCR
Quantitative RT-PCR was used to validate the differentially expressed genes. Expression of mRNA was determined in all 4 samples using Power SYBR® Green RNA-to-CT™ 1-Step Kit (Life Technologies, CA, USA) according to the manufacturer’s protocol. All of the primers used in qRT-PCR were purchased from Qiagen (Limburg, Netherland), and reference numbers used are: NPTX1 (PPH10301B), SLC20A1 (PPH11164A), DUSP15 (PPH18348B), HES7 (PPH20383B), NGFR (PPH00821A), PCDH18 (PPH10623A), CCL2 (PPH00192F), RASL11A (PPH14046A), FAM111B (PPH13923A), BMP5 (PPH00509A), GAPDH (PPH00150F), ACAT2 (PPH01591B), CYP51A1 (PPH01246A), DHCR7 (PPH06336F), EBP (PPH14187B), PMVK (PPH06350B), MVK (PPH06339A), DHCR24 (PPH02278F), FDPS (PPH01995A), LSS (PPH06366A), TM7SF2 (PPH08913B), LINGO1 (PPH08561A), EPHA7 (PPH05721F), FZD4 (PPH02427B), WNT7B (PPH02464C), PIK3R3 (PPH02312A), EFNB2 (PPH01144A), ROBO1 (PPH05962A), NRP1 (PPH01152A), PRKCA (PPH00977C), ABLIM3 (PPH15859A).
Western Blotting
The Western blotting for the selected proteins was performed, as previously described14. Briefly, the treated and untreated cells were rinsed with PBS and extracted on ice with cell lysis buffer (Cell signaling, Beverly, MA, USA). The protein concentrations were determined with BCA Protein Assay Kit (Thermo scientific, Waltham, MA, USA). 20 μg of total proteins from each sample were loaded and separated on a gradient 4–20% polyacrylamide gel and transferred to polyvinylidene difluoride (PVDF) membrane. Membranes were blocked with 5% fat-free milk in Tris-buffered saline-Tween 20 and incubated with the primary antibodies against RET and CCL2 from Cell Signaling Technology (Beverly, MA, USA), and antibody against ANGPTL4 from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Blots were subsequently washed and incubated with the appropriate HRP-conjugated secondary antibodies. The immunoreactive bands were visualized by enhanced chemiluminescence (Thermo Fisher, Rockford, IL USA) according to the manufacturer’s instructions. The levels of β-actin were estimated to check for equal samples loading.
Statistical Analysis
The results of RT-PCR were normalized to expression of GAPDH using the formula 2∆ CT. One-way ANOVA was used for comparing treatment with the combination of metformin and aspirin to the untreated control. A P value less than 0.05 was considered statistically significant.
Additional Information
How to cite this article: Yue, W. et al. Transcriptomic analysis of pancreatic cancer cells in response to metformin and aspirin: an implication of synergy. Sci. Rep. 5, 13390; doi: 10.1038/srep13390 (2015).
Supplementary Material
Acknowledgments
This work was supported by Rutgers Robert Wood Johnson Foundation research funds and the Functional Genomics Core Shared Resource of the Rutgers Cancer Institute of New Jersey (P30CA072720) and a grant (Grant No. 81470132) from the National Natural Science Foundation of China. We also thank RUCDR for the RNA sequencing.
Footnotes
Author Contributions W.Y., R.S.D. and X.L.T. designed the research; W.Y., T.W., E.Z., Y.L. and X.L.T. planned the statistical experimental design and analyzed data; W.Y., T.W., E.Z., Y.L., C.S.Y., Q.X. and X.L.T. conducted the research; W.Y., T.W., C.S.Y. and X.L.T. wrote the paper; W.Y. and X.L.T. had primary responsibility for the final content. All authors have reviewed and approved the final manuscript.
References
- Society, A. C. Cancer Facts & Figures 2014. Atlanta: American Cancer Society (2014).
- Yue W., Yang C. S., DiPaola R. S. & Tan X. L. Repurposing of metformin and aspirin by targeting AMPK-mTOR and inflammation for pancreatic cancer prevention and treatment. Cancer Prev Res (Phila) 7, 388–97 (2014). [DOI] [PubMed] [Google Scholar]
- Zhou G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108, 1167–74 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalender A. et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab 11, 390–401 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierotti M. A. et al. Targeting metabolism for cancer treatment and prevention: metformin, an old drug with multi-faceted effects. Oncogene 32, 1475–87 (2013). [DOI] [PubMed] [Google Scholar]
- Hirsch H. A., Iliopoulos D. & Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci USA 110, 972–7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch H. A., Iliopoulos D., Tsichlis P. N. & Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res 69, 7507–11 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley S. A. et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science 336, 918–22 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din F. V. et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology 142, 1504–15 e3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso L., Ai G., Spitale R. C. & Bhat G. J. Molecular targets of aspirin and cancer prevention. Br J Cancer 111, 61–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. R. et al. Aspirin induces apoptosis through the blockade of IL-6-STAT3 signaling pathway in human glioblastoma A172 cells. Biochem Biophys Res Commun 387, 342–7 (2009). [DOI] [PubMed] [Google Scholar]
- Deng X. S. et al. Metformin targets Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers. Cell Cycle 11, 367–76 (2012). [DOI] [PubMed] [Google Scholar]
- Wang Z., Gerstein M. & Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10, 57–63 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W. et al. Metformin combined with aspirin significantly inhibit pancreatic cancer cell growth in vitro and in vivo by suppressing anti-apoptotic proteins Mcl-1 and Bcl-2. Oncotarget (2015) [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovizio M., Bruno A., Tacconelli S. & Patrignani P. Mode of action of aspirin as a chemopreventive agent. Recent Results Cancer Res 191, 39–65 (2013). [DOI] [PubMed] [Google Scholar]
- Lalau J. D., Lemaire-Hurtel A. S. & Lacroix C. Establishment of a database of metformin plasma concentrations and erythrocyte levels in normal and emergency situations. Clin Drug Investig 31, 435–8 (2011). [DOI] [PubMed] [Google Scholar]
- Wilcock C. & Bailey C. J. Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica 24, 49–57 (1994). [DOI] [PubMed] [Google Scholar]
- Fantin V. R. & Leder P. Mitochondriotoxic compounds for cancer therapy. Oncogene 25, 4787–97 (2006). [DOI] [PubMed] [Google Scholar]
- Okochi-Takada E. et al. ANGPTL4 is a secreted tumor suppressor that inhibits angiogenesis. Oncogene 33, 2273–8 (2014). [DOI] [PubMed] [Google Scholar]
- Ng K. T. et al. Clinical relevance and therapeutic potential of angiopoietin-like protein 4 in hepatocellular carcinoma. Mol Cancer 13, 196 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani N. et al. Reduction of insulin signaling upregulates angiopoietin-like protein 4 through elevated free fatty acids in diabetic mice. Exp Clin Endocrinol Diabetes 120, 139–44 (2012). [DOI] [PubMed] [Google Scholar]
- Mulligan L. M. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer 14, 173–86 (2014). [DOI] [PubMed] [Google Scholar]
- Nair V. et al. Metformin inhibits pancreatic cancer cell and tumor growth and downregulates Sp transcription factors. Carcinogenesis 34, 2870–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair V. et al. Mechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: role of specificity protein (Sp) transcription factors. J Biol Chem 289, 27692–701 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuringa J. J. et al. MEN2A-RET-induced cellular transformation by activation of STAT3. Oncogene 20, 5350–8 (2001). [DOI] [PubMed] [Google Scholar]
- Borsig L., Wolf M. J., Roblek M., Lorentzen A. & Heikenwalder M. Inflammatory chemokines and metastasis–tracing the accessory. Oncogene 33, 3217–24 (2014). [DOI] [PubMed] [Google Scholar]
- Sanford D. E. et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res 19, 3404–15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baay M., Brouwer A., Pauwels P., Peeters M. & Lardon, F. Tumor cells and tumor-associated macrophages: secreted proteins as potential targets for therapy. Clin Dev Immunol 2011, 565187 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. W., Choi H. J., Ha S. J., Lee K. T. & Kwon Y. G. Recruitment of monocytes/macrophages in different tumor microenvironments. Biochim Biophys Acta 1835, 170–9 (2013). [DOI] [PubMed] [Google Scholar]
- Baldwin A. et al. Kinase requirements in human cells: V. Synthetic lethal interactions between p53 and the protein kinases SGK2 and PAK3. Proc Natl Acad Sci USA 107, 12463–8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S. et al. Specific peptide ligand for Grb7 signal transduction protein and pancreatic cancer metastasis. J Natl Cancer Inst 98, 491–8 (2006). [DOI] [PubMed] [Google Scholar]
- Oliveras-Ferraros C. et al. Acquired resistance to metformin in breast cancer cells triggers transcriptome reprogramming toward a degradome-related metastatic stem-like profile. Cell Cycle 13, 1132–44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar G. et al. Loss of WISP-2/CCN5 signaling in human pancreatic cancer: a potential mechanism for epithelial-mesenchymal-transition. Cancer Lett 254, 63–70 (2007). [DOI] [PubMed] [Google Scholar]
- Yang Z. et al. Epigenetic inactivation and tumor-suppressor behavior of NGFR in human colorectal cancer. Mol Cancer Res 13, 107–19 (2015). [DOI] [PubMed] [Google Scholar]
- Ma J., Jiang Y., Jiang Y., Sun Y. & Zhao X. Expression of nerve growth factor and tyrosine kinase receptor A and correlation with perineural invasion in pancreatic cancer. J Gastroenterol Hepatol 23, 1852–9 (2008). [DOI] [PubMed] [Google Scholar]
- Wang W. et al. Patterns of expression and function of the p75(NGFR) protein in pancreatic cancer cells and tumours. Eur J Surg Oncol 35, 826–32 (2009). [DOI] [PubMed] [Google Scholar]
- Hagihara A. et al. Identification of 27 5’ CpG islands aberrantly methylated and 13 genes silenced in human pancreatic cancers. Oncogene 23, 8705–10 (2004). [DOI] [PubMed] [Google Scholar]
- Mori Y. et al. Novel candidate colorectal cancer biomarkers identified by methylation microarray-based scanning. Endocr Relat Cancer 18, 465–78 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K., Jang S. W., Joshi J., Yoo M. H. & Ye K. Akt-phosphorylated PIKE-A inhibits UNC5B-induced apoptosis in cancer cell lines in a p53-dependent manner. Mol Biol Cell 22, 1943–54 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhang Z., Li Z. H. & Kong C. Z. Clinical significance of UNC5B expression in bladder cancer. Tumour Biol 34, 2099–108 (2013). [DOI] [PubMed] [Google Scholar]
- Okazaki S. et al. Clinical significance of UNC5B expression in colorectal cancer. Int J Oncol 40, 209–16 (2012). [DOI] [PubMed] [Google Scholar]
- Roth G. J., Machuga E. T. & Ozols J. Isolation and covalent structure of the aspirin-modified, active-site region of prostaglandin synthetase. Biochemistry 22, 4672–5 (1983). [DOI] [PubMed] [Google Scholar]
- Vane J. R. & Botting R. M. The mechanism of action of aspirin. Thromb Res 110, 255–8 (2003). [DOI] [PubMed] [Google Scholar]
- Alfonso L. F. et al. Aspirin inhibits camptothecin-induced p21CIP1 levels and potentiates apoptosis in human breast cancer cells. Int J Oncol 34, 597–608 (2009). [DOI] [PubMed] [Google Scholar]
- Marimuthu S. et al. Aspirin acetylates multiple cellular proteins in HCT-116 colon cancer cells: Identification of novel targets. Int J Oncol 39, 1273–83 (2011). [DOI] [PubMed] [Google Scholar]
- Kamble P., Selvarajan K., Aluganti Narasimhulu C., Nandave M. & Parthasarathy S. Aspirin may promote mitochondrial biogenesis via the production of hydrogen peroxide and the induction of Sirtuin1/PGC-1alpha genes. Eur J Pharmacol 699, 55–61 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passacquale G. et al. Aspirin-induced histone acetylation in endothelial cells enhances synthesis of the secreted isoform of netrin-1 thus inhibiting monocyte vascular infiltration. Br J Pharmacol 172, 3548–64 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim Mohammed A. M. T. Rilwanu Isah Tsamiya. Effects of acetylsalicylic acid and salicylic acid on the growth of HT3 cervical cancer cell line. American Journal of Pharmacy and Pharmacology 1, 32–44 (2014). [Google Scholar]
- Nelson E. R., Chang C. Y. & McDonnell D. P. Cholesterol and breast cancer pathophysiology. Trends Endocrinol Metab 25, 649–655 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabitova L., Gorin A. & Astsaturov I. Molecular pathways: sterols and receptor signaling in cancer. Clin Cancer Res 20, 28–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Daniels G., Lee P. & Monaco M. E. Lipid metabolism in prostate cancer. Am J Clin Exp Urol 2, 111–20 (2014). [PMC free article] [PubMed] [Google Scholar]
- Algire C., Amrein L., Zakikhani M., Panasci L. & Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr Relat Cancer 17, 351–60 (2010). [DOI] [PubMed] [Google Scholar]
- Bhalla K. et al. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prev Res (Phila) 5, 544–52 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren-Gluzer M., Aviram M. & Hayek T. Metformin inhibits macrophage cholesterol biosynthesis rate: possible role for metformin-induced oxidative stress. Biochem Biophys Res Commun 439, 396–400 (2013). [DOI] [PubMed] [Google Scholar]
- Nugent A. A., Kolpak A. L. & Engle E. C. Human disorders of axon guidance. Curr Opin Neurobiol 22, 837–43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P., Delloye-Bourgeois C. & Chedotal A. Novel roles for Slits and netrins: axon guidance cues as anticancer targets? Nat Rev Cancer 11, 188–97 (2011). [DOI] [PubMed] [Google Scholar]
- Rehman M. & Tamagnone L. Semaphorins in cancer: biological mechanisms and therapeutic approaches. Semin Cell Dev Biol 24, 179–89 (2013). [DOI] [PubMed] [Google Scholar]
- Gu C. & Giraudo E. The role of semaphorins and their receptors in vascular development and cancer. Exp Cell Res 319, 1306–16 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durinck S., Spellman P. T., Birney E. & Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc 4, 1184–91 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.