Abstract
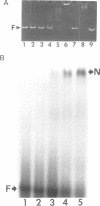
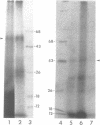
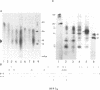
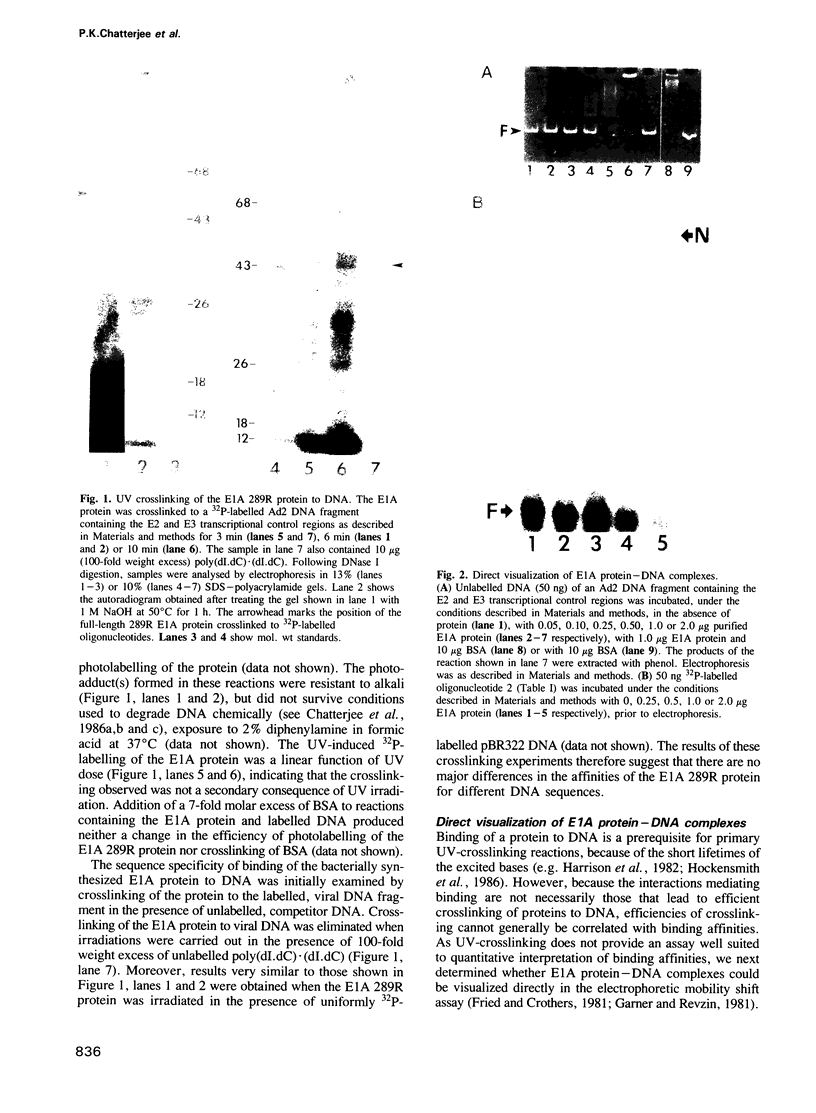
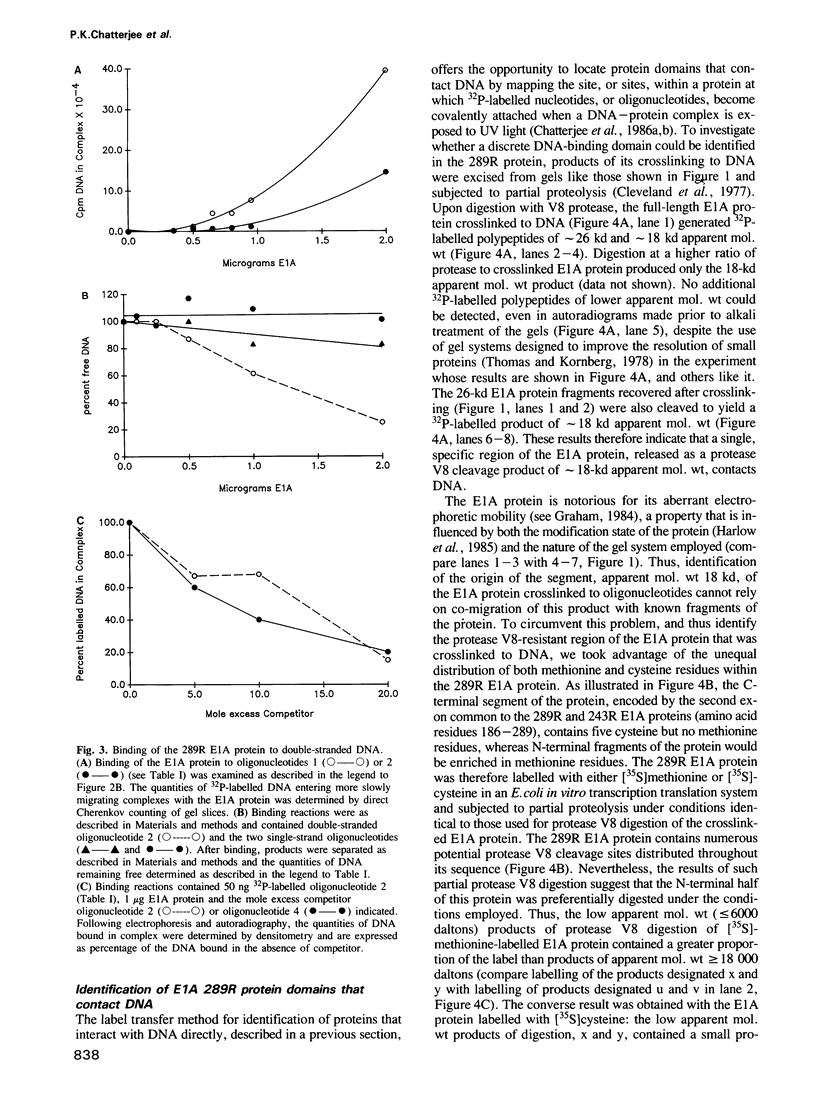
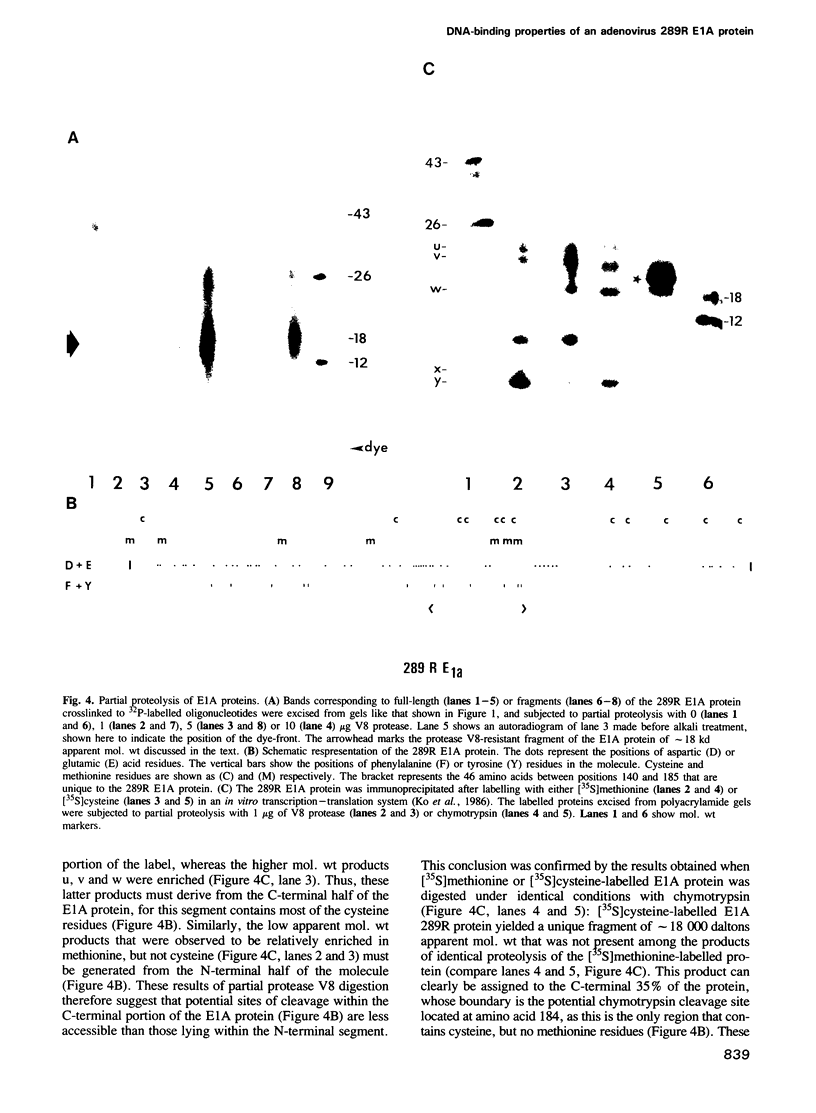
An adenovirus 2 289 amino acid (289R) E1A protein purified from Escherichia coli has been shown to interact with DNA by two independent methods. UV-crosslinking of complexes containing unmodified, uniformly 32P-labelled DNA and purified E1A protein induced efficient labelling of the protein with covalently attached oligonucleotides, indicating that the E1A protein itself contacts DNA. Discrete nucleoprotein species were also observed when E1A protein--DNA complexes were analysed by gel electrophoresis. Although the 289R E1A protein exhibited no significant binding to single-stranded DNA or to RNA, no evidence for its sequence-specific binding to double-stranded DNA was obtained with either assay. Identification of the sites of covalent attachment of 32P-labelled oligonucleotides by partial proteolysis of the crosslinked E1A protein indicated that the interaction of this protein with DNA is mediated via domain(s) in the C-terminal half of the protein. Such previously unrecognized DNA-binding activity is likely to contribute to the regulatory activities of this important adenoviral protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Chatterjee P. K., Flint S. J. Partition of E1A proteins between soluble and structural fractions of adenovirus-infected and -transformed cells. J Virol. 1986 Dec;60(3):1018–1026. doi: 10.1128/jvi.60.3.1018-1026.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P. K., Vayda M. E., Flint S. J. Adenoviral protein VII packages intracellular viral DNA throughout the early phase of infection. EMBO J. 1986 Jul;5(7):1633–1644. doi: 10.1002/j.1460-2075.1986.tb04406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P. K., Vayda M. E., Flint S. J. Identification of proteins and protein domains that contact DNA within adenovirus nucleoprotein cores by ultraviolet light crosslinking of oligonucleotides 32P-labelled in vivo. J Mol Biol. 1986 Mar 5;188(1):23–37. doi: 10.1016/0022-2836(86)90477-8. [DOI] [PubMed] [Google Scholar]
- Chatterjee P. K., Yang U. C., Flint S. J. Comparison of the interactions of the adenovirus type 2 major core protein and its precursor with DNA. Nucleic Acids Res. 1986 Mar 25;14(6):2721–2735. doi: 10.1093/nar/14.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Farmerie W. G., Loeb D. D., Casavant N. C., Hutchison C. A., 3rd, Edgell M. H., Swanstrom R. Expression and processing of the AIDS virus reverse transcriptase in Escherichia coli. Science. 1987 Apr 17;236(4799):305–308. doi: 10.1126/science.2436298. [DOI] [PubMed] [Google Scholar]
- Feldman L. T., Nevins J. R. Localization of the adenovirus E1Aa protein, a positive-acting transcriptional factor, in infected cells infected cells. Mol Cell Biol. 1983 May;3(5):829–838. doi: 10.1128/mcb.3.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B., Krippl B., Andrisani O., Jones N., Westphal H., Rosenberg M. E1A 13S and 12S mRNA products made in Escherichia coli both function as nucleus-localized transcription activators but do not directly bind DNA. Mol Cell Biol. 1985 Oct;5(10):2653–2661. doi: 10.1128/mcb.5.10.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J. Regulation of adenovirus mRNA formation. Adv Virus Res. 1986;31:169–228. doi: 10.1016/s0065-3527(08)60264-x. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer T. M., Erikson R. L. Rous sarcoma virus transforming protein, p60src, expressed in E. coli, functions as a protein kinase. Nature. 1981 Dec 24;294(5843):771–773. doi: 10.1038/294771a0. [DOI] [PubMed] [Google Scholar]
- Harlow E., Franza B. R., Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Whyte P., Franza B. R., Jr, Schley C. Association of adenovirus early-region 1A proteins with cellular polypeptides. Mol Cell Biol. 1986 May;6(5):1579–1589. doi: 10.1128/mcb.6.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C. A., Turner D. H., Hinkle D. C. Laser crosslinking of E. coli RNA polymerase and T7 DNA. Nucleic Acids Res. 1982 Apr 10;10(7):2399–2414. doi: 10.1093/nar/10.7.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockensmith J. W., Kubasek W. L., Vorachek W. R., von Hippel P. H. Laser cross-linking of nucleic acids to proteins. Methodology and first applications to the phage T4 DNA replication system. J Biol Chem. 1986 Mar 15;261(8):3512–3518. [PubMed] [Google Scholar]
- Kingston R. E., Baldwin A. S., Sharp P. A. Transcription control by oncogenes. Cell. 1985 May;41(1):3–5. doi: 10.1016/0092-8674(85)90049-2. [DOI] [PubMed] [Google Scholar]
- Ko J. L., Dalie B. L., Goldman E., Harter M. L. Adenovirus-2 early region IA protein synthesized in Escherichia coli extracts indirectly associates with DNA. EMBO J. 1986 Jul;5(7):1645–1651. doi: 10.1002/j.1460-2075.1986.tb04407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J. L., Harter M. L. Plasmid-directed synthesis of genuine adenovirus 2 early-region 1A and 1B proteins in Escherichia coli. Mol Cell Biol. 1984 Aug;4(8):1427–1439. doi: 10.1128/mcb.4.8.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Green M., Green M. R. An adenovirus E1a protein region required for transformation and transcriptional repression. Cell. 1986 Sep 26;46(7):1043–1051. doi: 10.1016/0092-8674(86)90704-x. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Loewenstein P. M., Green M. R., Green M. Functional domains of adenovirus type 5 E1a proteins. Cell. 1987 Sep 25;50(7):1091–1100. doi: 10.1016/0092-8674(87)90175-9. [DOI] [PubMed] [Google Scholar]
- Merrill B. M., Williams K. R., Chase J. W., Konigsberg W. H. Photochemical cross-linking of the Escherichia coli single-stranded DNA-binding protein to oligodeoxynucleotides. Identification of phenylalanine 60 as the site of cross-linking. J Biol Chem. 1984 Sep 10;259(17):10850–10856. [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Moran E., Zerler B., Harrison T. M., Mathews M. B. Identification of separate domains in the adenovirus E1A gene for immortalization activity and the activation of virus early genes. Mol Cell Biol. 1986 Oct;6(10):3470–3480. doi: 10.1128/mcb.6.10.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H., Kutter E., Nakanishi M. A restriction map of the bacteriophage T4 genome. Mol Gen Genet. 1980;179(2):421–435. doi: 10.1007/BF00425473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Paradiso P. R., Nakashima Y., Konigsberg W. Photochemical cross-linking of protein . nucleic acid complexes. The attachment of the fd gene 5 protein to fd DNA. J Biol Chem. 1979 Jun 10;254(11):4739–4744. [PubMed] [Google Scholar]
- Park C. S., Hillel Z., Wu C. W. DNA strand specificity in promoter recognition by RNA polymerase. Nucleic Acids Res. 1980 Dec 11;8(23):5895–5912. doi: 10.1093/nar/8.23.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt R. C., Fahnestock M. L., Lewis J. B. Differential nuclear localization of the major adenovirus type 2 E1a proteins. J Virol. 1987 Feb;61(2):247–255. doi: 10.1128/jvi.61.2.247-255.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J. F., Fisher F., Goding C. R., Jones N. C. Mutational analysis of the adenovirus E1a gene: the role of transcriptional regulation in transformation. EMBO J. 1987 Jul;6(7):2053–2060. doi: 10.1002/j.1460-2075.1987.tb02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R., Bruner M., Dalie B., Harter M. L. Activation of adenovirus promoters by the adenovirus E1A protein in cell-free extracts. Science. 1987 Aug 28;237(4818):1044–1046. doi: 10.1126/science.2956686. [DOI] [PubMed] [Google Scholar]
- Tanese N., Roth M., Goff S. P. Expression of enzymatically active reverse transcriptase in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4944–4948. doi: 10.1073/pnas.82.15.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. The study of histone--histone associations by chemical cross-linking. Methods Cell Biol. 1978;18:429–440. [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a proteins repress transcription from the SV40 early promoter. Cell. 1985 Mar;40(3):705–716. doi: 10.1016/0092-8674(85)90219-3. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Baltimore D. Localization of tyrosine kinase-coding region in v-abl oncogene by the expression of v-abl-encoded proteins in bacteria. J Biol Chem. 1985 Jan 10;260(1):64–71. [PubMed] [Google Scholar]
- Yee S. P., Branton P. E. Detection of cellular proteins associated with human adenovirus type 5 early region 1A polypeptides. Virology. 1985 Nov;147(1):142–153. doi: 10.1016/0042-6822(85)90234-x. [DOI] [PubMed] [Google Scholar]
- Zerler B., Moran B., Maruyama K., Moomaw J., Grodzicker T., Ruley H. E. Adenovirus E1A coding sequences that enable ras and pmt oncogenes to transform cultured primary cells. Mol Cell Biol. 1986 Mar;6(3):887–899. doi: 10.1128/mcb.6.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]