Abstract
An existing population pharmacokinetic model of darunavir in adults was updated using pediatric data from two studies evaluating weight-based, once-daily dosing of darunavir/ritonavir (ARIEL, NCT00919854 and DIONE, NCT00915655). The model was then used to provide once-daily dosing recommendations for darunavir/ritonavir in pediatric patients aged ≥3 to <12 years. The final model comprised two compartments with first-order absorption and apparent clearance dependent on the concentration of α1-acid glycoprotein. The recommended darunavir/ritonavir once-daily dosing regimens in children aged ≥3 to <12 years are: 35/7 mg/kg from 10 to <15 kg, 600/100 mg from 15 to <30 kg, 675/100 mg from 30 to <40 kg, and 800/100 mg for ≥40 kg. These doses should result in exposures similar to the adult exposure after treatment with darunavir/ritonavir 800/100 mg once daily, while minimizing pill burden and allowing a switch from suspension to tablet(s) as early as possible.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC? ☑ Darunavir/ritonavir is administered once daily in combination with other antiretrovirals for treatment-naïve adolescents aged >12 years and adults, and for treatment-experienced patients who have no darunavir RAMs. Dosing recommendations for darunavir/ritonavir were needed for once-daily dosing in children 3 to <12 years old. • WHAT QUESTION DID THIS STUDY ADDRESS? ☑ This analysis used a model-based approach to provide darunavir once-daily dose recommendations for treatment-naïve (or treatment-experienced with no darunavir RAMs) pediatric patients aged ≥3 to <12 years to achieve exposures comparable to those achieved with darunavir/ritonavir 800 mg/100 mg once daily in adults. • WHAT THIS ANALYSIS ADDS TO OUR KNOWLEDGE ☑ This analysis gives guidance for once-daily darunavir/ritonavir dosing for treatment-naïve (or treatment-experienced with no darunavir RAMs) children aged ≥3 to <12 years according to weight band. • HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY AND THERAPEUTICS ☑ This analysis used a model-based approach to interpolate once-daily dosing recommendations for children 6 to <12 years old using pharmacokinetic data from children 3 to <6 years old and adolescents 12 to <18 years old.
There has been a dramatic decrease in the morbidity and mortality of human immunodeficiency virus (HIV)-1-infected children since the introduction of HIV-1 protease inhibitor (PI)-containing antiretroviral regimens.1,2 However, more treatment options in suitable formulations are still needed for HIV-1-infected children, especially those aged under 6 years. Determination of appropriate dosing regimens in this age group requires specific trials, particularly as there are significant risks associated with underdosing,3 such as treatment failure and development of resistance.
Darunavir is a PI that, when used in combination with low-dose ritonavir or cobicistat, significantly reduces viral load in treatment-naïve and -experienced HIV-1-infected adults.4–10 Darunavir is available as an oral suspension (100 mg/ml), with comparable bioavailability to the tablet formulations.11 Tablets for pediatric administration are available in strengths of 75, 150, and 600 mg; an 800 mg tablet is also available.12 A fixed-dose combination tablet of darunavir 800 mg with cobicistat 150 mg is currently only available for use in adults6; there is no fixed-dose combination of darunavir and ritonavir. Ritonavir is available as an oral 80 mg/ml solution and 100 mg capsule and tablet. A study of darunavir/cobicistat for pediatric use is currently ongoing (GS-US-216-0128: NCT02016924).
DELPHI studied the pharmacokinetics, efficacy, and safety of darunavir/ritonavir twice-daily dosing in treatment-experienced children and adolescents (6 to <18 years old).13 Two further pediatric trials examining the safety and efficacy of darunavir/ritonavir given with ≥2 active antiretroviral agents, ARIEL (NCT00919854)14 and DIONE (NCT00915655),15 have been conducted. The pharmacokinetics of once-daily darunavir/ritonavir have also been evaluated in a substudy of ARIEL14,16 and in DIONE. Once-daily dosing for darunavir/ritonavir was initially developed for adults, based on darunavir's relatively long elimination half-life, the desirability of regimen simplification, and the reduction of the required ritonavir daily dose.17 Clinical trials have established the efficacy and safety of once-daily darunavir/ritonavir for treatment-naïve adults and for treatment-experienced adults who harbor no darunavir resistance-associated mutations.7–10 The ARIEL substudy included treatment-experienced, HIV-1-infected, pediatric patients aged ≥3 to <6 years, and weighing between 10 and <20 kg. DIONE included treatment-naïve, HIV-1-infected, adolescent patients aged ≥12 to <18 years and weighing ≥40 kg. Toxicity observed in juvenile rats prevents darunavir/ritonavir use in children aged <3 years old.18
A population pharmacokinetic model for darunavir/ritonavir was previously established in adults.19 The model was shown to have adequate predictive performance and was, for example, able to describe darunavir pharmacokinetics following administration of a different formulation when a correction factor for bioavailability was included.19 For the present study, the structural model was conserved and the model parameters were adjusted with a dataset containing richly sampled plasma concentration–time profiles from adult data from the DUET-1 and -2 studies (NCT00255099 and NCT00254046, respectively)20–23 and from children aged ≥3 to <18 years receiving twice-daily darunavir based on data from DELPHI (NCT00355524)13 and ARIEL.14 The final model comprised two compartments with first-order absorption and apparent clearance dependent on the concentration of α1-acid glycoprotein (AAG). In the current analysis, the model was further adjusted using richly sampled darunavir plasma concentration–time profiles in the ARIEL substudy and in DIONE. The main objective of this analysis was to evaluate and recommend a once-daily darunavir/ritonavir dosing regimen in children aged ≥3 to <12 years to achieve exposures within the limits of 80% to 130% of the geometric mean exposure in adults receiving darunavir/ritonavir 800/100 mg once daily.
Methods
Study objective
The main objective was achieved by: first, adjusting the population pharmacokinetic model of darunavir to include data from treatment-experienced, HIV-1-infected children between the ages of ≥3 to <6 years (ARIEL substudy)14,16 and treatment-naïve, HIV-1–infected, adolescent patients between the ages of ≥12 to <18 years (DIONE study),15 who received once-daily darunavir/ritonavir. Second, providing individual estimates and summary statistics of AUCtau, C0h, average concentration at steady state calculated from AUCtau (Css,ave), and CL/F for once-daily darunavir/ritonavir for these age groups. Lastly, performing simulations using the adjusted model parameters for various once-daily darunavir/ritonavir dosing scenarios to provide the pediatric dosing recommendations in HIV-1-infected children aged ≥3 to <12 years. For children aged 6 to <12 years, once-daily dosing recommendations were to be derived from model-based simulations.
Studies included in the analysis
The database used in this analysis included existing plasma concentration–time datasets from two pediatric studies: ARIEL;14 DELPHI13 after 2 weeks of treatment; two adult studies, DUET-1 and DUET-2 studies after 4 weeks of treatment,24 and also incorporated datasets from the pediatric DIONE study and the ARIEL once-daily substudy after 2 weeks of treatment14–16 (Table 1). Patients who had missing darunavir concentrations (one patient) or no pharmacokinetic observations (four patients) were omitted from the analysis. Darunavir concentrations without a time-matching quantifiable ritonavir concentration were excluded because such samples likely demonstrate nonadherence by the patient and no pharmacoenhancement of darunavir could, therefore, be expected.
Table 1.
Overview of the datasets used to adjust the darunavir population pharmacokinetic model
| Dataset | DUET-1 and DUET-2 | DELPHI | ARIEL | ARIEL substudy | DIONE |
|---|---|---|---|---|---|
| Patients (n) | 30 | 41 | 24 | 10* | 12 |
| Dose of darunavir/ritonavir | 600/100 mg BID | 300–600/50–100 mg BID | 20/3 mg/kg BID | 40/7 mg/kg QD for <15 kg | 800/100 mg QD |
| 600/100 mg BID for ≥15 kg | |||||
| Age range (years) | 18–66 | 6–17 | 3–5 | 3–5 | 12–17 |
| Bodyweight, range (kg) | 45–96 | 20–49 | 12–19 | 13–19 | 40–62 |
| AAG, range (mg/dL) | 54–347 | 48–312 | 63–156 | 56–125 | 52–120 |
| Samples per patient (n) | 8 | 5 | 5 | 6 | 6 |
| Time, range (h) | 0–12 | 0–12 | 0–12 | 0–24 | 0–24 |
| Darunavir formulation | Tablets 300 mg | Tablets 300 mg & 75 mg | Suspension 100 mg/mL | Suspension 100 mg/mL | Tablets 400 mg |
The 10 patients in the ARIEL QD substudy are also included in the 24 patients of the main ARIEL BID study.
The database included an adult dataset from the DUET-1 and DUET-2 trials because these data had similar sampling frequencies (five samples taken in children, eight in adults), and a similar timeframe after treatment (2 weeks in children, 4 weeks in adults) compared with the pediatric studies. The data from DUET came from patients who received darunavir/ritonavir without coadministration of etravirine (but with other concomitant antiretrovirals from the NRTI class with optional use of enfuvirtide).
Dosing
In the ARIEL trial,14 darunavir was given as an oral 100-mg/ml suspension while ritonavir was administered as an oral 80-mg/ml solution. The ARIEL substudy14,16 evaluated the pharmacokinetics of once-daily darunavir/ritonavir in treatment-experienced children ≥3 to <6 years old and weighing between 10 and <20 kg. The total darunavir/ritonavir dose was 40/7 mg/kg once daily for those weighing <15 kg and 600/100 mg once daily for those weighing ≥15 kg. This dosing was based on previous simulation of darunavir exposures using data from the main ARIEL trial, taking into account bodyweight, AAG levels, dosing convenience, and minimizing risk of underdosing. The DIONE study15,16 evaluated the pharmacokinetics of darunavir/ritonavir 800/100 mg once daily in treatment-naïve adolescent patients ≥12 to <18 years old, weighing ≥40 kg. Darunavir was given as 400 mg tablets and ritonavir was given as capsules. The dosing data for both the ARIEL and DIONE trials are presented in Table 2.
Table 2.
Dosing table for darunavir/ritonavir once daily intake per weight band for both the ARIEL and DIONE trials
| Bodyweight (kg) | Darunavir dose, QD (mg) | Ritonavir dose, QD (mg) |
|---|---|---|
| 10–10.9 | 400 | 66 |
| 11–11.9 | 440 | 72 |
| 12–12.9 | 480 | 76 |
| 13–13.9 | 520 | 86 |
| 14–14.9 | 560 | 92 |
| 15–19.9 | 600 | 100 |
| ≥40 | 800 | 100 |
Bioanalysis
Plasma concentrations of darunavir and ritonavir were assessed using a previously validated liquid chromatography-tandem mass spectrometry method with a lower limit of quantification of 5.00 ng/ml for both compounds.25
Model
The model was run using NONMEM 7 level 1.0 (ICON Development Solutions, Ellicott City, MD) with a GFortran (http://gcc.gnu.org/) compiler (Supplementary Information). Parameter estimations were done with the First-Order Conditional Estimation method with interaction using untransformed concentrations. All data management, evaluation of goodness-of-fit, and visual predictive checks and simulations were performed using R version 3.0.1.26
The initial model was an update of a previous population pharmacokinetic model of darunavir/ritonavir established in adults and adjusted for the clinical trial/commercial tablet formulation19 and for children ≥3 to <18 years old receiving twice-daily darunavir. It is a two-compartment model with first-order absorption (parameterized as KA, and fixed to the previously obtained value of 0.455 h−1). Apparent clearance was dependent on AAG levels and bodyweight. The distribution compartment was parameterized in terms of V3/F and Q/F (both fixed values of 84.3 l and 15 l/h, respectively).
The apparent oral clearance of darunavir is described by the following equation:
where CL/Fi = individual apparent oral clearance, CLint/F = population estimate of apparent intrinsic clearance, KAFF = affinity constant for AAG, given a fixed value of 0.0304, AAGi = individual AAG concentration, θ = influence of bodyweight [WTi] on apparent clearance, ηi = individual random effect, Frel = relative bioavailability correction for commercial tablet formulation and oral suspension compared with the formulation used in the previous model, given a fixed value of 1.18.
The apparent volume of distribution of the central compartment (V2/Fi) is assumed to be dependent on bodyweight, as described by the following equation:
After incorporating DIONE and ARIEL once-daily substudy datasets into the model, from the graphical exploration (Supplementary Figure 1) it was concluded that no modification of the structural model was deemed necessary. However, longer sampling times due to the 24-hour dose interval after once-daily darunavir administration allowed more reliable information on the distribution of the compound to be obtained, expressed as V2/F, Q/F, and V3/F. Hence, all the pharmacokinetic parameters were determined with the exception of KAFF and Frel, which were fixed to the values obtained during the original model development. The interindividual variability on CL/F, Q/F, and KA were determined, together with the multiplicative residual error.
The model-building dataset was then used for visual predictive check simulations (N = 100) using the newly estimated parameters, and CL/F was determined using noncompartmental approaches for both observed and simulated data. A standard visual predictive check would not be informative. However, this visual predictive check is deemed important because the purpose of the model is to investigate the influence of covariates on model-inferred clearance. The simulated and observed values of the parameters were compared for bodyweight, AAG concentration, age, and total once-daily darunavir dose.
Individual pharmacokinetic parameter estimates
Individual darunavir pharmacokinetic parameters for patients administered with once-daily darunavir/ritonavir were derived by means of post hoc estimates. Simulation records were added to the dataset at the exact time of trough (0h) to obtain a precise estimation of the darunavir plasma concentration at the trough for each visit where a plasma concentration was available and for all patients. This allows steady-state C0h to be captured without being biased by the actual sampling time relative to the dose intake, which can vary from one patient to another.
For the ARIEL once-daily substudy, only one visit where rich sampling occurred was available, and the individual pharmacokinetic parameters were determined directly during the model parameter estimation step. For the week 24 analysis of the DIONE trial, richly (2 weeks) and sparsely sampled (8 and 24 weeks) concentrations were used. The individual pharmacokinetic parameters were obtained using empirical Bayesian estimation, performed with NONMEM, using the MAXEVAL=0 option in the $ESTIMATION record of the adjusted model. The following pharmacokinetic parameter estimates were determined: CL/F (l/h), AUCtau (μg·h/ml), calculated as dose/apparent clearance (CL/F); C0h (ng/ml); and Css,ave, calculated as the AUCtau/dosing interval (24 hours).
Summary statistics were determined, and AUCtau was compared to the AUCtau in adults when treated with darunavir/ritonavir 800/100 mg once daily (mean value of 93.0 μg·h/ml and a geometric mean value of 89.7 μg·h/ml, determined from ARTEMIS data [data on file]). The ARTEMIS mean exposure was deemed an appropriate target exposure given the demonstrated efficacy and safety in this trial,7–9 and that pharmacokinetic extrapolation from adults to pediatrics is considered sufficient with regard to antiretrovirals. As the target is the virus, it can be assumed that the pharmacodynamic properties in the pediatric population will be the same compared to the adult population, therefore requiring a similar exposure in both populations.27 Darunavir pharmacokinetics do not differ between treatment-naïve (i.e., ARTEMIS) and -experienced (i.e., ODIN) patients.
Simulations
Simulations using the final model parameters were performed for various once-daily darunavir dosing scenarios in antiretroviral treatment-naïve children aged ≥3 to <18 years old, taking into account available darunavir dose strengths, pill burden, and optimizing the possibility to switch from suspension to tablet as early as possible. Based on the typical value of apparent clearance obtained from the population pharmacokinetic model, the expected darunavir exposures (AUCtau) for different once-daily doses were simulated for the 5th, 50th (median), and 95th percentiles of the observed AAG values in the pediatric studies, as a function of bodyweight (10 to 65 kg). The AAG data were derived from all visits with available values in the ARIEL, DIONE, and DELPHI studies, with the 5th, 50th, and 95th percentiles being 56.4, 91.2, and 157 mg/dL, respectively.
The following regimens were simulated for darunavir, assuming the presence of ritonavir (7 mg/kg or 100 mg once daily) and taking the available darunavir tablet strengths (75, 400, and 600 mg tablets) and the 100 mg/ml darunavir oral suspension into account: 30, 35, and 40 mg/kg for the 10 to 15 kg weight band; 30 and 35 mg/kg and 600 mg for the 15 to 20 kg weight band; 600 and 675 mg for the 20 to 30 kg weight band; 675 and 800 mg for the 30 to 40 kg weight band; 800 mg for the ≥40 kg weight band.
The dose recommendations were proposed based on comparable (80% to 130%) simulated total exposures (AUCtau) as in antiretroviral treatment-naïve adults receiving darunavir/ritonavir 800/100 mg once daily.
Results
Patients
Rich sampling data were taken from a pooled dataset of patients across five studies (ARIEL, DELPHI, DIONE, DUET-1, and DUET-2) (Table 1). The overall dataset for pharmacokinetic parameter estimation consisted of a total of 659 darunavir plasma concentration–time observations, of which 423 derived from pediatric patients. The observations were from 102 patients: 72 children (≥3 to <18 years old) and 30 adults (≥18 years old). The dataset used to evaluate the individual darunavir exposures in the DIONE study after 24 weeks of treatment consisted of 115 darunavir plasma concentration–time observations from 12 children.
Pharmacokinetic parameter estimation
All structural parameters were well estimated, and interindividual variability could be detected for population estimates of apparent intrinsic clearance (CLint/F), apparent intercompartmental clearance (Q/F), and the first-order absorption rate constant (KA) (Table 3). The similarities and differences between the published model (developed for children from ≥3 to ≤18 years old after twice-daily darunavir administration) and the model update reported here (developed for children aged ≥3 to <18 years after both twice-daily and once-daily darunavir administration) are as follows:
There were only slight changes in CLint/F (from 51.2 l/h to 51.0 l/h) and apparent central volume of distribution (V2/F) parameter estimates (from 127 l to 137 l).
There was no change in the effect of bodyweight on apparent oral clearance (CL/F) and V2/F.
There were substantial changes in apparent peripheral volume of distribution (V3/F) (from 84.3 l to 254 l) and Q/F (from 15.0 l to 19.1 l/h) due to an improved estimation achieved with the extra information gathered from the once-daily dosing.
The interindividual variability on V2/F and V3/F was too small to be compared with the previous model.
Table 3.
Darunavir population pharmacokinetic parameters following update with DIONE and ARIEL once-daily substudy datasets
| Parameter | Parameter estimate | Parameter SEE (CV%) | IIV estimate (CV%) | IIV SEE (CV%) |
|---|---|---|---|---|
| CLint/F (l/h) | 51.0 | 4.7 | 28 | 20 |
| Influence of WT on CL/F | 0.504 | 11 | ||
| KAFF of AAG (dl/mg) | 0.0304 | — | ||
| V2/F (l) | 137 | 21 | ||
| Influence of WT on V2/F | 0.774 | 18 | ||
| Q/F (l/h) | 19.1 | 16 | 64 | 59 |
| V3/F (l) | 254 | 41 | ||
| KA (1/h) | 0.528 | 17 | 50 | 66 |
| Frel | 1.18 | — | ||
| Multiplicative residual error | 0.0717 | 12 |
AAG, α1-acid glycoprotein; CL/F, apparent oral clearance; CLint/F, population estimate of apparent intrinsic clearance; CV%, percent coefficient of variation; Frel, population estimate of the relative bioavailability correction for the commercial tablet formulation and oral suspension compared with the formulation used in the previous model, given a fixed value of 1.18; IIV, interindividual variability; KA, first-order input rate constant; KAFF = affinity constant for AAG, given a fixed value of 0.0304; Q/F, apparent intercompartmental clearance; SEE, standard error of the estimate; V2/F, apparent central volume of distribution; V3/F, apparent peripheral volume of distribution; WT, bodyweight.
There were minimal differences between the observed and expected trend of the data, with no noticeable structural bias (Figure 1). Distributions for individual estimates for the random effects of CLint/F, Q/F, and KA are shown in Figure 2. Minimal shrinkage was detected for CLint/F, while shrinkage was more substantial for population estimates of Q/F and KA. This shrinkage was likely due to Q/F and KA being estimated at steady-state, which yields little information on these parameters. However, this had no impact on the exposure simulations and therefore the model was fit for purpose.
Figure 1.
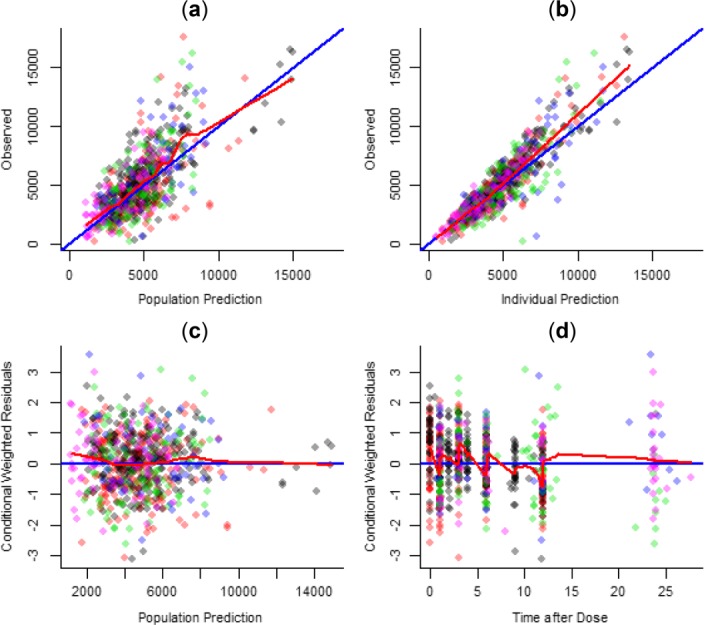
Basic goodness-of-fit plots for the model adjustment. (a) Observed vs. population prediction; (b) observed vs. individual prediction; (c) conditional weighted residuals vs. population prediction; (d) conditional weighted residuals vs. time after dose. Black dots represent adult DUET data, red dots DELPHI data, green dots ARIEL data, blue dots ARIEL QD substudy data, and pink dots DIONE data. Opacity is applied so that if points overlap, the overlapping area is darker.
Figure 2.
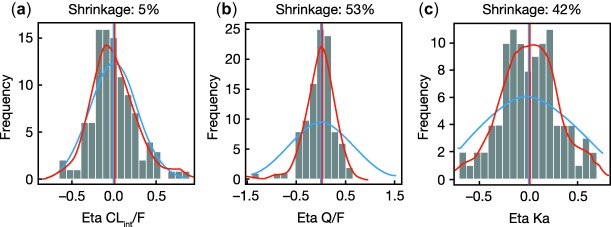
Random effects for (a) CLint/F, (b) Q/F, and (c) KA. The blue line represents the expected individual parameter distribution, and the red line the density line of the observed individual parameter distributions. CLint/F, population estimate of apparent intrinsic clearance; Q/F, intercompartmental clearance; KA, first-order absorption rate constant.
Visual predictive checks
The visual predictive checks of CL/F vs. all four covariates are shown in Figure 3. The model performed well over the range of covariates of interest: AAG concentration, bodyweight, darunavir dose, and age. Furthermore, the parameter values obtained from noncompartmental analysis based on the simulated profiles agreed well with model input data.
Figure 3.
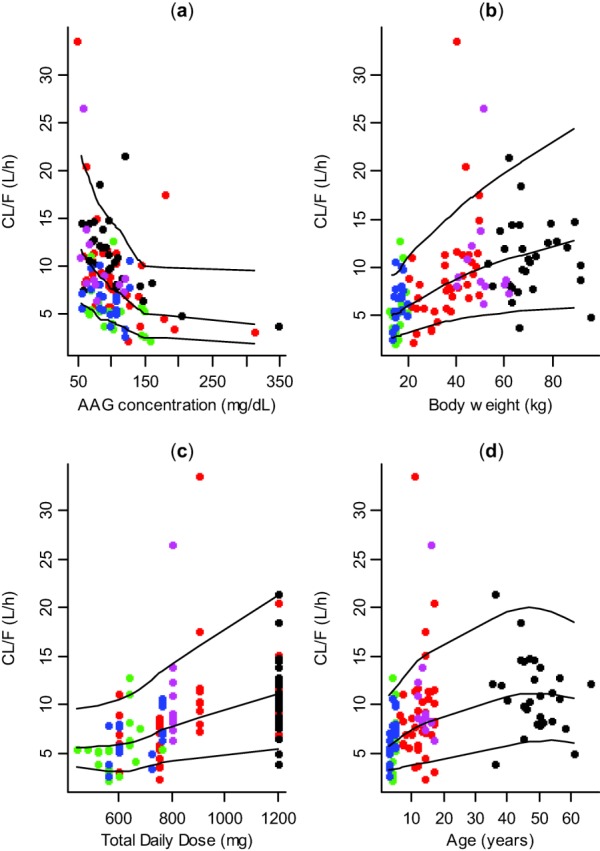
Visual predictive checks of CL/F vs. (a) α1-acid glycoprotein concentration (AAG); (b) bodyweight; (c) total daily darunavir dose; and (d) age. The dots represent the observed parameters determined by a noncompartmental analysis, the black lines represent the 5th, 50th (median), and 95th percentile (with smoothing applied) of the parameters based on simulated values. Black dots represent adult DUET data, red dots DELPHI data, green dots ARIEL data, blue dots ARIEL QD substudy data, and pink dots DIONE data.
Individual pharmacokinetic parameter estimates
The goodness-of-fit plots from the model adjustment containing only the data from the ARIEL once-daily substudy showed that the model predictions appeared to be accurate for children aged ≥3 to <6 years and weighing between 10 and <20 kg, with no detectable bias present for individual predictions, indicating that the model performed well (data on file).
The geometric mean darunavir area under the plasma concentration–time curve for each dosing interval (AUCtau) was 115 μg·h/ml, which represents 128% of the target geometric mean in adults treated with darunavir/ritonavir 800/100 mg once daily (89.7 μg·h/ml in the ARTEMIS trial, data on file). The mean exposure was on the upper side of the target because two individuals exhibited both high AAG values and low bodyweight, and therefore had low clearances and high exposures.
The goodness-of-fit plots for the Bayesian feedback analysis show that model predictions appeared to be accurate in treatment-naïve, adolescent patients ≥12 to <18 years old in the DIONE study treated for 24 weeks (data on file). The geometric mean darunavir AUCtau was 77.8 μg·h/ml, which represents 86.7% of the target geometric mean in adults treated with darunavir/ritonavir 800/100 mg once daily.
Exposure simulations
The expected darunavir exposures (AUCtau) are shown in Figure 4 with darunavir once-daily dosing of: 35 mg/kg dose, 10 to 15 kg bodyweight; 600 mg dose, 15 to 30 kg bodyweight; 675 mg dose, 30 to 40 kg bodyweight; 800 mg dose, 40 to 65 kg. The expected exposures with darunavir/ritonavir once-daily dosing that most closely matched adult exposure, based on visual inspection of the plots, formed the basis for treatment recommendations. The mean exposures of the regimens shown in Figure 4 are expected to be in the 80% to 130% range of the adult target (dosed with darunavir/ritonavir 800/100 mg once daily for average AAG concentrations). Simulation plots of all dosing strategies are shown in Supplemental Figure 2.
Figure 4.
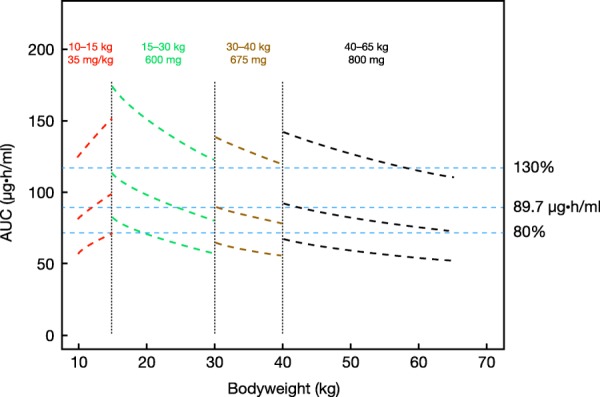
Expected darunavir exposures in children aged ≥3 to <18 years, weighing 10 to 65 kg at selected once-daily darunavir/ ritonavir regimens, given for the 5th, 50th (median), and 95th percentile of AAG concentrations, and compared to 80%, 100%, and 130% of the target exposure in adults (89.7 µg·h/ml, blue lines).
Discussion
A population pharmacokinetic model of darunavir was adjusted for children aged ≥3 to <18 years receiving twice-daily darunavir from a previous model in adults.19 The update to the model presented here was successfully performed using pharmacokinetic data from the DIONE (treatment-naïve adolescent patients ≥12 to <18 years old)15 and ARIEL substudy (treatment-experienced children ≥3 to <6 years old)14,16 to enable weight-based, once-daily darunavir dosing recommendations to be given in children from ≥3 to <12 years. While ritonavir levels were measured in DIONE and ARIEL and simultaneous population pharmacokinetic modeling of darunavir and ritonavir once daily has been described by other groups,28,29 ritonavir dosing was not modeled in the current analysis.
The model had adequate predictive performance and was deemed suitable for simulation purposes. The model was confirmed with pharmacokinetic parameters estimated using data from the ARIEL substudy and DIONE. The geometric mean exposure in pediatric patients would be deemed comparable to that in adults receiving darunavir/ritonavir 800/100 mg once daily if it fell within the predefined limits of 80% to 130% of the target adult exposure.30 This limits the risk of overexposure without compromising efficacy, because the lower range of AUCtau and trough plasma concentration (C0h) are within observations from adult data. The geometric mean darunavir AUCtau were 115 μg·h/ml in the ARIEL once-daily substudy and 77.8 μg·h/ml in DIONE, which were 128% and 87%, respectively, of the target geometric mean in adults treated with darunavir/ritonavir 800/100 mg once daily (89.7 μg·h/ml in the ARTEMIS trial). In the ARIEL once-daily substudy, two individuals exhibited high AAG values and low bodyweight giving low clearances and high exposures, which skewed the results for the dataset to the upper side.
As such, the mean exposure of these regimens is expected to be in the 80% to 130% of the adult target when treated with darunavir/ritonavir 800/100 mg once daily (ARTEMIS study) for average AAG concentrations. However, as shown in the estimated parameters from the ARIEL once-daily substudy, darunavir exposure could be higher in children with higher AAG concentrations. No association has been observed between darunavir exposure and the development of adverse events or laboratory abnormalities in previous trials of once-daily darunavir in adults.17,31
Simulations of potential darunavir/ritonavir once-daily regimens were applied to explore the resultant exposures. These, together with efficacy and safety data from the trials mentioned, were used to develop optimal once-daily darunavir/ritonavir dosing recommendations for treatment-naïve and treatment-experienced children aged ≥3 to <12 years old (with no darunavir resistance-associated mutations) (as in ODIN10).
For children weighing 10 to <15 kg, simulations from this model indicate that the dose should be 35/7 mg/kg darunavir/ritonavir once daily. From the ARIEL data, a darunavir/ritonavir dose of 40/7 mg/kg for children weighing 10 to <15 kg appeared to be appropriate to reach the target exposure in adults when treated with darunavir/ ritonavir 800/100 mg once daily. However, a darunavir/ ritonavir dose of 35/7 mg/kg is preferred in children weighing 10 to <15 kg, since the simulations show a better match with the adult exposure than those with 40/7 mg/kg, which may lead to overexposure (115% of geometric mean adult exposure vs. 100% of geometric mean adult exposure for 40/7 mg/kg and 35/7 mg/kg, respectively). In addition, limited safety data are available in this particular subpopulation as studied in ARIEL. For children weighing 15 to <30 kg, the dose should be 600/100 mg darunavir/ritonavir once daily to reach the target exposure in adults. Although this dose may seem high in patients weighing 15 to 20 kg, this regimen offers the opportunity to switch from a darunavir suspension to the tablet for patients weighing >15 kg, using only one 600 mg tablet. For children weighing 30 to <40 kg, the dose should be 675/100 mg darunavir/ritonavir once daily (75 and 600 mg tablets). A higher dose (800 mg) leads to high AUCtau and C0h and could exceed values observed in adults receiving darunavir/ritonavir 800/100 mg once daily. For children weighing ≥40 kg, the dose should be 800/100 mg darunavir/ritonavir once daily. This dose was deemed appropriate for these children (≥12 to <18 years) to reach the target exposure in adults.
In conclusion, we have adapted a pharmacokinetic two-compartment model with first-order absorption, and containing bodyweight and AAG as covariates, to provide recommendations on dosing in children aged ≥3 to <12 years dosed once daily with darunavir boosted with ritonavir and given with two other antiretroviral agents for the treatment of HIV-1. The model performed well, and allowed regimens to be recommended that should result in target pediatric exposure close to that observed in adults treated with darunavir/ritonavir 800/100 mg once daily. Using the model to interpolate once-daily dose recommendations for children aged 6 to <12 years, allowed optimal doses to be derived without the need to conduct a specific once-daily dosing study in this age group. Recommended pediatric doses are 35/7 mg/kg from 10 to <15 kg, 600/100 mg from 15 to <30 kg, 675/100 mg from 30 to <40 kg, and 800/100 mg for ≥40 kg.
Acknowledgments
We thank the patients and their families, investigators, study center staff, and Janssen study personnel, in particular those who reviewed and provided input into the analysis and/or article but who are not listed as authors. This study was sponsored by Janssen Research & Development, Beerse, Belgium. Medical writing support was provided by Ian Woolveridge, PhD, of Zoetic Science, an Ashfield company, Macclesfield, UK; this support was funded by Janssen Pharmaceuticals.
Author Contributions
A.B., T.N.K., T.V.D.C., M.O., F.L.T., A.V., and P.V. wrote the manuscript; A.B., T.N.K., T.V.D.C., M.O., F.L.T., A.V., and P.V. designed the research; A.B., T.N.K., T.V.D.C., M.O., F.L.T., A.V., and P.V. performed the research; A.B., T.N.K., T.V.D.C., M.O., F.L.T., A.V., and P.V. analyzed the data.
Conflict of Interest
A.B., T.N.K., T.V.D.C., M.O., F.L.T., and A.V. are full-time employees of Janssen. P.V. was a full-time employee of Janssen at the time of the analyses.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
References
- DHHS. 2014. Guidelines for the use of antiretroviral agents in pediatric HIV infection. < http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf > ( ). Accessed 19 November 2014.
- Brady MT, et al. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J. Acquir. Immune. Defic. Syndr. 2010;53:86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menson EN, et al. Underdosing of antiretrovirals in UK and Irish children with HIV as an example of problems in prescribing medicines to children, 1997-2005: cohort study. BMJ. 2006;332:1183–1187. doi: 10.1136/bmj.332.7551.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotet B, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369:1169–1178. doi: 10.1016/S0140-6736(07)60497-8. [DOI] [PubMed] [Google Scholar]
- Madruga JV, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomized controlled phase III trial. Lancet. 2007;370:49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- Tashima K, et al. Cobicistat-boosted darunavir in HIV-1-infected adults: Week 48 results of a Phase IIIb open-label single-arm trial. AIDS Res. Ther. 2014;11:39. doi: 10.1186/1742-6405-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz R, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naïve HIV-1-infected patients at week 48. AIDS. 2008;22:1389–1397. doi: 10.1097/QAD.0b013e32830285fb. [DOI] [PubMed] [Google Scholar]
- Mills AM, et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naïve, HIV-1-infected patients: 96-week analysis. AIDS. 2009;23:1679–1688. doi: 10.1097/QAD.0b013e32832d7350. [DOI] [PubMed] [Google Scholar]
- Orkin C, et al. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naïve patients in the ARTEMIS trial. HIV Med. 2013;14:49–59. doi: 10.1111/j.1468-1293.2012.01060.x. [DOI] [PubMed] [Google Scholar]
- Cahn P, et al. Week 48 analysis of once-daily vs. twice-daily darunavir/ritonavir in treatment-experienced HIV-1-infected patients. AIDS. 2011;25:929–939. doi: 10.1097/QAD.0b013e328345ee95. [DOI] [PubMed] [Google Scholar]
- Kakuda TN, et al. Pharmacokinetics of darunavir after administration of an oral suspension with low-dose ritonavir and with or without food. Clin. Pharmacol. Drug Dev. 2014;3:346–352. doi: 10.1002/cpdd.88. [DOI] [PubMed] [Google Scholar]
- Kakuda TN, et al. Bioavailability and bioequivalence of a darunavir 800-mg tablet formulation compared with the 400-mg tablet formulation. Int. J. Clin. Pharmacol. Ther. 2014;92:805–816. doi: 10.5414/cp202066. [DOI] [PubMed] [Google Scholar]
- Blanche S, et al. Pharmacokinetics, safety and efficacy of darunavir/ritonavir in treatment-experienced children and adolescents. AIDS. 2009;23:2005–2013. doi: 10.1097/QAD.0b013e328330abaa. [DOI] [PubMed] [Google Scholar]
- Violari A, et al. Safety and efficacy of darunavir/ritonavir in treatment-experienced pediatric patients: week 48 results of the ARIEL trial. Pediatr. Infect. Dis. J. 2015;34:e132–137. doi: 10.1097/INF.0000000000000644. [DOI] [PubMed] [Google Scholar]
- Flynn P, et al. Efficacy and safety of darunavir/ritonavir at 48 weeks in treatment-naïve, HIV-1–infected adolescents. Results from a phase 2 open-label trial (DIONE) Pediatr. Infect. Dis. J. 2014;33:940–945. doi: 10.1097/INF.0000000000000308. [DOI] [PubMed] [Google Scholar]
- Kakuda TN, Brochot A, van de Casteele T, Opsomer M. Tomaka F. 2013. Establishing darunavir dosing recommendations in treatment-naïve and treatment-experienced pediatric patients. 14th International Workshop on Clinical Pharmacology in HIV, 22–24 April, Amsterdam, The Netherlands. [DOI] [PMC free article] [PubMed]
- Kakuda TN, Brochot A, Tomaka FL, Vangeneugden T, Van De Casteele T. Hoetelmans RMW. Pharmacokinetics and pharmacodynamics of boosted once-daily darunavir. J. Antimicrob. Chemother. 2014;69:2591–2605. doi: 10.1093/jac/dku193. [DOI] [PubMed] [Google Scholar]
- Prescribing information. PREZISTA® (darunavir) tablet, film coated for oral use. < http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021976s007lbl.pdf >.
- Vis P, Sekar V, van Schaick E. Hoetelmans R. 2006. Development and application of a population pharmacokinetic model of TMC114 in healthy volunteers and HIV-1 infected subjects after administration of TMC114 in combination with low-dose ritonavir. 15th Annual Meeting of the Population Approach Group in Europe, 14–16 June, Bruges, Belgium. Abstract 964.
- Lazzarin A, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370:39–48. doi: 10.1016/S0140-6736(07)61048-4. [DOI] [PubMed] [Google Scholar]
- Madruga JV, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370:29–38. doi: 10.1016/S0140-6736(07)61047-2. [DOI] [PubMed] [Google Scholar]
- Katlama C, et al. Efficacy and safety of etravirine in treatment-experienced, HIV-1 patients: pooled 48 week analysis of two randomized, controlled trials. AIDS. 2009;23:2289–2300. doi: 10.1097/QAD.0b013e3283316a5e. 23. [DOI] [PubMed] [Google Scholar]
- Katlama C, et al. Efficacy and safety of etravirine at week 96 in treatment-experienced HIV type-1-infected patients in the DUET-1 and DUET-2 trials. Antivir. Ther. 2010;15:1045–1052. doi: 10.3851/IMP1662. [DOI] [PubMed] [Google Scholar]
- Kakuda TN, et al. Pharmacokinetics and pharmacodynamics of the non-nucleoside reverse-transcriptase inhibitor etravirine in treatment-experienced HIV-1-infected patients. Clin. Pharmacol. Ther. 2010;88:695–703. doi: 10.1038/clpt.2010.181. [DOI] [PubMed] [Google Scholar]
- Schöller-Gyüre M, et al. Pharmacokinetics of darunavir/ritonavir and TMC125 alone and coadministered in HIV-negative volunteers. Antivir. Ther. 2008;12:789–796. [PubMed] [Google Scholar]
- The R Project for Statistical Computing. http://www.r-project.org/ >. Accessed 19 November 2014.
- FDA Guidance for Industry. 2003. Exposure-Response Relationships—Study Design, Data Analysis, and Regulatory Applications, prepared by the U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). < http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm072109.pdf > ( ). Accessed 27 March 2015.
- Dickinson L, et al. Simultaneous population pharmacokinetic modeling of darunavir and ritonavir once daily in HIV-infected patients: evaluation of lower ritonavir dose. J. Int. AIDS Soc. 2012;15(Suppl 4):18331. [Google Scholar]
- Moltó J, et al. Simultaneous pharmacogenetics-based population pharmacokinetic analysis of darunavir and ritonavir in HIV-infected patients. Clin. Pharmacokinet. 2013;52:543–553. doi: 10.1007/s40262-013-0057-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jadhav PR, Lala M. Gobburu JV. Clarification on precision criteria to derive sample size when designing pediatric pharmacokinetic studies. J. Clin. Pharmacol. 2012;52:1601–1606. doi: 10.1177/0091270011422812. [DOI] [PubMed] [Google Scholar]
- Kakuda T, Brochot A, Tomaka F, van de Casteele T. Vangeneugden T. 2012. Generalized additive model analysis of the relationship between darunavir pharmacokinetics and pharmacodynamics following once–daily darunavir/ritonavir 800/100mg treatment in the Phase III trials ARTEMIS and ODIN. 11th International Congress on Drug Therapy in HIV Infection, 11–15 November, Glasgow, UK.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


