Abstract
Mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes comprises a number of mitochondrial disorders with a wide range of clinical presentations. We present the case of a 32-year-old patient with an m.3243A>T mitochondrial DNA mutation who presented with rhabdomyolysis after 2 days of excessive physical work. The case presented here demonstrates a new clinical phenotype associated with this pathogenic mtDNA mutation.
Background
Mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) comprises a number of mitochondrial disorders with a wide range of clinical presentations.1 Common symptoms in childhood follow a period of normal development and include muscle weakness, fatigue, recurrent migraine-like headaches, bowel problems, seizures and neuropsychological deficits. Stroke-like episodes often begin before the age of 40.2 We present the case of a 32-year-old patient with an m.3243A>T mitochondrial DNA mutation who presented with rhabdomyolysis after 2 days of excessive physical work.
Case presentation
A 32-year-old man reported with muscle ache in arms and legs after 2 days of excessive labour. The pain first occurred after a few hours of continuous work and persisted after returning home. At the same time, the patient noticed a darkening of his urine. The symptoms worsened during the following day and our patient experienced a pain in his right flank.
On clinical examination the patient had not shown any objective weakness or sensory loss. Neuropsychological and cognitive functions were normal. Reflexes in his arms and legs were normal. Serum creatine kinase was elevated to 23 360 U/l (reference <170 U/l) on the first day after admission and decreased to 10 080 U/l on the second day. The level normalised during the next 2 weeks. There was myoglobinuria. Electromyographic and nerve conduction studies revealed an increased insertion activity and small, polyphasic potentials with short duration which we interpreted as signs of myopathy. More detailed re-evaluation of the patient's history revealed muscle ache after physical exercise in the gym and that this symptom had been experienced during the past 3 years. This pain persisted despite continuous muscle training. However, the patient had not experienced muscle weakness, headache, loss of appetite and seizures until the episode before admission. Family history evaluation indicated that two cousins were diagnosed with diabetes mellitus, none of whom had complained about muscle ache or neurological problems.
Investigations
In order to narrow down the differential diagnosis of his myopathy, a muscle biopsy was taken from the musculus rectus femoris. Histological examination revealed changes compatible with mitochondrial myopathies (figure 1). Consecutive genetic analysis was performed by PCR and Sanger sequencing on DNA extracted from skeletal muscle. Direct sequencing revealed the m.3243A>T mutation in the tRNALeu (MT-TL1) gene of the mitochondrial DNA at an estimated heteroplasmy level of 30%.
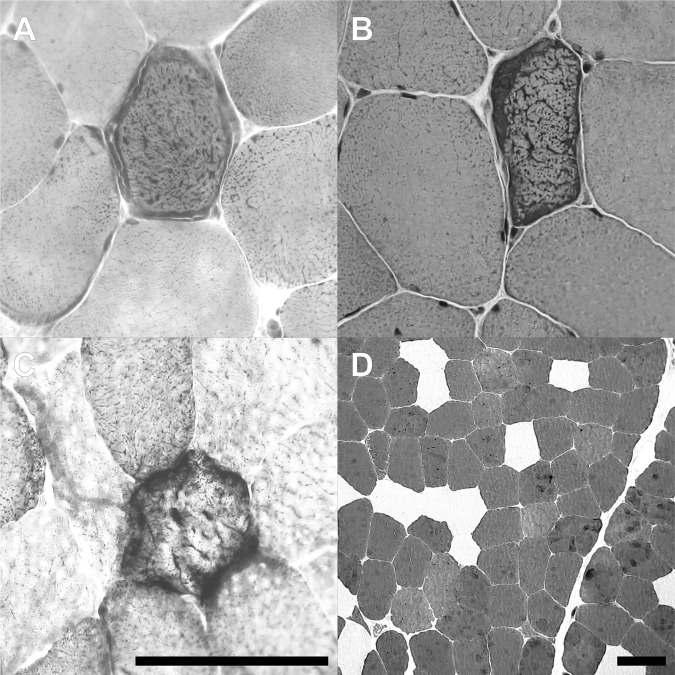
Figure 1.
Histochemical aspects of the muscle biopsy. Routine staining of frozen sections reveals multiple ragged red fibres in Gömöri trichrome (A) and H&E (B). Multiple fibres are negative for cytochrome C oxidase (COX) and hyper-reactive for succinate dhydrogenase (SDH) as demonstrated by combined COX/SDH enzymactic histochemistry (C). ATPase differentiation at pH 9.4 indicates a fibre type 2 predominance (dark fibres, D). Scale bars: 100 µm.
Differential diagnosis
Traumatic injuries
Viral infections
Myalgias from other aetiologies
Bacterial infections
Cold exposure
Malignant hyperthermia
Muscle phosphorylase deficiency
Phosphofructokinase deficiency
Carnitine palmityl transferase deficiency
Phosphoglycerate mutase deficiency
Hyperosmotic conditions
Guillain-Barré syndrome
Inflammatory myositis
Outcome and follow-up
As part of our counselling we suggested to avoid excessive physical workout for a month and our patient was compliant. However, he still reported exercise-induced myalgia even after mild physical activity.
Discussion
We present a patient with an m.3243A>T mutation in MT-TL1 who exhibited exercise induced muscle pain over 3 years, followed by an episode of acute rhabdomyolysis after excessive physical workout. This particular mtDNA mutation has previously been described in four patients only: a 9-year-old girl with lactic acidosis and multiple respiratory chain deficiencies,3 a 7-year-old girl with recurrent cerebral infarcts,4 a 22-year-old man with sensorineural deafness and short stature and a 41-year-old woman with chronic progressive external ophtalmoplegia (CPEO) and ptosis.5 Approximately 80% of patients with a MELAS syndrome exhibit an m.3243A>G mutation in MT-TL1. This mutation causes a wide range of phenotypes, including CPEO, cardiomyopathy, isolated myopathy, maternally inherited diabetes, deafness and isolated gastrointestinal complications. Muscle ache and rhabdomyolysis have only rarely been reported in MELAS patients and have previously been observed only in combination with other symptoms, in particular hypacusis, impaired glucose tolerance and stroke-like episodes.6–8
The case presented here demonstrates a new clinical phenotype associated with this pathogenic mtDNA mutation.
Learning points.
The rare m.3243A>T mutation is associated with lactic acidosis, multiple respiratory chain deficiencies, recurrent cerebral infarcts, sensorineural deafness and short stature and chronic progressive external ophtalmoplegia.
The case report broadens the spectrum of phenotype of this rare mtDNA mutation: muscle pain and rhabdomyolysis are also associated with this rare m.3243A>T mutation.
In patients with muscle pain and rhabdomyolysis in whom no other cause could be found a genetic analysis may be useful.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet 2005;6:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavlakis SG, Phillips PC, DiMauro S, et al. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol 1994;16:481–8. [DOI] [PubMed] [Google Scholar]
- 3.Shaag A, Saada A, Steinberg A, et al. Mitochondrial encephalomyopathy associated with a novel mutation in the mitochondrial tRNA leu(UUR) gene (A3243T). Biochem Biophys Res Commun 1997;233:637–9. [DOI] [PubMed] [Google Scholar]
- 4.Longo N, Schrijver I, Vogel H, et al. Progressive cerebral vascular de generation with mitochondrial encephalopathy. Am J Med Genet 2008;46:361–7. [DOI] [PubMed] [Google Scholar]
- 5.Alston CL, Bender A, Hargreaves IP, et al. The pathogenic m.3243A>T mitochondrial DNA mutation is associated with a variable neurological phenotype. Neuromusc Dis 2010;20:403–6. [DOI] [PubMed] [Google Scholar]
- 6.Deschauer M, Wieser T, Neudecker S, et al. Mitochondrial 3243 G mutation (MELAS mutation) associated with painful muscle stiffness. Neuromuscul Disord 1999;9:305–7. [DOI] [PubMed] [Google Scholar]
- 7.Chariot P, Abadia R, Agnus D, et al. Simvastatin-induced rhabdomyolysis followed by a MELAS syndrome. Am J Med 1993;94:109–10. [DOI] [PubMed] [Google Scholar]
- 8.Kwon JH, Kim JS. Rhabdomyolysis in a patient with MELAS syndrome. Eur Neurol 2003;50:123–4. [DOI] [PubMed] [Google Scholar]