Abstract
The remarkable health benefits of the chemical cocktails occluded within Vites vinefera (grapes) have been broadly used as dietary supplements and as natural pharmaceuticals in the treatment of various diseases including human cancer. Current discovery demonstrates the rapid formation of gold nanoparticles with the phytochemicals present in grapes, which serve a dual role as synergistic reducing agents to reduce gold salts into gold nanoparticles and also as stabilizers to provide a robust coating on the gold nanoparticles in a single step. Furthermore, the grape-generated gold nanoparticles (GAuNPs), have demonstrated remarkable in vitro stability on specific functionalization with peptides (GSH) and thiol-containing compounds (lipoic acid) followed by the induction of cell-specific response. In addition, the grape-generated gold nanoparticles (GAuNPs, GSH-GAuNPs, LA-GAuNPs) have demonstrated remarkable affinity towards human breast cancer cells (HBL-100) in the present study. These studies thus signified the cellular internalization of GAuNPs and its conjugates by transmission electron microscopy through endocytosis into cancer cells. Notably, at higher concentration of gold nanoparticles conjugate, there was an asymmetric accumulation of gold nanoparticles in the periphery of the cell nucleus of the HBL-100 cells which was confirmed by fluorescence microscopy. Other than gold salts, no “manmade” chemicals are used in this truly biogenic, green nanotechnological process which thereby paves the way for outstanding opening for their application in molecular imaging and cancer therapy.
Keywords: Green synthesis, Grape-gold nanoparticles, GSH-grape-gold nanoparticles, Lipoic acid-grape-gold nanoparticles, HBL-100 cells, Phytochemicals
Introduction
Cancer induction, growth, and progression are multistep events, and numerous studies have demonstrated that various dietary agents interfere with these stages of cancer of various organ sites thereby sharing great promise in our conquest to control human malignancies. Based on these encouraging observations, research efforts from across the globe have focused on identifying, characterizing, and providing scientific basis to the efficacy of various dietary phytonutrients in an effort to develop effective strategy to block malignancies. Fruits and vegetables represent untapped reservoir of various nutritive and nonnutritive phytochemicals with potential cancer chemopreventive activity. From the clue of “French paradox,” polyphenolics from grapes (Vites vinefera) and red wines attracted the attention of scientists to define their chemical composition and their properties for human health (Urpi et al. 2009). The reported evidences of beneficial health effects of phenolic compounds (Chacona et al. 2009) include inhibiting some degenerative diseases, such as cardiovascular diseases (Olas et al. 2008) and certain types of cancers (God et al. 2007) reducing plasma oxidation stress and slowing aging (Meyer et al. 1997). The antioxidative characteristics of phenolic compounds are mainly ascribed to their free-radical scavenging and metal-chelating properties, as well as their effects on cell signaling pathways and on gene expression (Soobrattee et al. 2005). Although grape polyphenols are widely used for the prevention and treatment of cancer, its therapeutic effects are always limited by severe adverse effects, such as the stability of biological activity in tissue, bioavailability in vivo, etc. (Chen and Dou 2008). There has been reported that the biological activity of polyphenols might depend on the form of their administration (Henning et al. 2005). To overcome these disadvantages and improve chemotherapeutic activity, researchers have focused on the development of nano-sized drug carriers (Forrest and Kwon 2008).
Nanoparticles have a specific capacity for drug loading, have efficient photoluminescence ability, and are therefore important materials in the targeted delivery of imaging agents and anticancer drugs. (Horcajada et al. 2010; Sajja et al. 2009). Drug carriers made up of nanoparticles (NP) are able to overcome biological barriers, accumulate preferentially in tumors, and specifically recognize single cancer cells for detection and treatment. As the nanorevolution in the realms of medical and technological applications unfolds, it is imperative to develop environmentally benign and biologically friendly green chemical processes (Studer et al. 2010). The utility of plant-based phytochemicals in the overall synthesis and the architecture of nanoparticles and various nanoparticle-embedded products are highly attractive as they bring an important symbiosis between natural or plant sciences and nanotechnology. Continuous demand for new anticancer drugs has stimulated chemotherapeutic research based on the use of metals since potential drugs developed in this way may be less toxic and more prone to exhibit antiproliferative activity against tumors (Shankar et al. 2004). The naturally grown plants which occlude phytochemicals may serve as long-lasting and environmentally benign reservoirs for the production of a myriad of gold nanoparticles. Recently, synthesis of Au and Ag nanoparticles using extracts of Cinnamomum camphora leaf (Huang et al. 2007), phyllanthin (Kasthuri et al. 2009), and Alfalfa sprouts (Gardea et al. 2003b) as a reducing and capping agent has been reported.
The utilities of NP strongly depend upon their physiochemical characteristics and their interaction with various surface moieties. Nanoparticles have to be surface modified to make them stable, biodegradable, biocompatible, and with high specificity for preparation of bioconjugate and some functional groups, such as cyano, thiol (Yonezawa et al. 2006), glutathione (Basu and Pal 2007), and amino groups (Subramaniam et al. 2005; Aslam et al. 2004) which are known to have high affinity for gold can be used as capping agents for gold nanoparticles. Such systems can limit the release of encapsulated materials more effectively (Chen et al. 2007). A major advantage of using these short peptide motifs is that they home in to the tumor vasculature, which is less dependent on the variability of receptors expressed directly on the tumor cell surface (Ruoslahti 2000). Hence, by incorporating appropriate peptides and thiol-rich molecules into a linkage between carrier and drug, it is possible to develop rapid release without appreciably contributing to drug loss during circulation in the central blood compartment.
While the tremendous health benefits of chemical cocktails present within grapes is beyond doubt, the actual applications of the chemical reduction power of the myriad of chemicals present in herbs and spices is still in infancy. Therefore, we investigated the synergistic potentials of polyphenols, flavonoids, catechins, and various phytochemicals present grape extract for the reduction reactions of gold salts to produce AuNps which have potential applications in the diagnosis and therapy of various deadly diseases including cancer. In this study, we synthesized a kind of novel gold nanoparticles conjugates using glutathione and lipoic acid as encapsulant materials for entrapment of grape polyphenols. The morphology, structure, and characteristic of the glutathione capped grape gold nanoparticles and lipoic acid capped grape gold nanoparticles were confirmed by UV–spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and Fourier transform infrared (FTIR). We also extended our communication to study the application of gold nanoparticles as carriers of grape polyphenols by determining its cytotoxicity in vitro against human breast cancer (HBL-100) cell lines and such studies are missing up to date to the best of our knowledge.
Experimental
Synthesis of GAuNPs, GSH-GAuNPs, and LA-GAuNPs
Grapes were washed with distill water to remove any traces of contaminants. Grapes were cut into small pieces and added into a conical flask containing 100 ml of Millipore water and boiled for 10 min and filtered using Whatmann filter paper. To 10 ml of grape extracts, 10 ml of 1-mM aurochlorate was added and heated to 75°C for 10 min. The color of the mixture changes from pale purple to dark purple. To the grape-gold colloid solution (25 mL), glutathione (20 mg) was added, and the mixture was stirred for 10–15 min. After the stirring was completed, the mixture was centrifuged at 4,500 rpm to separate the capped gold nanoparticles. The pellet obtained was resuspended in 1 mL of phosphate buffer (pH 7). A 600-μmol portion of α-lipoic acid in 10 mL of NaOH 0.5 M solution was added to 25 mL of freshly prepared citrate-capped Au NPs under stirring at room temperature (20–23°C). After 24 h, AuNPs capped with dihydrolipoic acid (DHLA), obtained from α-lipoic acid reduction, were dialyzed against phosphate-buffered saline (PBS) for 48 h using a 10-kDa cut-off dialysis bag (Interchim®, France). The dialysis medium was changed once to fresh PBS after 24 h. The resulting Au at DHLANPs solution was stored in the dark at 4°C for a maximum period of 2 months. The gold nanoparticles thus formed were separated immediately using a 5-μm filter and were characterized by UV–vis absorption spectroscopy FTIR and SEM analysis.
Characterization of GAuNPs, GSH-GAuNPs, and LA-GAuNPs
The stability and the identity of the grape-initiated gold nanoparticles (GAuNPs) were measured by recording UV absorbance after 30 min. The Plasmon resonance band at ∼535 nm confirmed the retention of nanoparticulates in all the above mixtures. X-ray diffraction pattern of dry nanoparticles powder was obtained using Siemens D 5005 X-ray diffractometer with CuKa radiation (l = 0.1542 nm).The FTIR spectra were obtained on a Nicolet 5700 FTIR instrument with the sample as KBr pellets. The morphology of the nanoparticles was analyzed using the high-resolution images obtained with a JEOL 3010 transmission electron microscope and scanning electron microscope.
In vitro studies: HBL-100 cell culture and maintenance
The cells were maintained in Dulbecco’s modified eagle’s medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 12.5% horse serum, 2.5% fetal bovine serum, 50 U/mL penicillin, and 5 mg/mL streptomycin—at an incubator setting of 5% CO2 and 37°C. All the experiments were carried out 24–48 h after cells were seeded. The cells were routinely harvested by trypsinization 0.25% when the cells approached sub-confluent stage and were placed on 25-cm culture flasks split into 1:6. Human breast cancer cell (HBL-100) cell line was purchased from NCCS Pune. GAuNPs was dissolved in PBS followed by ultra-sonification for five mints, and concentrations of 8 mg/10 ml were used for the various experiment.
Assay of in vitro cytotoxicity of GAuNPs, GSH-GAuNPs, and LA-GAuNPs in HBL-100 cells
The 3-(4,5-dimethylthazol-2-yl)-2,5-diphenyltetrazolium bromide blue-indicator dye (MTT)-based assay is a simple nonradioactive colorimetric assay to measure cell cytotoxicity, proliferation, or viability. MTT reduction was examined according to the methods of Mosmann (1983), with modification. HBL-100 cells were used for the analysis of cytotoxicity in vitro. The cells (1 × 106cells/mL) were placed into 96-well tissue-culture plates and incubated at 37°C. After 24 h, cells were treated with three different concentrations of GAuNPs, glutathione-capped grape gold nanoparticles (GSH-GAuNPs), and lipoic acid-capped-grape gold nanoparticles (LA-GAuNPs) (10, 50, 100, and 150 μg/mL, respectively). Untreated cells were used as controls. Plates were incubated in a humidified 5% CO2 balanced-air incubator at 37°C for 24, 48, and 72 h, respectively. Then, 10 μL of 5 mg/mL MTT solution was added to each well, and the plates were incubated for another 4 h, and then the medium was discarded. Dimethyl sulfoxide (100 μL) was added to each well, and the solution was vigorously mixed to dissolve tetrazolium dye. The absorbance of each well was measured by enzyme-linked immunosorbent assay reader (BioTek; Austria) at a test wavelength of 570 nm. Percentages of surviving cells to untreated controls were calculated by using the formula as % viability = [(At/As) × 100] %, where At and As indicate the absorbance of the sample and control, respectively.
Determinination of the integrity of cell membranes: the LDH leakage
The lactate dehydrogenase (LDH) assay is used to evaluate cell-membrane integrity because the release of this large (9–160 kDa) enzyme from the cytoplasmic compartment to the supernatant of cells is indicative of membrane damage. Based on the reduction of NAD by the action of LDH to form a tetrazolium dye, the amount of LDH was measured spectrophotometrically at 492 nm. The background absorbance measured at 660 nm was subtracted from the reading at 492 nm. After cells were exposed to different concentrations, GAuNPs, GSH-GAuNPs, and LA-GAuNPs (10, 50, 100, and 150 μg/mL, respectively) for 48 h, the medium was collected, and the amount of LDH release into the medium and the total LDH were determined, respectively.
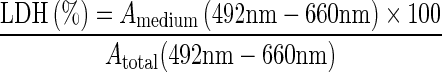 |
TEM analysis of surface characteristics of HBL-100 cells during internalization of GAuNPs, GSH-GAuNPs, and LA-GAuNPs
HBL-100 cells with a density of 1 × 106 cells/mL were grown in DMEM (Invitrogen) supplemented with 12.5% horse serum, 2.5% fetal bovine serum, 50 U/mL penicillin, and 5 mg/mL streptomycin—at an incubator setting of 5% CO2 and 37°C. After trypsinization and centrifugation, cell pellets were resuspended in Hank’s balanced salt solution for the exposure of 100 μg/mL of GAuNP’s and further incubated for 24 h at 37○C. The medium was then aspired, and the cell layer was rinsed three times with growth medium to remove any traces of uninternalized GAuNPs. About 0.5 mL of 0.1 M Trypsin-EDTA solution was added to each well to detach the cell layer from the plastic. Detached cells were dispersed in 4 mL of complete growth medium and gently pipette out of the well. The cell suspension was transferred into a centrifuge tube and centrifuged at approximately 125×g for 5 min. Supernatant was discarded, and cell pellet was embedded in paraffin, sectioned, and examined under transmission electron microscopy.
Assay of apoptosis induced by GAuNPs, GSH-GAuNPs, and LA-GAuNPs in HBL-100 cells DAPI staining assay
The fluorescent dye 4′,6-diamidino-2-phenylindole (DAPI) was used to detect the nuclear fragmentation that is a characteristic of apoptotic cells. PC12 cells (5 × 103 cells/well in 12-well plates) were incubated at 37°C with GSH-GAuNPs and LA-GAuNPs (50 or 100 μg/mL) and then washed with PBS and fixed with 70% ethanol for 20 min. The fixed cells were washed with PBS and stained with the DNA-specific fluorochrome DAPI (1 μg/ml). Following 10 min of incubation, the cells were washed with PBS, and the plates were observed under a fluorescence microscope (Olympus Optical, Tokyo, Japan).
DNA fragmentation analysis
An apoptotic cell is characterized by its unique ladder of nucleotide fragments in DNA-agarose gel electrophoresis. HBL cells (5 × 103 cells/well in a 12-well plates) were lysed with lysis buffer (10 mM Tris–HCl, 5 mM EDTA, 200 mM NaCl, 0.2% SDS, and incubated at 60°C for 5 min); the sample was digested with 2.5 μl of proteinase K (more than 3 μl−1; Sigma) and 5 μl of RNase A(Iu μl−1) (Fermentas) and was further incubated at 50°C for 1 h. After centrifugation at 1,000×g for 15 min, the supernatants were extracted with an equal volume of phenol, chloroform, and isoamyl alcohol. The DNA was then mixed with 4 M sodium chloride and 100% ethanol and stored at −70°C overnight. Each DNA sample was loaded onto a 1.8% Tris–boric acid–EDTA agarose gel and electrophoresed at 100 V for 30 min.
Results and discussion
The chemical inertness of gold has been used internally in humans for the past 50 years, from its use in teeth to implants to radioactive gold used in cancer treatment. The primary rationale for selecting gold nanoparticles is their biocompatibility, very high surface area (large amount of drugs can be loaded), ease of characterization, and surface modification (i.e., organic molecules such as drugs, peptides, antibodies, etc. can be easily conjugated to gold nanoparticles). Choosing the right ligand for nanoparticle synthesis is key in forming AuNPs with desirable properties. In this study, we chose the naturally occurring peptide ligand, GSH, and thiol-rich ligand, lipoic acid, because of the favorable properties such as the presence of thiol, carboxylic acid and amino groups, water solubility at relevant biological pH, biological compatibility, and ease of functionalization, thereby making water-soluble nanoparticles for biological applications.
The presence of carboxylic acid and amino groups on GSH ligand complexed with Au (I) has the potential advantage of being a pH-sensitive compound, which can adopt different conformational states and sizes depending on the pH of solution. Whetten et al. synthesized the Au NPs via sodium borohydride reduction of the mixture of tetrachloroauric acid and GSH in methanol–water (2:3) and obtained gold nanoparticles with most abundant component having a diameter of 0.9 nm. We have initiated to design such nanoparticles on the direct intervention of phytochemicals for the production of gold nanoparticles which may provide a new method and an important opportunity for improvement in breast cancer treatments, the most common form of cancer in women worldwide. Such nanoparticles coupled with the specific targeting agents have the ability to track and eliminate breast cancer cells. In this paper, we present grape-gold-based nanoparticles for breast cancer diagnosis and treatment for which we used human breast lymphoma cells.
Synthesis of the biocompatible nano gold from V. vinefera
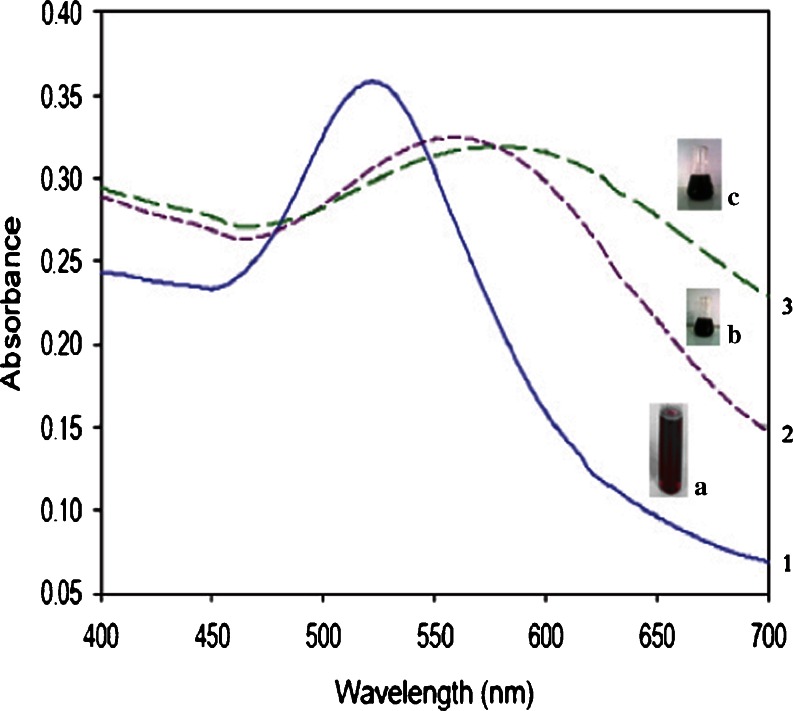
Figure 1, a–c depicts the change in color of GAuNPs before and after capping. The color of the grape extract changed pink to wine red (1a), to blue upon addition of glutathione (1b), and to dark blue after capping with lipoic acid (1c). Synthetic conditions have been optimized for the quantitative large-scale conversions of HAuCl4 to the corresponding AuNPs using grape extract. The foremost phytochemicals present in grape extract consist of water-soluble catechins (catechin, epicatechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, etc.,) and thearubigins which are oligomers of catechins of unknown structure. As the generation of AuNPs using grape extract involves aqueous media, the water-soluble phytochemicals of grape extract may be playing a major role in the overall reduction reactions of HAuCl4. Interestingly, the systematic investigation of Satish et al. (2009) granted the role of polyphenols (catechins and theaflavins) for the generation and stabilization of AuNPs through independent experiments. Normally, thiols containing organic compounds are employed to stabilize AuNPs against agglomeration and strong interaction (Brust et al. 1994). It has been shown that all the catechins act as outstanding reducing agents to reduce the Au (III) to the corresponding gold nanoparticles (Satish et al. 2009). The nanoparticles thus generated were coated with GSH and lipoic acid stabilizing agent and showed significant stability. These experiments have decidedly confirmed that catechin and epigallocatechin gallate hand out dual roles as reduction and stabilizing agents, whereas epigallocatechin and epicatechin can be used only for the reduction of gold salts and require GSH and lipoic acid as an external stabilizing mediator. Thus, our study provided an evidence for the better stability by GSH and lipoic acid when coupled to biomolecules to obtain new delivery platforms (Roux et al. 2005).
Fig. 1.
UV–visible spectroscopic analysis of 1 GAuNPs, 2 GSH, and 3 lipoic acid-stabilized GAuNPs 1 the blue line indicates the peak at a wave length of 500–550 nm fir GAuNPs, 2 the dashed line indicates the peak at a wave length of 550–600 nm for GSH-GAuNPs, 3 the green dotted line indicates the peak at a wave length of 600–650 nm for LA-GAuNPs. The beakers a, b, and c imply the color change after the addition of auro chloric acid to grape extract, GSH, and lipoic acid a AUNPs synthesized from grape extract, b GAuNPs stabilized and capped with glutathione, c GAuNPs stabilized and capped with lipoic acid
UV–visible spectroscopy studies
The gold nanoparticles synthesized from grape extract were relatively monodisperse in colloidal solution, which was confirmed by a single peak in the absorbance spectra (Fig. 1). Gold nanoparticles exhibit some special optical properties such as Plasmon resonance, which is primarily a quantum phenomenon operative on the nanoscale. Absorption measurements indicated that the Plasmon resonance wavelength of GAuNPs was 535 nm. As shown in Fig. 1, the peaks 2 and 3 are shifted towards the higher wavelength after capping with glutathione (540–580 nm) and lipoic acid (560–620 nm), respectively. The λmax shift in the absorbance spectra was mainly due to the surface modification of the gold nanoparticles (Figs. 2, 3). Fascinatingly, the surface Plasmon resonance, the major cause for the absorption, may be affected by surface modification with covalent coupling thereby increasing their size (Sheetal et al. 2008). The covalent coupling may also be due to the protective coating of the organic molecule, glutathione—a tripeptide (glutamic acid, cysteine, and glycine), which has many binding points for the gold nanoparticles (two carboxylic groups, one thiol group, and three amino groups). More effectively, the thiol group is involved in the attachment with the AuNP. In the case of lipoic acid-capped nanoparticles, the disulfides are reduced by polyphenols to two thiol groups (−S–S– → –SH + −SH), which are involved in the binding of lipoic acid to gold nanoparticles. And the coupling can be extended via either the carboxylic or the amino groups (of glutathione/lipoic acid). It is conceivable that the cocktail of phytochemicals in grapes along with nontoxic antioxidants lipoic acid and GSH are acting synergistically in stabilizing gold nanoparticles from any agglomeration in solution. A similar study by Gautham et al. (2009) created borohydride-reduced AuNPs capped with glutathione and lipoic acid that was covalently linked to horse radish peroxidase, provided some insight in the application of biosensors as tools for diagnostics.
Fig. 2.
SEM images of gold nanoparticles obtained using a GAuNPs, b GSH-GAuNPs, c lipoic acid-GAuNPs
Fig. 3.
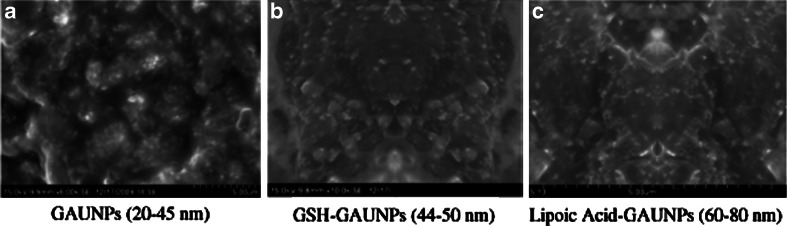
TEM images of gold nanoparticles obtained using a GAuNPs, b GSH-GAuNPs, c lipoic acid-GAuNPs
Size, morphology, and stability properties
The techniques for the characterization of nanoparticle size and morphology are SEM (Bilati et al. 2005) and TEM (Teixeira et al. 2005). Figures 4 and 5 indicate the size and morphology as observed under SEM and TEM on AuNPs synthesized using grapes as spherical in shape within the size range of 20–45 nm (Figs. 3a and 4a). Investigations on experiments using commercially available catechins have unambiguously confirmed that catechins are excellent reducing and stabilizing agents to reduce Au (III) to the corresponding gold nanoparticles (Satish et al. 2009). In addition, the effect of pH (Gardea et al. 2003b), time (Huang et al. 2007), temperature (Groning et al. 2004), and measurement of charges (Shankar et al. 2004a) may also play a major role in the determination of shape and size of nanoparticles. However, the presence of some phytochemicals (Brust et al. 1994) might render a minimum stability by failing to provide effective coating to shield the nanoparticles from agglomeration studies to GAuNPS. In order to capitalize on the reduction powers of such phytochemicals, we have utilized GSH, a tripeptide and lipoic acid, an antioxidant as naturally available stabilizing agent in our reactions. Thus, GSH-GAuNPs (Figs. 3b and 4b) and LA-GAuNPs (Figs. 3c and 4c) sizes as measured by SEM and TEM are in good agreement and are in the range of 40–80 nm suggesting that thiols and peptides are capped on grape phytochemically reduced gold nanoparticles. Such size distribution analysis of capped and non-capped GAuNPs confirms that particles are well dispersed. This extra stability rendered by GSH and lipoic acid-capped AUNP arises due to the chemical inertness by the complete coverage of the gold core by GSH ligands. Gold nanoparticles can be stabilized by anionic ligands such as carboxylic acid derivatives like citrate, tartrate, and lipoic acid (Peng et al. 2007). Earlier studies showed that glutathione used for capping gold quantum clusters (AU-n-SG-m) (−SG, glutathione thiolate) is one such group of compounds which has been well known for the stability of the AUNPs synthesized chemically (Habeeb and Pradeep 2007). Moreover, he confirmed in his results that the bigger clusters, n > 25, can be converted into AU25SG18 by adding excess GSH. In addition, the six free electrons present in the conduction band of nanoparticulate gold make them potential candidates to bind with thiols and amines. Therefore, by changing the size and shape of AuNps, the SPB and scattering may be tuned for application in cellular imaging, drug delivery, and therapy.
Fig. 4.
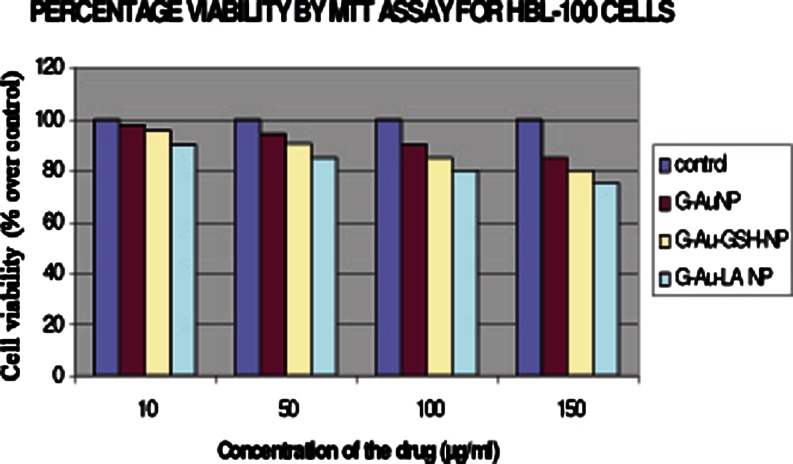
Dose-dependent cytotoxicity of GAuNPs, GSH-GAuNPs, and LA-GAuNPs in HBL-100-cells after 24 h of exposure to different concentrations GAuNPs, GSH-GAuNPs, and LA-GAuNPs (10, 50, 100, and 150 μg/mL, respectively) using MTT assay. Values are expressed as percent over control
Fig. 5.
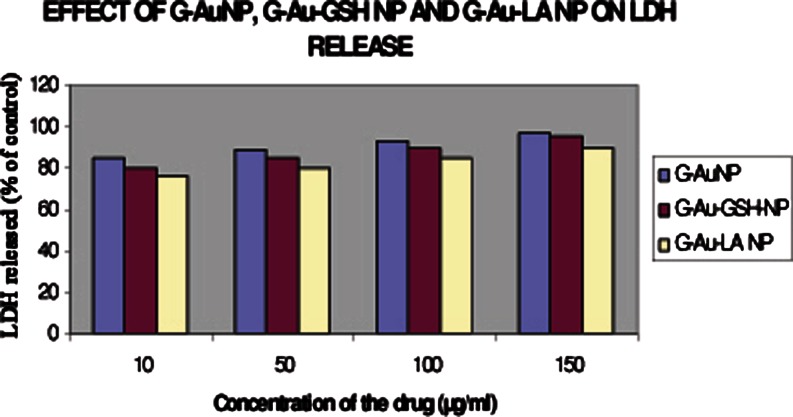
LDH release LDH leakage after HBL-100 cells exposed to different concentrations GAuNPs, GSH-GAuNPs, and LA-GAuNPs (10, 50, 100, and 150 μg/mL, respectively)
In vitro uptake and localization characterization
Characterization of in vitro nanoparticle uptake and localization is intrinsically linked to cytotoxicological studies because uptake provides evidence of nanoparticle–cell interaction, wherein the delicate intracellular machinery is exposed to nanoparticles. Compared to in vivo studies, in vitro studies benefit from being faster, lower cost, allowing greater control, and minimizing ethical concerns by reducing the number of laboratory animals required for testing. The most commonly used in vitro assessment techniques generally evaluate either viability (live/dead ratio) or toxicity mechanism. Herein, the major viability-based assays are organized into the categories of proliferation, necrosis, or apoptosis DNA damage detection techniques. In our present communication, the cytotoxicty of GAuNPs, GSH-GAuNPs, and LA-GAuNPs under in vitro conditions in HBL-100 cells was examined using two cytotoxicity markers, including MTT reduction, and LDH leakage is used to study the effects of gold nanopatricle conjugates on cellular viability. To check the cytotoxicity of cells treated with GAuNPs (10–150 μL), GSH-GAuNPs (10–150 μL), and LA-GAuNPs (10–150 μL) for 24 h, and a number of viable cells were enumerated by colorimetric MTT assay. After 24 h of post-treatment, HBL-100 cells showed excellent viability even up to 150 μL of GAuNPs, GSH-GAuNPs, and LA-GAuNPs (Fig. 5). Results of MTT assays clearly revealed the cytotoxic effect of GAuNPs in a dose-dependent manner for HBL-100 cell lines and LA-GAuNPs exerted slightly better cytotoxic effect towards HBL-100 cells in comparison to GSH-GAuNPs. Although GAuNPs also showed cytotoxicity towards cancer cells, the effect was much less compared to GSH-GAuNPs and LA-GAuNPs. Treatment with 150 μL or higher doses of GAuNPs and GSH-GAuNPs and LA-GAuNPs to HBL-100 cell increased LDH leakage into culture media (Fig. 6), signifying an AUNP-induced compromise of plasma membrane integrity, and the IC50 of AgNPs was 150 μL. Henceforth, the release of LDH (Fig. 6) in our study is in agreement with the excellent viability of the cells treated with GAuNPs and GSH-GAuNPs and LA-GAuNPs as proven by MTT assay. It is also important to recognize that a vast majority of gold (I) and gold (III) compounds exhibit varying degrees of cytotoxicity to a variety of cells (Basset et al. 2003; Hamer 2007). GAuNPs pretreatment at a concentration of 150 μL reduced the LDH leakage to a minimum, and this concentration is used in subsequent studies.
Fig. 6.
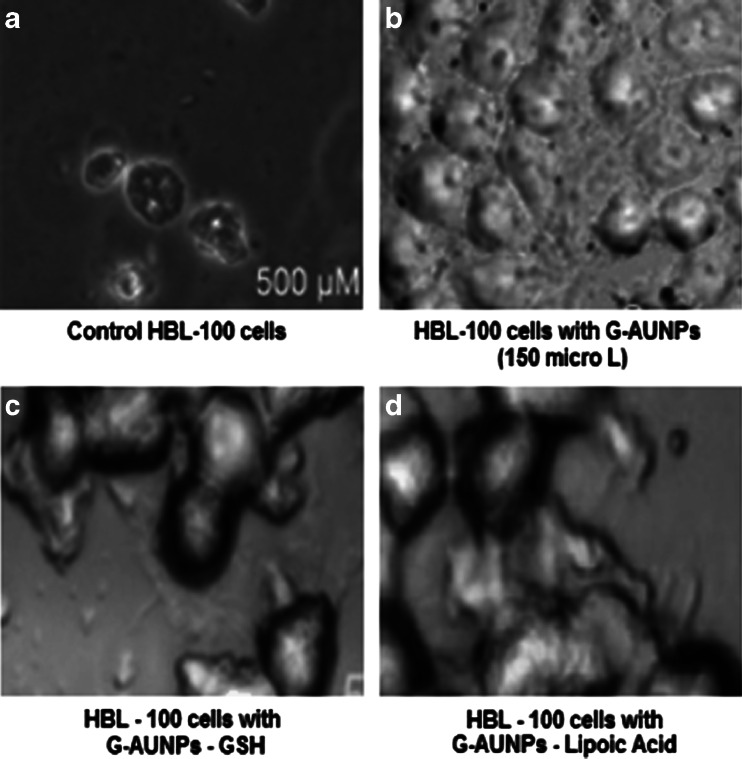
Phase contrast microscopic pictures of HBL-100 cells a untreated and treated b with 500 μM GAuNPs, c GSH-GAuNPs, and d liopic acid-GAuNPs
Induction of apoptosis by GAuNPs, GSH-GAuNPs, and LA-GAuNPs in HBL-100 cells
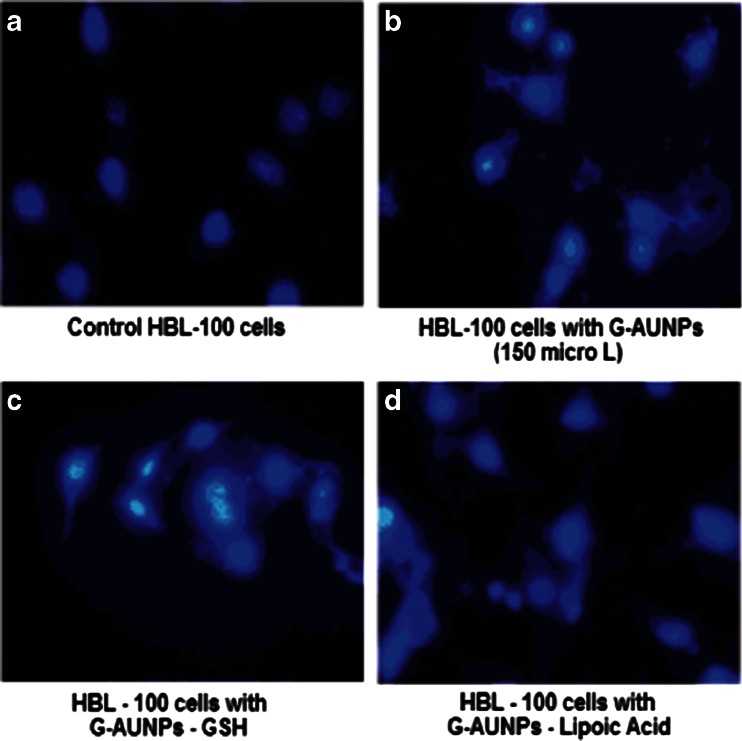
Surface reactivity, chemical composition, and large specific surface area have been deemed important properties in nanoparticle-mediated toxicity (Wallace et al. 2007). HBL-100 cells, after treatment with nanometer-sized GAuNPs, GSH-GAuNPs, and LA-GAuNPs, exhibited ultra structure and biochemical features that are characteristic of apoptosis, as shown by chromatin condensation and inter nucleosomal DNA fragmentation. The phase-contrast microscopic pictures of altered morphology of HBL-100 cells which is characteristic of apoptotic cell stage when treated with GSH-GAuNPs, and LA-GAuNPs (40–80 nm) are shown in Fig. 7a–d. In addition, the nuclear fragmentation, a hallmark of cellular apoptosis, was clearly exhibited by fluorescent microscopic studies after DAPI staining of untreated and GSH-GAuNPs and LA-GAuNPs (40–80 nm)-treated HBL cells (Fig. 8a–d). A minimum of 200 cells were counted and classified as follows: (1) live cells (normal nuclei: blue chromatin with organized structure); (2) stressed cells (bright-blue chromatin, which is highly condensed, margined, or fragmented).
Fig. 7.
Fluroscent microscopic pictures of HBL-100 cells a untreated and treated b with 500 μM GAuNPs, c GSH-GAuNPs, and d liopic acid-GAuNPs
Fig. 8.
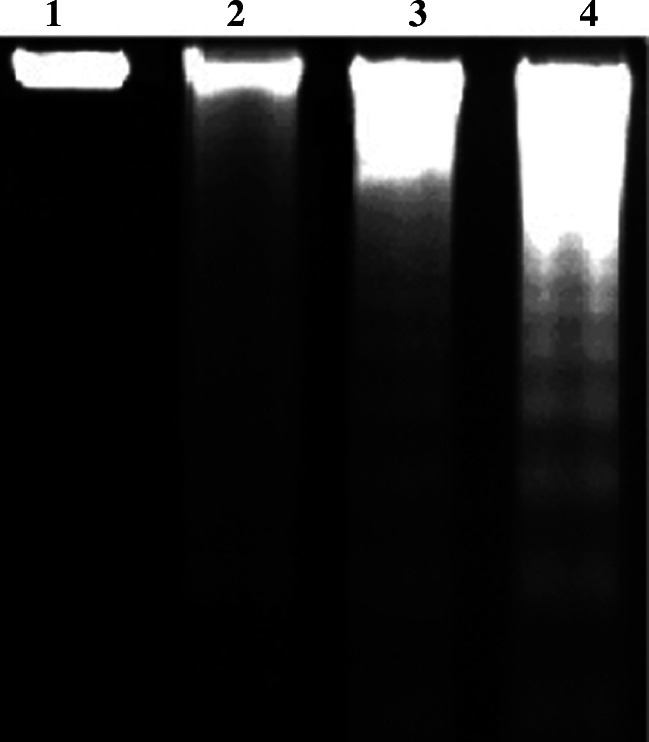
HBL-100 cells were treated for 24 h with 500 μM untreated cells (lane 1) GAuNPs (lane 2) GSH-GAuNPs (lane 3), and LA-GAuNPs (lane 4) for inter-nucleosomal DNA fragmentation analyzed by electrophoresis on a 1.6% Tris-Borate-EDTA agarose gel electrophoresis
Metal complexes have been extensively studied for their nuclease-like activity using the redox properties of the metal and dioxygen to produce reactive oxygen species to promote DNA cleavage by direct strand scission or base modification in cancer cells (Burrows and Muller 1998). A more current development in this area has been testing of metal nanoparticles such as gold and platinum nanoparticles for DNA degradation studies (Shen et al. 2009; López et al. 2010). Use of metal nanoparticles can be in particular advantageous in generating singlet oxygen [42 and 43] (Lipovsky et al. 2009; Portolés et al. 2010)
A recent report by Geddes and coworkers demonstrated that the presence of metal nanoparticles can enhance singlet oxygen generation (Zhang et al. 2008). The enhanced electromagnetic fields in proximity to metal nanoparticles are the basis for the increased absorption predicts the extent of absorption and the relative increase in singlet oxygen generation from photosensitizers (Barber et al. 1983; Yang et al. 1995)
A very recent study by Midander and coworkers reported the effect of metal nanoparticles inducing single-stranded breaks in the human lung cells (Midander et al. 2009). Previous studies illustrated the potent cytotoxic, genotoxic, and toxicological activities of nanoparticles in vivo (Midander et al. 2009; Chen et al. 2006) and in cultured cancer cell lines (Sengupta et al. 2007). However, a methodical study using GAuNPs on DNA degradation and cytotoxicity towards breast cancer cells are missing up to date to the finest of our information. Thus, 40–80-nm-sized GSH-GAuNPs and LA-GAuNPs (lanes 2 and 3) treated HBL-100 cells in our study displayed a ladder pattern of inter-nucleosomal DNA fragmentation on TBE-agarose gel electrophoresis in DNA ladder assay (Studer et al. 2010) as revealed in Fig. 9 which is also another characteristic of apoptosis. All these results confirm that treatment with GSH-GAuNPs and LA-GAuNPs induce apoptosis in human breast cancer cells compared to GAuNPs.
Fig. 9.
Confocal microscopic image of control HBL-100 cells (a); HBL-100 cells treated with 500 μM of GSH-GAuNPs (b) arrows indicate agglomerated GAuNPs; c HBL-100 cells treated with 500 μM of LA-GAuNPs for 14 h. Arrows indicate agglomerated AuNPs in a membrane-bound vesicle (probably perinuclear lysosome)
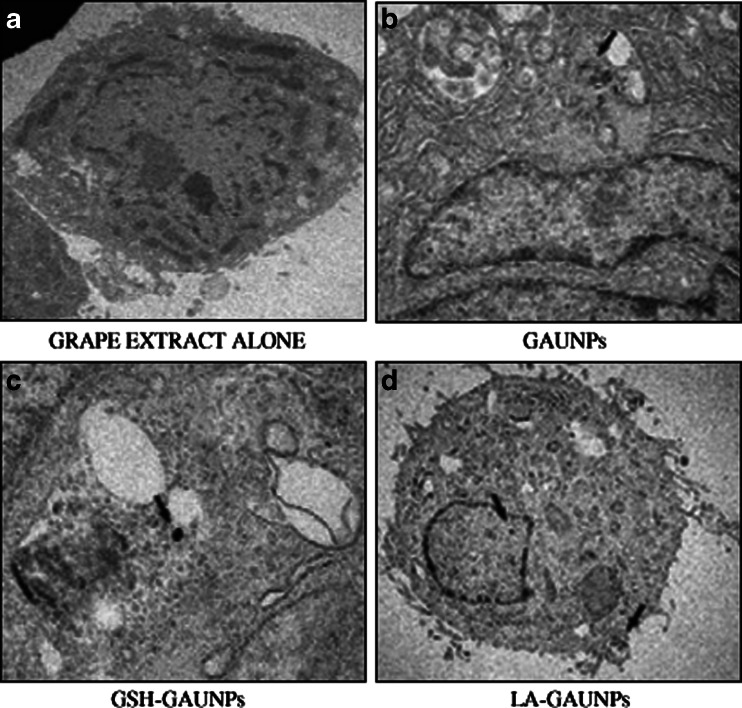
Cellular internalization studies of GAuNPs, GSH-GAuNPs, and LA-GAuNPs in HBL-100 cells
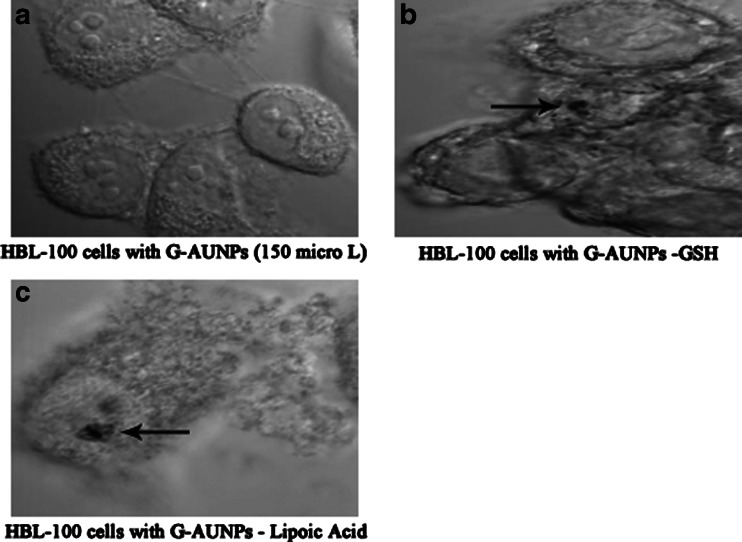
Membrane integrity is another cellular characteristic commonly used to determine viability during in vitro nanotoxicology experiments. Surprisingly, cancer cells are highly metabolic and porous in nature and are known to internalize solutes rapidly compared to normal cells (Sun et al. 2007). Do the cells internalize the particles or do the particles remain bound to the cell membrane? Results of cellular internalization studies of AuNps solutions are keys to provide insights into their use in biomedicine. Their selective cell and nuclear targeting will provide new pathways for their site-specific delivery as diagnostic/therapeutic agents. To address these issues, confocal microscopic studies confirmed the uptake of GSH-GAuNPs and LA-GAuNPs inside the HBL-100 cells (Fig. 10a–c) with the presence of agglomerated gold nanoparticles; a similar observation was also reported by Stark and coworkers with copper nanoparticles for Hela cells (Studer et al. 2010).
Fig. 10.
a–d TEM images of HBL-100 cells showing the internalization of GAuNPs depicting the arrival of a GAuNPs at the cells membrane, binding of the nanoparticles to surface receptors, membrane wrapping of the nanoparticles, and finally internalization into the cell nucleus
Further, TEM images of breast tumor (HBL-100) cells treated with GSH-GAuNPs and LA-GAuNPs unequivocally validated our hypothesis. Significant internalization of GSH-GAuNPs and LA-GAuNPs via endocytosis within the HBL-100 cells was observed (Fig. 11). GSH-GAuNPs and LA-GAuNPs were detected within larger endocytic compartments of diverse morphology. These include peripherally both early and late endosomes and lysozomes. The internalization of nanoparticles within cells could occur via processes including phagocytosis, fluid-phase endocytosis, and receptor-mediated endocytosis. The viability of HBL-100 cells post-internalization suggests that the phytochemical coating and the size of the nanoparticles renders the nanoparticles nontoxic to cells. A number of studies have supported our study, demonstrating that phytochemicals have the ability to penetrate the cell membrane and internalize within the cellular matrix (Sun et al. 2007; Mizuno et al. 2007).
Fig. 11.
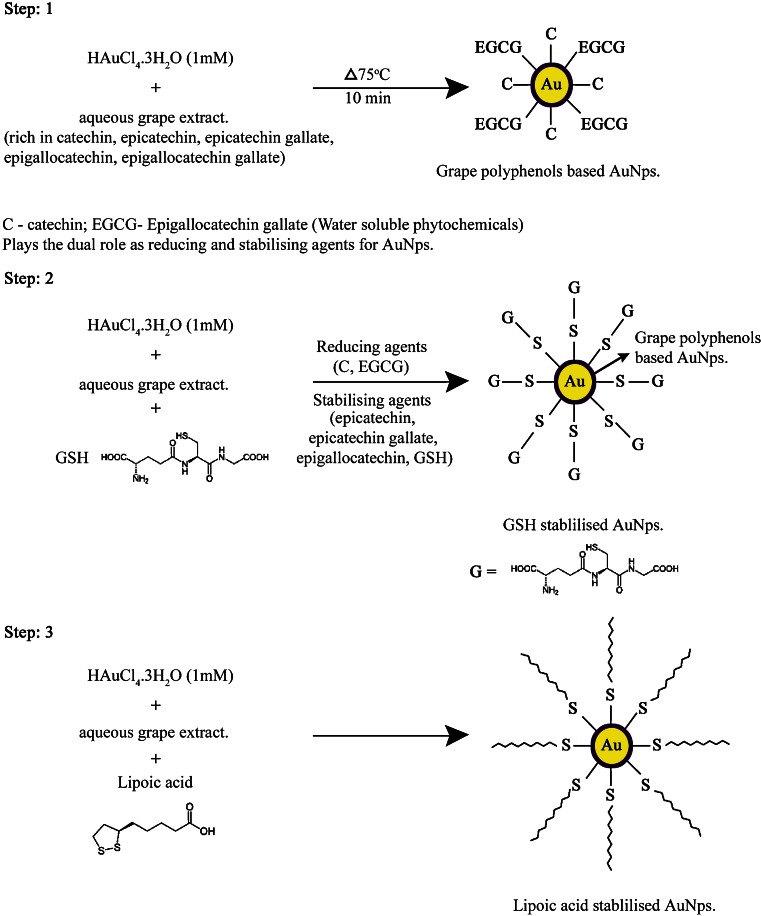
Schematic representation of biosynthesis of grape-based gold nanoparticles (Step 1) and ligand (GSH and lipoic acid) stabilized grape gold nanoparticles (Steps 2 and 3)
Therefore, we hypothesized that grape-derived phytochemicals and other antioxidants, if coated on gold nanoparticles, will show internalization within cancer cells. Such a harmless internalization of gold nanoparticles will provide new opportunities for probing cellular processes via nanoparticulate-mediated imaging.
The present investigation resulted in the development of environment-friendly green methodology to produce biologically benign gold nanoparticles stabilized with biologically relevant thiol-rich antioxidants. Here, the gold nanoparticles are generated by reduction of gold precursor (a source of Au3+ ions) by a reducing agent (grape polyphenols) in the presence of stabilizers (GSH and Lipoic acid) that keeps nanoparticles apart, thus avoiding their aggregation. Although there are reports for the anticancer activity of phytochemically stabilized AUNPs, this is the first report to investigate the cytotoxicity of GSH and lipoic acid (in addition to phytochemicals) stabilized AUNPs. Herein, the major viability-based assays, such as proliferation, necrosis, or apoptosis and DNA damage detection of GSH-GAuNps and LA-GAuNps in HBL-100 cells have been proved as keys to provide insights into their use in biomedicine. In addition, GSH-GAuNps and LA-GAuNps selective cell and nuclear targeting compared to GAuNps will provide new pathways for their site-specific delivery as diagnostic/therapeutic agents.
References
- Aslam A, Fu L, Su M, Vijayamohanan K, Dravid VP. Novel one-step synthesis of aminestabilized aqueous colloidal gold nanoparticles. J Mater Chem. 2004;14:1795–1797. doi: 10.1039/b402823f. [DOI] [Google Scholar]
- Barber PW, Chang RK, Massoudi H. Electrodynamic calculations of the surface- enhanced electric intensities on large Ag spheroids. Phys Rev B. 1983;27:7251–7261. doi: 10.1103/PhysRevB.27.7251. [DOI] [Google Scholar]
- Basset C, Vadrot J, Denis J, Poupon J, Zafrani ES. Prolonged cholestasis and ductopenia following gold salt therapy. Liver Int. 2003;23:89–93. doi: 10.1034/j.1600-0676.2003.00806.x. [DOI] [PubMed] [Google Scholar]
- Basu S, Pal T. Glutathione-induced aggregation of gold nanoparticles: electromagnetic interactions in a closely packed assembly. J Nanosci Nanotechnol. 2007;7:1904. doi: 10.1166/jnn.2007.739. [DOI] [PubMed] [Google Scholar]
- Bilati U, Allemann E, Doelker E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 2005;24:67–75. doi: 10.1016/j.ejps.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol derivatised gold nanoparticles in a two phase liquid/liquid system. J Chem Soc Chem Commun. 1994;7:801–802. doi: 10.1039/c39940000801. [DOI] [Google Scholar]
- Burrows CJ, Muller JG. Oxidative nucleobase modifications leading to strand scission. Chem Rev. 1998;98:1109–1152. doi: 10.1021/cr960421s. [DOI] [PubMed] [Google Scholar]
- Chacona MR, Ceperuelo MV, Maymo ME, Mateo-Sanzb JM, Arolac L, Guitierreza C, Fernandez-Reald JM, Ardevolc A, Simona I, Vendrella J. Grape-seed procyanidins modulate inflammation on human differentiated adipocytes in vitro. Cytokine. 2009;47:137–142. doi: 10.1016/j.cyto.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Chen D, Dou QP. Tea polyphenols and their roles in cancer prevention and chemotherapy int. J Mol Sci. 2008;9:1196–1206. doi: 10.3390/ijms9071196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Meng H, Xing G, Chen C, Zhao Y, Jia G, Wang T, Yuan H, Ye C, Zhao F, Chai Z, Zhu C, Fang X, Ma B, Wan L. Acute toxicological effects of copper nanoparticles in vivo. Toxicol Lett. 2006;163:109–120. doi: 10.1016/j.toxlet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Chen F, Zhang ZR, Huang Y. Evaluation and modification of N-trimethyl chitosan chloride nanoparticles as protein carriers. Int J Pharm. 2007;336:166–173. doi: 10.1016/j.ijpharm.2006.11.027. [DOI] [PubMed] [Google Scholar]
- Forrest ML, Kwon GS. Impact of nanoscience and nanotechnology on controlled drug delivery. Adv Drug Deliv. 2008;60:861–862. doi: 10.1016/j.addr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Gardea TJL, Gomez E, Jose YM, Parsons JG, Peralta VJR, Tioani H. Alfalfa sprouts: anatural source for the synthesis of silver nanoparticles. Langmuir. 2003;19:1357–1361. doi: 10.1021/la020835i. [DOI] [Google Scholar]
- Gautham KA, Mitra CK. Direct electrochemistry of horseradish peroxidase-gold nanoparticles conjugate. Sensors. 2009;9:881–894. doi: 10.3390/s90200881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- God JM, Tate P, Larcom LL. Anticancer effects of four varieties of muscadine grape. J Med Food. 2007;10:54–59. doi: 10.1089/jmf.2006.699. [DOI] [PubMed] [Google Scholar]
- Groning R, Adesina S, Muller RS. Formation of particles in aqueous infusions of medicinal plant Harungana madagascariensis. Pharmazie. 2004;59:279–281. [PubMed] [Google Scholar]
- Habeeb MMA, Pradeep T. Au25@SiO2: quantum clusters of gold embedded in silica. Chem Phys Lett. 2007;449:186. doi: 10.1016/j.cplett.2007.10.065. [DOI] [Google Scholar]
- Hamer M. The beneficial effects of tea on immune function and inflammation: a review of evidence from in vitro, animal, and human research. Nutr Res. 2007;27:373–379. doi: 10.1016/j.nutres.2007.05.008. [DOI] [Google Scholar]
- Henning SM, Niu Y, Liu Y, Lee NH, Hara Y, Thames GD, Minutti RR. Bioavailability and antioxidant effect of epigallocatechin gallate administered in purified form versus as green tea extract in healthy individuals. J Nutr Biochem. 2005;16:610–616. doi: 10.1016/j.jnutbio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T, Eubank JF, Heurtaux D, Clayette P, Kreuz C, Chang JS, Hwang YK, Marsaud V, Bories PN, Cynober L, Gil S, Ferey G, Couvreur P, Gref R. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater. 2010;9:172–178. doi: 10.1038/nmat2608. [DOI] [PubMed] [Google Scholar]
- Huang J, Li Q, Sun, D, Lu Y, Su Y, Yang X, Wanh H, Wang Y, Shao W, He N, Hong J, Chen C (2007) Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum canphora leaf. Nanotechnology 18:1–11
- Kasthuri J, Kathiravan K, Rajendiran N (2009) Phyllanthin assisted biosynthesis of silver and gold nanoparticles: a novel biological approach. J Nanopart Res 11:1075–1085
- Lipovsky A, Tzitrinovich Z, Friedmann H, Applerot G, Gedanken A, Lubart R. EPR study of visible light-induced ROS generation by nanoparticles of ZnO. J Phys Chem C. 2009;113:15997–16001. doi: 10.1021/jp904864g. [DOI] [Google Scholar]
- López T, Figueras F, Manjarrez J, Bustos J, Alvarez M, Silvestre AJ, Rodriguez R, Martínez FA, Martínez E. Catalytic nanomedicine: a new field in antitumor treatment using supported platinum nanoparticles. In vitro DNA degradation and in vivo tests with C6 animal model on Wistar rats. Eur J Med Chem. 2010;45:1982–1990. doi: 10.1016/j.ejmech.2010.01.043. [DOI] [PubMed] [Google Scholar]
- Meyer AS, Yi OS, Pearson DA, Waterhouse AL, Frankel EN. Inhibition of human low density lipoprotein oxidation in relation to composition of phenolic antioxidants in grapes (Vitis vinifera) J Agric Food Chem. 1997;45:1638–1643. doi: 10.1021/jf960721a. [DOI] [Google Scholar]
- Midander K, Cronholm P, Karlsson HL, Elihn K, Möller L, Leygraf C, Wallinder IO. Surface characteristics, copper release, and toxicity of nanoand micrometer-sized copper and copper(II) oxide particles: a cross disciplinary study. Small. 2009;5:389–399. doi: 10.1002/smll.200801220. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Cho YY, Zhu F, Ma WY, Bode AM, Yang CS, Ho CT, Dong ZG. Theaflavin-3,3′-Digallate Induces Epidermal Growth Factor Receptor Down-Regulation. Mol Carcinog. 2007;45:204–212. doi: 10.1002/mc.20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal Immunological Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Olas B, Wachowicz B, Tomczak A, Erler J, Stochmal A, Oleszek W. Comparative antiplatelet and antioxidant properties of polyphenol-rich extracts from: berries of Aronia melanocarpa, seeds of grape and bark of Yucca schidigera in vitro. Platelets. 2008;19:70–77. doi: 10.1080/09537100701708506. [DOI] [PubMed] [Google Scholar]
- Peng Z, Chen Z, et al. A novel immunoassay based on the issociation of immunocomplex and fluorescence quenching by gold nanoparticles. Analytica Chimica Acta. 2007;583:40–44. doi: 10.1016/j.aca.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Portolés MJL, Gara PMD, Kotler ML, Bertolotti S, Román ES, Rodríguez HB, Gonzalez MC. Photophysical properties of blue-emitting silicon nanoparticles. Langmuir. 2010;26:10953–10960. doi: 10.1021/la100980x. [DOI] [PubMed] [Google Scholar]
- Roux S, Garcia B, Bridot JL, Salome M, Marquette C, Lemelle L, Gillet P, Blum L, Perriat P, Tillement O. Langmuir. 2005;21:2526–2536. doi: 10.1021/la048082i. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Targeting tumor vasculature with homing peptides from phage display. Semin Cancer Biol. 2000;10:435–442. doi: 10.1006/scbi.2000.0334. [DOI] [PubMed] [Google Scholar]
- Sajja HK, et al. (2009) Development of multifunctional nanoparticles for targeted drug delivery and noninvasive imaging of therapeutic effect. Curr Drug Discov Technol 6:43–51 [DOI] [PMC free article] [PubMed]
- Satish K, Nune Nripen Chanda Ravi S, Kavita K, Rajesh R, Kulkarni Subramanian T. Green nanotechnology from tea: phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J Mater Chem. 2009;19:2912–2920. doi: 10.1039/b822015h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta TK, Leclerc GM, Hsieh KTT, Leclerc GJ, Singh I, Barredo JC. Cytotoxic effect of 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) on childhood acute lymphoblastic leukemia (ALL) cells: implication for targeted therapy. Mol Cancer. 2007;10:46. doi: 10.1186/1476-4598-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar SS, Ahmed A, Akkamwar B, Sastry M, Rai A, Singh A. Biological synthesis of triangular gold nanoprisms. Nature. 2004;3:482–488. doi: 10.1038/nmat1152. [DOI] [PubMed] [Google Scholar]
- Sheetal DE, Maheswara R, Anjali S, Varsha P, Prasad BL. Chem Natural Gum Reduced/Stabilized Gold Nanoparticles for Drug Delivery Formulations. Eur. J. 2008;14:10244–10250. doi: 10.1002/chem.200801093. [DOI] [PubMed] [Google Scholar]
- Shen Q, Nie Z, Guo M, Zhong CJ, Lin B, Li W, Yao S. Simple and rapid colorimetric sensing of enzymatic cleavage and oxidative damage of single-stranded DNA with unmodified gold nanoparticles as indicator. Chem Commun. 2009;28:929–931. doi: 10.1039/b818081d. [DOI] [PubMed] [Google Scholar]
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat Res. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Studer AM, Limbach LK, Van Duc L, Krumeich F, Athanassiou EK, Gerber LC, Moch H, Stark WJ (2010) Nanoparticle cytotoxicity depends on intracellular solubility: Comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol Lett 197:169–174 [DOI] [PubMed]
- Subramaniam C, Tom RT, Pradeep T. On the formation of protected gold nanoparticles from AuCl4- by the reduction using aromatic amines. J Nanopar Res. 2005;7:209–217. doi: 10.1007/s11051-005-0315-0. [DOI] [Google Scholar]
- Sun DJ, Liu Y, Lu DC, Kim W, Lee JH, Maynard J, Deisseroth A. Endothelin-3 growth factor levels decreased in cervical cancer compared with normal cervical epithelial cells. Hum Pathol. 2007;38:1047–1056. doi: 10.1016/j.humpath.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Teixeira M, Alonso MJ, Pinto MMM, Barbosa CM. Development and characterization of PLGA nanospheres and nanocapsules containing xanthone and 3-methoxyxanthone. Eur J Pharm Biophar. 2005;59:491–500. doi: 10.1016/j.ejpb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Urpi SM, Monagas M, Khan N, Lamuela RRM, Santos BC, Sacanella E, Castell M, Permanyer J, Andre LC. Epicatechin, procyanidins, and phenolic microbial metabolites after cocoa intake in humans and rats. Anal Bioanal Chem. 2009;394:1545–1556. doi: 10.1007/s00216-009-2676-1. [DOI] [PubMed] [Google Scholar]
- Wallace WE, Keane MJ, Murray DK, Chisholm WP, Maynard AD, Ong TM. Phospholipid lung surfactant and nanoparticle surface toxicity: Lessons from diesel soots and silicate dusts. J Nanoparticle Res. 2007;3:923–938. [Google Scholar]
- Yang WH, Schatz GC, Duyne RPV. Discrete dipole approximation for calculating extinction and Raman intensities for small particles with arbitrary shapes. J Chem Phys. 1995;103:869–875. doi: 10.1063/1.469787. [DOI] [Google Scholar]
- Yonezawa T, Nomura T, Kinoshita T, Koumoto K. Preparation and characterization of polypeptide-stabilized gold nanoparticles. J Nanosci Nanotechnol. 2006;6:1649–1651. doi: 10.1166/jnn.2006.222. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Aslan K, Previte MJ, Geddes CD. Plasmonic engineering of singlet oxygen generation. PNAS. 2008;105:1798–1802. doi: 10.1073/pnas.0709501105. [DOI] [PMC free article] [PubMed] [Google Scholar]