Abstract
Atrial involvement is an uncommon feature of advanced non-small-cell lung cancer, occurring in up to 10% of patients with bronchogenic carcinoma. Additionally, cardiac metastases from other sources are documented in up to 7% of cancer patients at autopsy. Because atrial invasion can lead to systemic embolisation and/or outflow obstruction, it is treated regardless of the overall prognosis. While the gold standard treatment has historically been surgical resection, advances in radiotherapy allow for the safe treatment of cardiac disease. Here we present the case of a woman with pulmonary adenocarcinoma of the left lower lobe that progressed to invade the pulmonary vein and left atrium while maintained on standard chemotherapy. She was treated with intensity-modulated radiotherapy and had a complete response in terms of her atrial disease within 3 months. She suffered no acute toxicity or complications as a result of the radiation therapy.
Background
Neoplastic atrial invasion is a feared complication of primary lung cancer and is also a consequence of cardiac metastases from other sources. This can lead to widespread systemic embolisation and/or outflow tract obstruction. As a result, treatment is always warranted. The historical gold standard has been surgical resection, particularly for pulmonary neoplasms in which an en bloc resection may be possible. However, advances in radiation oncology allow the utilisation of radiotherapy with reduced risk of radiation-related heart disease. This is an illustrative case of this principle, as we present a patient with advanced lung cancer with atrial invasion who received fractionated intensity-modulated radiation therapy. Within 3 months, the atrial invasion had resolved and the patient experienced no acute radiation-related side effects.
Case presentation
A 63-year-old woman with stage IV (T3N2M1) adenocarcinoma of the lung presented to radiation oncology follow-up with complaints of haemoptysis. A year prior to this appointment, she had developed a persistent dry cough and heaviness in her chest. She had been previously healthy, with no significant family history. She presented to her primary care physician 3 months later when home remedies failed to help, and a chest x-ray showed a 9×7 cm left lower lobe (LLL) mass. A subsequent CT scan confirmed the presence of an 8×7×6 cm LLL mass, and an immediate CT-guided lung biopsy revealed a poorly differentiated carcinoma. On immunohistochemistry, the neoplasm was cytokeratin 7/cytokeratin 53/p63/TTF-1 positive and cytokeratin 20 negative, consistent with an adenocarcinoma. EGFR and K-ras were wildtype and there was no EML4-ALK fusion gene. Notably, she had a 10-pack-year smoking history, although she quit 24 years prior to diagnosis.
Unfortunately, a staging positron emission tomography/CT showed abnormal uptake in the right adrenal gland and multiple lymph nodes in the ipsilateral mediastinal, left paraesophageal, gastric, hepatic, retroperitoneal, aortocaval and periaortic regions as well as was a destructive lesion in the right iliac wing. She was thus diagnosed with T3N2M1 (AJCC stage IV) disease and was referred to radiation oncology for palliative radiation to the right hip; this proved effective in palliating her pain, and deemed her disease radiosensitive. Simultaneously, she was treated with carboplatin and taxol, and interval CT scans at 2 and 4 months showed partial response in the chest but no change in disease burden elsewhere. Unfortunately, a follow-up MRI of the brain a month later, as part of a protocol to enroll her in a clinical trial, revealed a 5 mm enhancing nodule in the left cerebellum and a 4 mm leptomeningeal nodule along the right temporal lobe. As such, she was referred to radiation oncology again for consideration of stereotactic radiosurgery for her intracranial lesions.
However, in the interval she developed haemopytsis associated with heartburn and deep, dull, non-radiating chest pain. A CT showed interval development of tumour thrombus measuring 3.6×1.6 mm extending into the left inferior pulmonary vein and into the left atrium, with an increase in size of the LLL mass to 6.7×6.3 cm (from 5.2×4.7 cm 2 months prior). She had progression of her extra-thoracic disease as well.
On examination, she was thin but otherwise well appearing. Her lungs were clear to auscultation bilaterally, with no wheezing, rhonchi or rales. Her heart rate was regular, with a regular rhythm. She had no other findings on physical examination.
Investigations
CT chest (as above): Non-small-cell lung carcinoma (NSCLC) with notable radiographic increase in the dominant left lower lobe mass (now measuring 6.7×6.3 cm) with new tumour thrombus (measuring 3.6×1.6 cm) extending into the left atrium and new regional mediastinal lymphadenopathy; unchanged small left pleural and new trace to small pericardial effusions (see figure 1).
Figure 1.
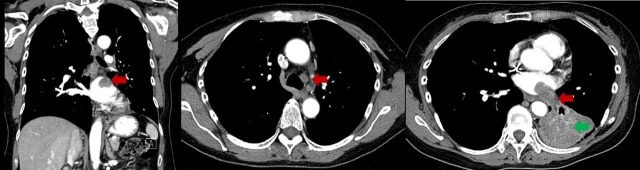
CT scan of the chest showing pulmonary vein invasion and left atrial tumour thrombus (red arrows) and left lower lobe primary tumour (green arrow).
Treatment
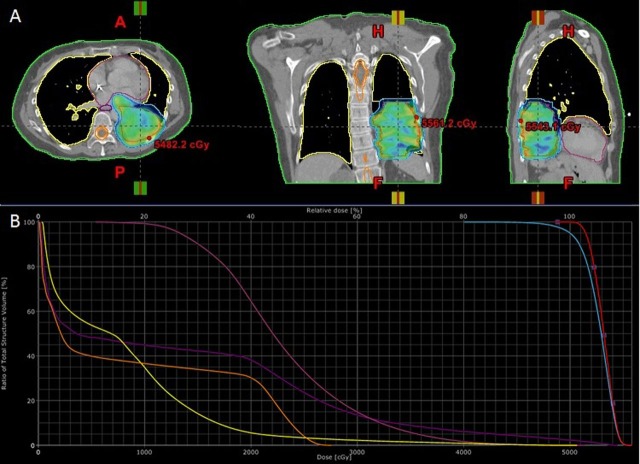
The institutional thoracic oncology tumour board convened to discuss this patient's case. While surgery is the most common treatment for extension of primary lung cancer into the great vessels and atria, this patient was not deemed a surgical candidate as the risk of surgery outweighed its benefit for this patient with metastatic NSCLC, and radiotherapy was recommended by the thoracic radiation oncologist. The patient received 5000 cGy in 20 fractions over 28 days (figure 2). A four-dimensional CT scan to assess tumour and cardiac motion was obtained along with the contrast-containing free-breathing CT simulation scan of the entire thorax for radiation planning, and both were reconstructed with 1.5 mm thick axial slices. An internal target volume (ITV) of the tumour was segmented by accounting for tumour motion from the four-dimensional scan. A margin of 6–8 mm was added to the ITV to form the planning target volume (PTV). A dose of 50 Gy in fraction size of 2.5 Gy per fraction was delivered to cover at least 95% of the PTV. Inverse-planning algorithms were utilised to achieve dosimetric parameters to the organs at risk (total lung, normal heart, spinal cord and oesophagus; see figure 2 for dose-wash and dose volume histogram, and table 1 for specific dosimetric parameters). Treatment was planned and delivered on an advanced linear accelerator (Novalis TX, Varian Medical Systems, Palo Alto, CA, USA and BrainLab Medical Systems, Westchester, IL, USA.) using the Volumetric-modulated Arc Therapy (VMAT) technique. Stereoscopic x-ray imaging as well as cone-beam CT was done during each treatment for image-guided radiotherapy to optimise daily treatment accuracy.
Figure 2.
Intensity-modulated radiation treatment plan. (A) Dose-wash images showing dose to intra-atrial lesion in three planes. (B) Dose–volume histogram showing dose to various structures (orange, spinal cord; yellow, total lung; deep purple, oesophagus; magenta, heart). See table 1 for additional dosimetric parameters.
Table 1.
Dosimetric parameters of radiation treatment plan
| Structure | Dosimetric parameter |
|---|---|
| ITV | D100=50 Gy |
| Max dose=55.8 Gy | |
| PTV | D95=50 Gy |
| Max dose=55.8 Gy | |
| Lung | V20=5.6% |
| Mean dose: 7.9 Gy | |
| Esophagus | D33≤21.9 Gy |
| D66≤1 Gy | |
| Mean dose: 13.4 Gy | |
| Spinal cord | Max dose=27.6 Gy |
| Uninvolved heart | D33≤24.8 Gy |
| D66≤19.7 Gy | |
| Mean dose: 23 Gy |
ITV, internal target volume; PTV, planning target volume; Dx, dose delivered to x% of target structure; Vx, volume (in %) receiving x Gy.
Outcome and follow-up
The patient tolerated radiotherapy well, with no acute complications. Her symptoms improved significantly with resolution of haemoptysis and diminution of her persistent cough. She subsequently received stereotactic radiosurgery to her intracranial lesions and began second-line chemotherapy with gemcitabine. A follow-up CT scan 3 months after radiotherapy showed an interval decrease in the LLL mass (now measuring 5.5×4.5 cm) and resolution of the tumour component that previously extended into the left atrium; the tumour thrombus occluding the left inferior pulmonary had also diminished in size (figure 3). The patient tolerated gemcitabine well, but a month later, she suddenly presented to the emergency room with 5 days of progressive, debilitating shortness of breath. She was afebrile and normoxic; however, a CT chest showed multiple ground glass opacities. She was diagnosed with Pneumocystis jiroveci pneumonia and rapidly developed sepsis. Despite aggressive antibiotic and pressor support, the patient passed away within several days of her presentation to the emergency room.
Figure 3.
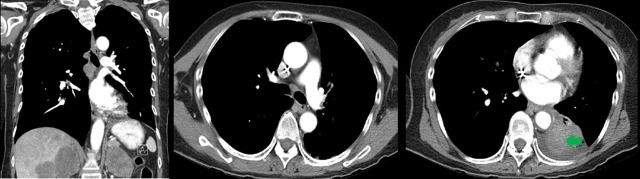
Three-month follow-up CT scan showing interval resolution of the atrial tumour burden and decrease in the size of the left lower lobe primary tumour (green arrow).
Discussion
Atrial extension is a relatively uncommon complication of advanced NSCLC, and makes the primary tumour a T4 lesion by TNM staging. Vascular invasion alone has been observed in 77% of cases in a histopathological series of 87 patients,1 and in 45% of cases in an MRI angiography series of 20 patients.2 In the latter study, tumour thrombus in the pulmonary vein extended to within 1.5 cm of the left atrium in 10% of the cases. Reports of metastatic cancers following a similar pattern of left atrial invasion (via the pulmonary vein) are much rarer, with less than 15 cases reported in the past 50 years.3 4 However, an increasing number of cardiac metastases not involving the pulmonary vasculature is being observed, with autopsy incidences of 2.3% and 7.1% within the general population and among cancer patients, respectively, which is higher than the rates observed before 1970.5
Invasion of the pulmonary vein can cause multiple arterial emboliaations, which can manifest clinically as embolic strokes or acute arterial occlusions.6 Atrial invasion can additionally lead to paroxysmal obstruction of the mitral valve, mimicking an atrial myxoma and presenting with a diastolic ‘plop’, platypnea and progressive outflow obstruction.7 Because of the catastrophic sequelae of intra-atrial tumour, aggressive treatment is typically pursued regardless of the patient's overall prognosis.
Historically, the gold standard treatment has been surgical resection. In cases with extension of non-metastatic primary NSCLC, complete en bloc resection of the primary tumour and left atrium with or without the great vessels is favoured. In a series of 101 NSCLC patients undergoing en bloc resection, the 5-year survival rate was 13%, with a median survival of 9.2 months (including operative death); patients in whom a complete resection was achieved had significantly higher 5-year survival rate and median survival time of 19% and 13.8 months, respectively.8 Preoperative radiotherapy to doses of 26–50 Gy has been associated with improved prognosis following complete resection.9 Notably, detection of vascular invasion prior to surgical resection is critical, as failure to do so can create massive intraoperative embolisation of the tumour, causing stroke, acute arterial occlusion and possibly death.2 Surgery has also been employed in patients with metastatic extension of non-pulmonary tumours.3
Radiotherapy for cardiac lesions has been employed for years, with successful reports dating back to the early 1940s10 11 However, it has generally been utilised primarily in the instance of patient refusal or unsuitability, or to palliate large effusions.12–14 A concern with radiation monotherapy has been the development of radiation-related heart disease (RRHD) as a consequence of the irradiation of large cardiac volumes with the doses needed to achieve high tumour control probabilities15 16 (for further review, see17–19). At doses ≥30 Gy, RRHD can manifest within 2 years of treatment with the risk increasing with higher doses, younger age at irradiation and presence of other cardiac risk factors. At lower doses, the latency period can be over a decade. A lower threshold below which there is no risk has not been identified. RRHD can manifest in the pericardium, myocardium, valves, conduction system or coronary arteries. Chronic pericarditis is the most common form or RRHD with an incidence of 20% with doses >40 Gy; acute pericarditis is also a common form of RRHD. Due to lack of myocyte cell division, the myocardium is more radioresistant, and clinically significant disease (classically a restrictive cardiomyopathy) is only seen at high doses (>60 Gy) and/or in concert with anthracycline therapy. Valvular disease secondary to endocardial fibrosis presents late (>10 years latency) and is more commonly seen with doses >30 Gy in concert with anthracycline. Mixed aortic stenosis/regurgitation is the most common valvular disease, likely due to the high pressures and associated blood flow velocities across the aortic valve. In contrast, right-sided conduction system abnormalities, which also manifest late after doses >30 Gy, are more common because of the anterior location of the right bundle branches. The effect on the coronary arteries can be significant, with the relative risk of a myocardial infarction ranging from 2.2 to 2.8 for patients irradiated for Hodgkin's disease20 and 2.2 for women with left-sided breast cancer.21 Myocardial infarctions begin to occur 5–10 years after treatment, with the risk increasing with the presence of typical coronary artery disease risk factors, such as male gender, hypertension, cigarette use and hypercholesterolaemia.
Modern radiation oncology techniques such as intensity-modulation have greatly reduced the risk of RRHD by delivering a high dose to the tumour with sharp dose-gradient to the adjacent normal heart and other organs. Furthermore, due to the increased accuracy and precision of modern radiotherapy, less margin is applied to the gross tumour volume to overcome daily set-up or tumour/organ motion uncertainty. In particular, the risk of pericardial disease and of coronary artery disease has been significantly limited with strategies like deep inspiratory breath hold.22 Preclinical studies suggest that gating on the cardiac cycle may further reduce cardiac toxicity.23 Improved dose-delivery techniques can also increase the utility of radiation. In fact, intensity-modulated radiotherapy was recently employed to treat a cardiac metastases from small-cell lung cancer14 and anaplastic thyroid carcinoma.24 In the latter case, the patient was also pacemaker-dependent and the use of intensity modulated radiation therapy (IMRT) allowed the pacemaker to receive a maximum dose 0.37 Gy while the tumour received a maximum dose of 43.1 Gy. This underscores the high degree of precision and accuracy afforded by modern radiation oncology techniques. In our case, intensity-modulated radiotherapy was used to deliver 50 Gy to the lesion, with the maximum dose to the intra-atrial lesion reaching 55.8 Gy and a mean dose of 23 Gy to the uninvolved heart. The patient had no acute side effects from radiation, and in fact had complete resolution of her intracardiac disease within 3 months. The positive outcomes of these recent reports, with respect to cardiac disease burden, suggest that radiation can be safely used to treat neoplastic involvement of the atria from extracardiac neoplasms, whether it is secondary to primary lung cancer or not.
Learning points.
Atrial involvement is an uncommon but not rare feature of advanced non-small-cell lung carcinoma (NSCLC). Additionally, cardiac metastases from other primary tumours are increasing in frequency, occurring in up to 7% of cancer patients at autopsy.
Atrial involvement can lead to massive systemic embolisation or catastrophic outflow obstruction, and thus treatment is important even in patients in whom there is no hope of overall cure.
For NSCLC, complete en bloc resection of the primary tumour as well as involved great vessels and left atria can afford 5-year survival rates of up to 19%.
Advances in radiation oncology technology can allow for the successful, non-invasive relief of intra-atrial disease burden while minimising the risk of radiation-related heart disease.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Roberts TE, Hasleton PS, Musgrove C, et al. Vascular invasion in non-small cell lung carcinoma. J Clin Pathol 1992;45:591–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi K, Furuse M, Hanaoka H, et al. Pulmonary vein and left atrial invasion by lung cancer: assessment by breath-hold gadolinium-enhanced three-dimensional MR angiography. J Comput Assist Tomogr 2000;24:557–61. [DOI] [PubMed] [Google Scholar]
- 3.Funakoshi Y, Mukohara T, Kataoka T, et al. Left atrial extension of metastatic lung tumor via pulmonary vein: report on the first case of Ewing sarcoma. Rare Tumors 2010;2:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dogan U, Zamani A, Gormus N, et al. The first case report of a metastatic myxoid liposarcoma invading the left atrial cavity and pulmonary vein. Heart Surg Forum 2011;14:E261–63. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mamgani A, Baartman L, Baaijens M, et al. Cardiac metastases. Int J Clin Oncol 2008;13:369–72. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi AK, Pearson AC, Orsinelli DA. Tumor invasion of the pulmonary veins: a unique source of systemic embolism detected by transesophageal echocardiography. J Am Soc Echocardiogr 1995;8:97–9. [DOI] [PubMed] [Google Scholar]
- 7.Koo BC, Woldenberg LS, Kim KT. Pulmonary vein tumor thrombosis and left atrial extension in lung carcinoma. J Comput Tomogr 1984;8:331–6. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchiya R, Asamura H, Kondo H, et al. Extended resection of the left atrium, great vessels, or both for lung cancer. Ann Thorac Surg 1994;57:960–5. [DOI] [PubMed] [Google Scholar]
- 9.Fukuse T, Wada H, Hitomi S. Extended operation for non-small cell lung cancer invading great vessels and left atrium. Eur J Cardiothorac Surg 1997;11:664–9. [DOI] [PubMed] [Google Scholar]
- 10.Shelburne SA, Aronson HS. Tumors of the heart. II. Report of a secondary tumor of the heart involving the pericardium and the bundle of his with remission following deep roentgen-ray therapy . Ann Intern Med 1940;14:728–36. [Google Scholar]
- 11.Blotner H, Sosman MD. X-ray therapy of the heart in a patient with leukemia, heart block, and hypertension. N Engl J Med 1944;230:793–6. [Google Scholar]
- 12.Woodring JH, Bognar B, van Wyk CS. Metastatic chondrosarcoma to the lung with extension into the left atrium via invasion of the pulmonary veins: presentation as embolic cerebral infarction. Clin Imaging 2002;26:338–41. [DOI] [PubMed] [Google Scholar]
- 13.Lemus JF, Abdulhay G, Sobolewski C, et al. Cardiac metastasis from carcinoma of the cervix: report of two cases. Gynecol Oncol 1998;69:264–8. [DOI] [PubMed] [Google Scholar]
- 14.Orcurto MV, Delaloye AB, Letovanec I, et al. Detection of an asymptomatic right-ventricle cardiac metastasis from a small-cell lung cancer by F-18-FDG PET/CT. J Thorac Oncol 2009;4:127–30. [DOI] [PubMed] [Google Scholar]
- 15.Terry LN, Jr, Kligerman MM. Pericardial and myocardial involvement by lymphomas and leukemias. The role of radiotherapy. Cancer 1970;25:1003–8. [DOI] [PubMed] [Google Scholar]
- 16.Catterall M, Evans W. Myocardial injury from therapeutic irradiation. Br Heart J 1960;22:168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darby SC, Cutter DJ, Boerma M, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys 2010;76:656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidenreich PA, Kapoor JR. Radiation induced heart disease: systemic disorders in heart disease. Heart 2009;95:252–8. [DOI] [PubMed] [Google Scholar]
- 19.Gaya AM, Ashford RF. Cardiac complications of radiation therapy. Clin Oncol (R Coll Radiol) 2005;17:153–9. [DOI] [PubMed] [Google Scholar]
- 20.Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA 1993;270:1949–55. [PubMed] [Google Scholar]
- 21.Paszat LF, Mackillop WJ, Groome PA, et al. Mortality from myocardial infarction after adjuvant radiotherapy for breast cancer in the surveillance, epidemiology, and end-results cancer registries. J Clin Oncol 1998;16: 2625–31. [DOI] [PubMed] [Google Scholar]
- 22.Remouchamps VM, Vicini FA, Sharpe MB, et al. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys 2003;55: 392–406. [DOI] [PubMed] [Google Scholar]
- 23.Gladstone DJ, Flanagan MF, Southworth JB, et al. Radiation-induced cardiomyopathy as a function of radiation beam gating to the cardiac cycle. Phys Med Biol 2004;49:1475–84. [DOI] [PubMed] [Google Scholar]
- 24.Dasgupta T, Barani IJ, Roach M., III Successful radiation treatment of anaplastic thyroid carcinoma metastatic to the right cardiac atrium and ventricle in a pacemaker-dependent patient. Radiat Oncol 2011;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]