Abstract
A 23-year-old woman developed massive pulmonary haemorrhage in the 19th week of pregnancy. Essential invasive ventilation was seriously impaired by the mechanical properties of the blood-filled lungs. Consecutive severe respiratory failure (pO2 10 mm Hg, pCO2 320 mm Hg, pH 6.73) induced a cardiac arrest. Bronchoscopy could not identify the source of bleeding. During 45 min of cardiopulmonary resuscitation, veno-venous extracorporeal membrane oxygenation (ECMO) was installed. Subsequently, neither a high-resolution CT (HRCT) scan nor pulmonary angiography could identify the origin of the haemorrhage. Finally, the excessive pulmonary bleeding was controlled by placing an endobronchial blocker in the middle lobe bronchus. However, pulmonary haemorrhage reoccurred and this time HRCT revealed an isolated bronchiectasis in the middle lobe. Based on this finding, surgical lobectomy was performed. The patient recovered fully without any neurological sequelae. A solitary bronchiectasis has not previously been described as a cause of massive pulmonary haemorrhage in pregnancy.
Background
Severe acute respiratory failure is associated with high mortality. Veno-venous extracorporeal membrane oxygenation (ECMO) provides an extracorporeal blood circuit to enable oxygenation and carbon dioxide removal in the circulating blood (figure 1). Over more than three decades ECMO has been used as rescue bridging therapy when conventional ventilation has failed.1 The use of ECMO during or immediately after cardiopulmonary resuscitation (CPR) has been described in a few clinical series and case reports with encouraging results. But more detailed information about the neurological outcome and other major complications are lacking.2 The prognosis in prolonged hypoxic cardiac arrest still remains poor. Therapeutic anticoagulation with unfractionated heparin is essential for ECMO therapy. However, heparin is contraindicated in severe ongoing bleeding. Therefore, the usefulness of ECMO in life-threatening pulmonary haemorrhage has been questioned. However, in the absence of other therapeutic options, ECMO has been used in a few cases of pulmonary haemorrhage.3
Figure 1.
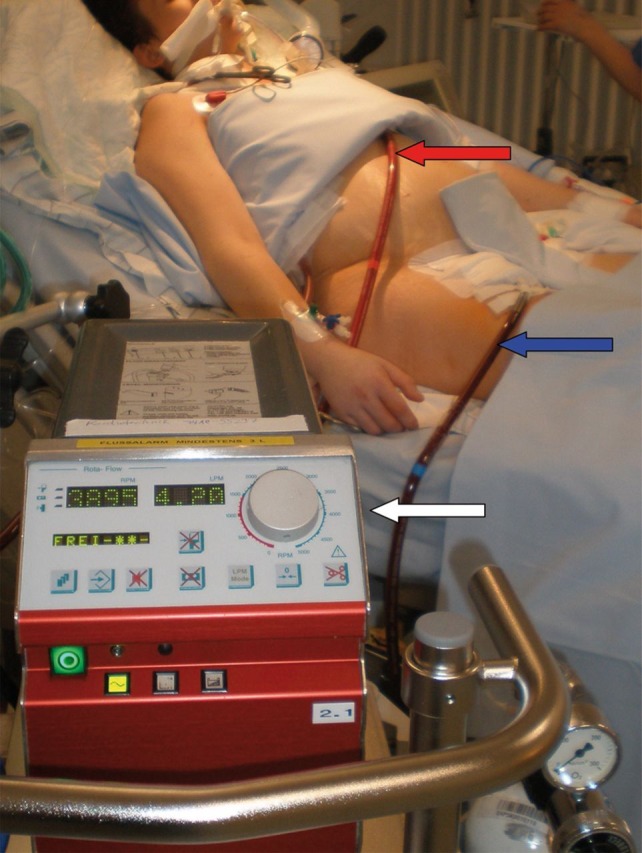
Extracorporeal membrane oxygenation (ECMO) in a pregnant woman on the intensive care unit (ICU). A venous outflow cannula (23F, 50 cm) inserted in the right femoral vein (blue arrow) delivers deoxygenated blood for the ECMO circuit. After oxygenation via an extracorporeal membrane, the blood is reperfused via a central venous inflow cannula (17F, 18 cm) inserted in the vena subclavia sinistra (red arrow). In this figure, the ECMO device (white arrow) produces an extracorporeal circulation with 4.2 l/min driven by a pump frequency of 3895 rotations per minute.
Case presentation
A 23-year-old woman at 19 weeks gestation was treated in a community hospital because of a respiratory infection and mild haemoptysis. She had no pre-existing illnesses and the pregnancy had proceeded normally. Two weeks after these initial symptoms, the patient developed severe pulmonary haemorrhage. Due to progressive respiratory failure, endotracheal intubation and mechanical ventilation were required and the patient was transferred to a university hospital. Around midnight she was admitted to the emergency department with extreme hypoxia (pulse oxymetric oxygen saturation, SaO2 73% despite ventilation with 100% oxygen).
Invasive ventilation was seriously impaired because of the mechanical properties of the blood-filled lungs. Despite volume-controlled ventilation with high ventilation pressures (inspiratory pressure 45 cm H2O, positive end-expiratory pressure (PEEP) 5 cm H2O) a maximum tidal volume of only 150 ml could be generated. Immediate bronchoscopy showed the bronchial system completely filled with fresh blood and some clots. Under these circumstances, it was impossible to identify an isolated source of bleeding. Finally, the pulmonary haemorrhage caused ventilatory arrest with severe hypoxic and hypercapnic respiratory failure (pO2 10 mm Hg, pCO2 320 mm Hg, pH 6.73) and secondary hypoxic cardiac arrest. CPR was performed using a mechanically controlled chest compression device (LUCAS, Jolife, Lund, Sweden). Simultaneously, the decision was made to implant a veno-venous ECMO (PLS-System and Rotaflow, Marquet, Rastatt, Germany) in this apparently unpromising clinical situation (figure 1). It took 45 min until femoral and jugular venous cannulas were inserted and an ECMO circuit was established. Meanwhile, CPR was continuously performed in response to the persistent hypoxia. At this time, ultrasound showed a viable fetus without relevant bradycardia. Attempts to localise bleeding via rigid bronchoscopy and to re-establish ventilation by lung separation with a double lumen tube were unsuccessful.
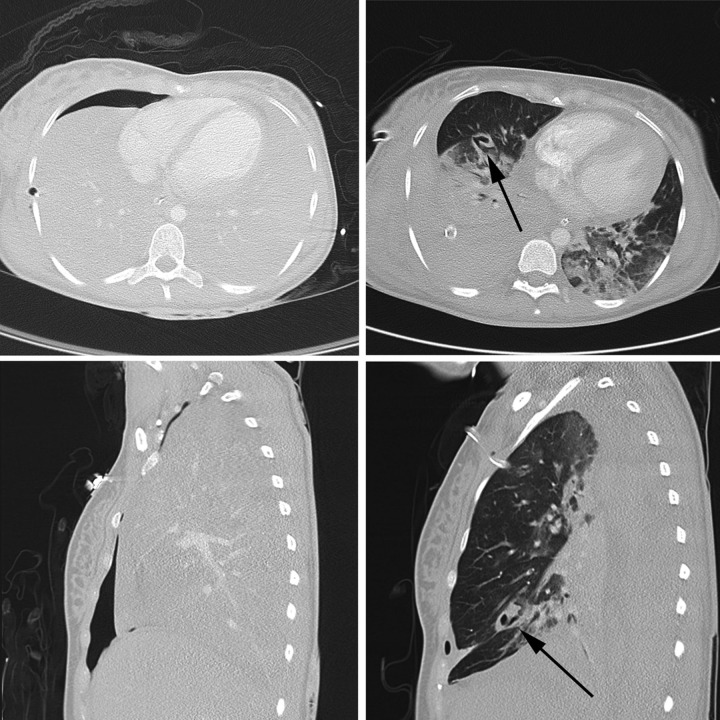
After successful resuscitation and ongoing ECMO support, arterial oxygenation and carbon dioxide elimination (pO2 62 mm Hg, pCO2 39 mm Hg and pH 7.45) normalised without mechanical ventilation. Under these conditions, neither a high-resolution CT (HRCT) scan nor a pulmonary angiogram revealed the source of bleeding because the bronchial system was completely filled and tamponated with blood (figure 2). The patient was transferred to the intensive care unit (ICU). A total of 16 units of packed erythrocytes had to be transfused within the first 24 h. Meanwhile, repeated bronchoscopy localised the source of bleeding predominantly in the middle lobe bronchus. The haemorrhage was controlled by placing a selective endobronchial blocker in the middle lobe bronchus for 24 h. The respiratory function gradually improved and adequate mechanical ventilation could be restarted.
Figure 2.
Thoracic CT scan in transverse (above) and sagittal (below) projection; left: after admission to the emergency department: massive intrabronchial and intraparenchymal blood and ventral pneumothorax; right: 13 days later CT clearly showed a solitary bronchiectasis (black arrow) in the middle lobe.
Despite the life-threatening pulmonary bleeding, therapeutic anticoagulation with unfractionated heparin for the extracorporeal circuit was started, guided by an activated partial thromboplastin time target of 50 s. After 3 days of ECMO therapy, a huge haematothorax on the right side caused a haemodynamically relevant mediastinal shift and jugular-venous congestion and was treated with multiple chest drains and pleural irrigation. Furthermore, the venous inflow ECMO cannular was relocated to the left vena subclava (figure 1).
Several bronchoscopies were required over the next few days to clear the bronchial system of blood clots. In the early period after removal of the endobronchial blocker, no further bleeding occurred. The pulmonary function recovered, ventilation further improved and ECMO therapy was stopped on day 8. Due to an anticipated prolonged weaning, percutaneous dilatational tracheostomy was performed the following day. On day 12, a second massive pulmonary haemorrhage occurred. Once again, the patient had to be resuscitated because of severe hypoxia while another endobronchial blocker was placed in the middle lobe via bronchoscopy.
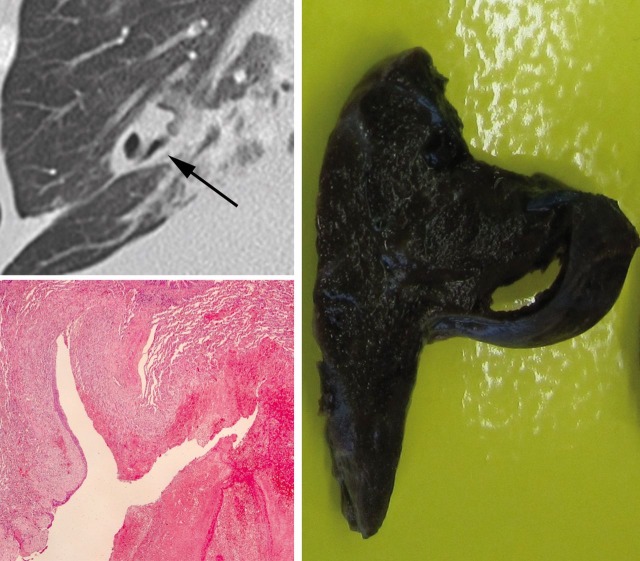
This time a CT scan clearly revealed a solitary bronchiectasis in the right middle pulmonary lobe (figure 3) as the source of bleeding. This had been covered with blood in the first CT scan. Based on these findings the middle pulmonary lobe was surgically resected. Subsequently, no pulmonary bleeding occurred, the patient was weaned from the mechanical ventilation and the tracheal cannula was removed 30 days after admission.
Figure 3.
Left above: magnification of the CT scan showing a bronchiectasis in the middle pulmonary lobe (black arrow); left below: micrograph showing an ectatic bronchus with focal epithelial cell metaplasia, ulceration of the mucosa, some haemorrhage and granulating inflammation (H&E; magnification × 2.5) right: correlating 7.2 × 4.7 × 1.2 cm macroscopic lung resectate.
Differential diagnosis
Extended haemostasiological investigations showed neither a thrombopathy nor a plasmatic coagulopathy. Autoimmune causes of the coagulation disorder such as antiphospholipid syndrome, lupus erythematosus, Wegener's granulomatosis, Goodpasture syndrome or genetic causes such as haemophilia or von Willebrand disease were excluded. Toxicology did not identify a drug-induced haemorrhagic diathesis. Bronchoalveolar lavage, urine culture, or blood samples did not reveal positive microbiological results. Further, extensive investigations for viral or bacterial pneumonia, tuberculosis, mycosis or autoimmune disease all remained negative. Until the second CT scan was performed, the aetiology of this life-threatening pulmonary haemorrhage remained unknown.
Outcome and follow-up
On day 10, fetal ultrasound suddenly showed bilateral cerebral ventriculomegaly which strongly suggested hypoxic–ischaemic cerebral alterations. After a multidisciplinary ethical conference and perinatal counselling, both parents decided to abort the fetus. The patient was discharged home 37 days after admission without any respiratory or neurological sequelae. At 6 and 12 months follow-up visits, the patient returned to our department with her husband and her 4-year-old son in excellent health and had returned to work. She appeared intellectually unimpaired and denied any neurological deficits. In a recent follow-up telephone interview she reported that she was pregnant again.
Discussion
In pregnant women, haemoptysis is generally mild and has an identifiable pathogenesis. But frequently the cause of pulmonary haemorrhage in pregnancy cannot be identified and is therefore classified as idiopathic.4 The close association with the duration of pregnancy suggests that pulmonary haemorrhage may be related to hormonal changes. An association with abnormally dilated and tortuous bronchial arteries has been described in pregnant women with massive haemoptysis and some authors have questioned whether idiopathic haemoptysis in pregnant women is a distinct entity.4 A solitary bronchiectasis responsible for massive pulmonary haemorrhage in pregnancy has not been described until now. During the clinical course, we generated the hypothesis that a pre-existing solitary bronchiectasis might have been aggravated by a respiratory viral infection and hormonal changes. But that was highly speculative and a viral infection was never confirmed in this case.
The presented case demonstrates that even sophisticated technical approaches such as pulmonary angiography and HRCT may fail to identify the source of pulmonary haemorrhage. This limitation in the diagnostic workup of severe acute pulmonary haemorrhage might lead to an incomplete diagnosis or imprecise clinical hypothesis.
Patients with severe pulmonary bleeding are best treated in centres with ECMO facilities.1 Successful use of veno-venous ECMO in pregnancy has been reported5 but to our knowledge ECMO therapy for massive pulmonary bleeding with hypoxic cardiac arrest in pregnancy has not been described until now. This unique case describes a solitary bronchiectasis causing severe pulmonary haemorrhage in a pregnant woman successfully treated with veno-venous ECMO for prolonged resuscitation, selective endobronchial blocking and surgical lobectomy.
Learning points.
A solitary bronchiectasis is a very rare differential diagnosis for pulmonary haemorrhage in pregnancy.
Emergency treatment options are lung separation with a double lumen tube, selective endobronchial blocking and surgical lobectomy.
The prognosis in prolonged hypoxic (paO2 10 mm Hg) and hypercapnic (pCO2 320 mm Hg) cardiac arrest is poor but not hopeless.
Veno-venous ECMO is a useful last therapeutic resort in hypoxic cardiac arrest caused by pulmonary bleeding in certain carefully selected cases.
Even sophisticated diagnostic approaches (high-resolution CT, pulmonary angiography) may fail to localise the source of pulmonary haemorrhage.
Acknowledgments
We thank Hans Klose, Head of the Section Pneumology, Department of Internal Medicine II, University Medical Centre Hamburg-Eppendorf, for his support in clinical treatment and decision-making and for providing clinical and scientific pneumological expertise when reviewing this case report.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351–63. [DOI] [PubMed] [Google Scholar]
- 2.Cardarelli MG, Young AJ, Griffith B. Use of extracorporeal membrane oxygenation for adults in cardiac arrest (E-CPR): a meta-analysis of observational studies. ASAIO J 2009;55:581–6. [DOI] [PubMed] [Google Scholar]
- 3.Arokianathan D, Trower K, Pooboni S, et al. Leptospirosis: a case report of a patient with pulmonary haemorrhage successfully managed with extra corporeal membrane oxygenation. J Infect 2005;50:158–62. [DOI] [PubMed] [Google Scholar]
- 4.Peyrat E, Chabbert V, Escamilla R, et al. Idiopathic hemoptysis in pregnant women: a distinct entity?. Respir Med 2007;101:2221–3. [DOI] [PubMed] [Google Scholar]
- 5.King PT, Rosalion A, McMillan J, et al. Extracorporeal membrane oxygenation in pregnancy. Lancet 2000;356:45–6. [DOI] [PubMed] [Google Scholar]