Abstract
Background:
Alzheimer's disease (AD) is an age-related progressive neurodegenerative disease, which is characterized clinically by serious impairment in memory and cognition. Current medications only slow down the dementia progression and the present treatment one-drug one-target paradigm for anti-AD treatment appears to be clinically unsuccessful. Therefore, alternative therapeutic strategies are urgently needed. With respect to multifunctional and multitargeted characteristics of Rosa damascena via its effective flavonoids, we investigated the effects of R. damascena extract on behavioral functions in a rat model of amyloid-β (A-β)-induced Alzheimer's disease.
Materials and Methods:
After preparation of the methanolic extract of the R. damascena, HPLC analysis and toxicity studies, median lethal dose (LD50) and dose levels were determined. For evaluation of baseline training behavioral performance, Morris water maze and passive avoidance tests were used. A-β was injected bilaterally into CA1 area of the hippocampus. Twenty-one days after injection of A-β, the first probe trial of the behavioral tests were used to confirm learning and memory impairment. To examine the potential effects of the extract on behavioral tasks, the second probe trials were performed after one month administration of R. damasena extract.
Results:
Results showed that the R. damascena extract significantly improved the spatial and long-term memories in the extract- treated groups in a dose-dependent manner, as in the middle and high doses it had significant effect.
Conclusion:
According to these results, we concluded that R. damascena can reverse behavioral deficits caused by A-β, and may provide a new potential option for prevention and treatment of the cognitive dysfunction in Alzheimer's disease.
Keywords: Alzheimer's disease, learning and spatial memory, long-term memory, Rosa damascena
INTRODUCTION
Alzheimer's disease (AD) is one of the most serious neurodegenerative diseases and the most common cause of dementia in aging population affecting approximately 26 million people worldwide, and whose prevalence has been calculated to triplicate by 2050.[1,2] AD is characterized clinically by progressive impairment in memory, cognition, and behavioral functions.[3] It is believed that AD initiates with synaptic impairment, neuronal loss, microglial cell proliferation, and is followed by inflammation.[4,5] The neuropathological feature of AD is complex, including cerebral accumulation of extracellular amyloid-β (A-β) plaques and intraneuronal neurofibrillary tau tangles composed of dystrophic neuritis and hyperphosphorylated tau protein.[6] According to the “amyloid hypothesis,” the soluble A-β in the brain, plays an important role in the development of AD.[7] Despite catastrophic increase of dementia patients worldwide, no effective treatment is available yet, although several acetylcholine esterase inhibitors such as donepezil, rivastigmine, and galantamine usually were used, but these medicines can only slow down the progression of dementia, rather than restoring brain function in a real clinical situation. Therefore, alternative and multitargeted therapeutic strategies are urgently needed to prevent or treat Alzheimer's disease.[8,9] Because AD arises via multiple pathological or neurotoxic pathways, herbal medicines as a result of multifunction, multitarget characteristics have potential of optimum pharmaceutical and nutraceutical effects on AD patients.[10]
Medicinal herbs-derived agents have different mechanisms from conventional drugs, which can be effective in clinical treatment. Herbs contain some of the most powerful natural antioxidants and bioactive secondary metabolites such as flavonoids, phenols, or phenolic components that make them valuable in their antioxidant and antiaging effects.[11] Rosa damascena is a plant that belongs to genus Rosa and family Rosaceae. Rosaceae are well known as ornamental plants and have referred to the king of flowers. Some members of the Rosaceae family have long been used for food and medical purposes. Most of the studies showed that R. damascena as a natural plant with high source of flavonoids such as quercetin, kaemferol, myricetin, gallic acid, and their glycoside derivatives may have curable effect on treatment of diseases.[11,12] It has been shown that essential oil of R. damascena retards the development of behavioral seizures in amygdale electrical kindling and possesses ability of contract kindling acquisition.[13] There is considerable evidence suggesting that extract of the R. damascena significantly induces the neurite outgrowth and inhibits the A-β fibrilization and deposition in brain.[14] Also it is shown that the R. damascena oil can relieve human stress and depression.[15] Flavonoids perform a multiplicity of neuroprotective functions within the brain, including neural protection against neurotoxin injuries, neuroinflammative suppression and promotion of memory, learning, and cognitive function. These effects could be mediated by flavonoids that inhibit apoptosis, promote neuronal survival and synaptic plasticity. They can also promote cerebrovascular blood flow, angiogenesis, neurogenesis, and neuronal morphology.[16] The aim of the current study was to determine the effects of methanolic extract of R.damascena on behavioral functions in a rat model of amyloid- β (1- 42) induced Alzheimer's disease.
MATERIALS AND METHODS
Methanolic extraction of R. damascena
Dried fresh flowers of R. damascena Mill was purchased from Isfahan Pharmaceutical Sciences Research Center (Iran). It was ground to a fine powder (2 kg) and then macerated with methanol (10 L). Extraction procedure was performed three times and each time for 4 consecutive days with intermittent stirring (1 h stirring at every 12 h interval) using magnetic stirrer until the extract was light colored. The combined extracts were filtered and concentrated under reduced pressure in a rotary vacuum evaporator (200 mbar) in a water bath at 40°C. Dried gummy extract (380 g) was stored in 4°C until use.[11]
HPLC analysis and extract standardization
High-performance liquid chromatographic (HPLC) analysis was performed on a Waters system, equipped with 515 HPLC pump, UV–Visible detector (2487 dual absorbance) operated at 365 nm, and millennium software for the determination of quercetin in the extract. After acid hydrolysis of 100 mg of the extract (1 h in 2N HCl, at 95°C), the hydrolyzed flavonoids were extracted through ethyl acetate to 5 mL. Then 20 μl of the sample injected into a Nova-Pak C18, 3.9 × 150 mm (Waters, Milford, MA, USA) using H3PO4 10 mM in water (solvent A) and acetonitrile (solvent B) with gradient elution at a flow rate of 0.8 mL/min. A standard calibration curve in the range of 100–1000 μg/mL for quantitative analysis was prepared using different concentrations of quercetin (Sigma Aldrich, USA) as standard material (100, 200, 500, and 1000 μg/mL). The relationship between the concentration and peak-area of standard was measured using the minimum square method (R2 value).[17]
Acute toxicity studies and determination of median lethal dose (LD50)
Toxicity is defined as “the potential of a substance to exert a harmful effect on humans or animals, and a description of the effect and the conditions or concentration under which the effect takes place.”[18]
Acute toxicity is involved in the estimation of LD50 (the dose which has proved to be lethal (causing death) to 50% of the tested group of animals). Determination of acute oral toxicity is usually an initial screening step in the assessment and evaluation of the toxic characteristics of all compounds.[19,20]
Assignment and housing of the animals for toxicity studies
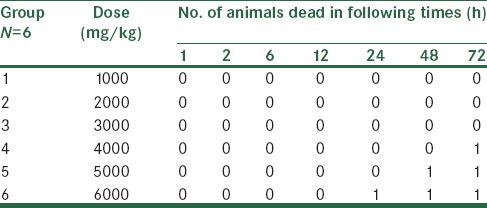
Each animal was assigned a unique identification number. Thirty-six male Wistar rats (200–250 g) were housed six per cage. Six rats in each group at each dose level were chosen. In the present study, rats were gavaged via a gastric tube with increasing six series doses of R. damascena extract to get 50% lethality according to table 1. One dose being used per group. The animals were observed in several times up to 72 h for survival and morbidity.[21,22] Median lethal dose (LD50) was calculated as given in table 1.
Table 1.
Determination of (LD50)
Dose levels and dose selection
This is based on the results of (LD50) finding test. At least three dose levels were used, spaced appropriately to produce test groups. For dose selection, 20% of (LD50) 6000 mg/kg was determined as the highest dose (1200 mg/kg) and decreasing spaced doses of 600 and 300 mg/kg were considered as middle and low doses, respectively, in our experimental study.[23]
Animals and stereotaxic surgery
Male Wistar rats (200–250 g) were housed four per cage and maintained on a 12 h light–dark cycle in an air-conditioned constant temperature (23 ± 1°C) room, with food and water made available ad libitum. The Ethics Committee for Animal Experiments at Isfahan University of Medical Sciences approved the study, and all experiments were conducted in accordance with the international guiding principles for biomedical research involving animals, revised in 1985. Initially, all animals were randomly divided into 6 groups (n = 10) for evaluation of baseline training performance in Morris water maze and passive avoidance tests. After spatial acquisition phase of Morris water maze and training (learning) phase of the passive avoidance test, animals were grouped as following: First group = control, Second group = sham, Third group = Alzheimer's disease + Normal saline, Fourth group = Alzheimer's disease + 300 mg of R. damascena extract, Fifth group = Alzheimer's disease + 600 mg of R. damascena extract and sixth group = Alzheimer's disease + 1200 mg of R. damascena extract. The protocol of experimental design is summarized in Figure 1.
Figure 1.
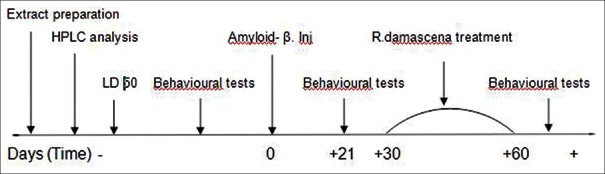
Experimental schedule of the research design during the course of the study
Rats (groups 3rd to 6th) were anesthetized by intraperitoneal injection of chloral hydrate (350 mg/kg) and placed into stereotaxic device (Stoelting, Kiel, WI, USA). A heating pad was used to maintain body temperature at 36.5 ± 0.5°C. An incision was made along the midline, the scalp was retracted, and the related area was cleaned and dried. In addition, lidocaine and epinephrine solution (0.4 mL) were injected in several locations surrounding the incision. In order to make minimum brain trauma during infusion, injections were performed very slowly (1 μL/min) using a microinjection pump. Amyloid-β (1-42) (Sigma) was dissolved in phosphate buffered saline (PBS 0.1 M) and aliquots at a concentration of 1 μg/μL were stored at −20°C until used. For induction of aggregation, aliquots of amyloid-β (1-42) were incubated for 5 days at 37°C before administration. Then 3 μg/3 μL of fibrillar A-β (1–42) or vehicle (PBS 0.1) were injected into the CA1 area of hippocampus at the following coordinates: Incisor bar −3.3 mm, −3.6 mm anterior-posterior to the bregma, 2.4 mm lateral to the sagittal suture, 2.8 mm dorsoventral from top of the skull bilaterally according to the atlas by Paxinos and Watson.[24] The needle was kept in place for 5 min to allow the injected solutions and tissue to equilibrate and avoid the possible reflux through the needle track. Incisions were ligated with silk thread. In sham group, same surgery was performed except that PBS was injected in CA1. No surgery was done in the control group.
Morris water maze
The circular tank (180 cm in diameter) was filled with water (22 ± 2°C) made opaque and was surrounded by a variety of extramaze cues. The tank was divided into four equal quadrants (northeast, northwest, southwest, and southeast) and four start positions were located at the intersection of the quadrants [Figure 2a]. A platform (12.5 cm in diameter) was placed in the northeast (the target quadrant) and submerged 2.0 cm below the water surface where it remained for all spatial trials.[25,26,27] Twenty-four hours before water maze training, all rats were habituated to the water and apparatus. In the spatial acquisition phase, the rats learned to find a submerged platform using extramaze cues. Each rat participated in eight trials that were organized into two blocks of four trials (one trial/start position within a block). Each block was considered a separate test session and blocks were separated by 30 min. For each trial, the rat was given a maximum time of 60 s to locate the platform. After mounting the platform, the animals were allowed to remain there for 30 s, and then were placed in a holding cage for 30 s until the start of next trial. If the rat did not locate the platform within 60 s, it was guided to it by the experimenter. After completion of spatial acquisition phase, the animals were returned to their home cages until the initiation of probe trials in the test days. During the different phases of the maze, the animals were monitored by digital camera fixed 2 m above the maze and different parameters were analyzed using computer-based software (Radiab 1). Swim time (in s), swim distance (in cm), and swim speed (in cm/s) were recorded.
Figure 2.
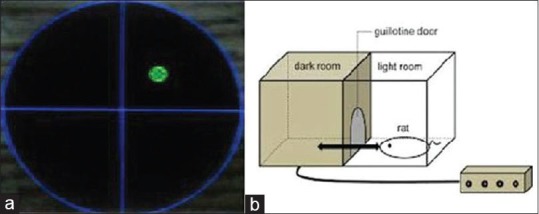
(a) Tank of the Morris water maze and site of the platform. (b) Schematic representation of the passive avoidance test apparatus
The first and second probe trials (spatial retention) 21 days after surgery and following extract treatment were performed, respectively. In the probe trials, the hidden platform was removed and animals were allowed to swim freely for 60 s. During the probe trials, the percentage time in target quadrant was calculated. Cued acquisition task conducted 20 min after completion of the probe trials in order to test their nonmnemonic aspects of water maze performance such as swimming ability, motivation, and visual ability. In this phase, the platform was elevated above the water surface and was placed in different positions, and rats were allowed to swim freely toward visible platform for 60 s. Twenty-one days after operation the 1st probe trial of Morris water maze and passive avoidance tests were performed and memory impairment was confirmed in groups 3–6.
Then dried R. damascena extract was dissolved in normal saline 0.9% +1% Tween 20, and then was administrated orally at 300, 600, 1200 mg/kg/day for 1 month via gavage in groups 4–6. Sham and 3rd groups received only normal saline at the same time. After R. damascena extract treatment 2nd probe trial of Morris water maze and passive avoidance tests were performed to evaluate the effects of extract on learning and spatial and long-term memories.
Passive avoidance test
The apparatus for the step-through passive avoidance test is an automated shuttle-box divided into an illuminated chamber and a dark chamber of the same size (20 × 25 × 30 cm each) by a wall with a guillotine door[28,29,30] [Figure 2b]. In the present study, passive avoidance test was used as an index of long-term memory.
In the adaptation trial, each rat was trained to adapt to the step-through passive avoidance apparatus. The animal was placed into the illuminated chamber, facing away from the dark chamber. After 60 s, the door between these two boxes was opened and the rat was allowed to move into the dark chamber. The training trial was performed 24 h after the adaptation trial. In training trial, each rat was placed individually in the illuminated chamber, and once it entered the dark chamber an electric shock (40 V, 0.3 A, 2 s) was delivered to its feet through the floor grid. The rat was immediately removed and returned to cage. The first probe trial (retention trial) was conducted 21 days after surgery, in which rats were placed again in illuminated chamber and the interval (step-through latency) between placement in illuminated chamber and entry to the dark one was recorded. If the animal did not enter the dark chamber within 5 min, the test was terminated and the step through latency was recorded as 300 s. Memory impairment was confirmed in groups 3–6. Then R. damascena extract was applied as described previously. After R. damascena extract treatment, 2nd probe trial of passive avoidance was performed to evaluate the effects of extract on long-term memory.
Statistical analysis
Data were expressed as mean ± SEM (standard error of mean) and were analyzed using either SPSS version 19 or GraphPad Prism® 5.0. software. Statistical analysis was performed using the following tests. The escape latencies, swim distance, and swim speed in the water maze were analyzed by two-way analysis of variance (ANOVA) for between-subject differences between groups and repeated measures (within subjects) for effects across block interval 1–2 (“BLOCK” effect). The probe trials data for percentage of time in target quadrant were analyzed by multivariate ANOVA followed by Tukey's honestly significant difference (HSD) test as a post hoc analysis. One-way ANOVA followed by Tukey's HSD multiple comparison test as a post hoc analysis were performed for evaluation of training trial and retention trials of passive avoidance test. A value of P < 0.05 was considered statistically significant.
RESULTS
HPLC standardization of the extract
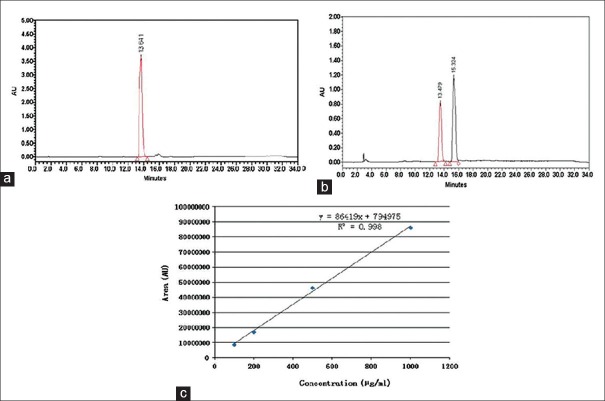
Methanolic extraction of the R. damascena was performed and standardized using quercetin as the bioactive marker for standardization of the extract. Quercetin peak of extract appeared at a retention time of 13.48 min and using calibration curve, the extract was standardized to contain 548.89 ± 20.23 mg/100 g of the total quercetin (0.55% w/w) [Figure 3a and b]. By using of millennium processing software, the calibration curve was determined by linear regression in the range of 100–1000 μg/mL. The regression equation was y = 86419x + 794975, where x was the concentration of total quercetin in the extract (μg/mL) with the correlation co-factor (R2) of 0.998 [Figure 3c].[14]
Figure 3.
(a) HPLC chromatogram of quercetin standard at 365 nm; 20 μL of the quercetin standard (Sigma Aldrich, USA) in the range of 100–1000 μg/mL was injected into a Nova-Pak C18, 3.9 × 150 mm (Waters, Milford, MA, USA) using H3PO4 10 mM in water (solvent A) and acetonitrile (solvent B) with gradient elution at a flow rate of 0.8 mL/min. Quercetin peak appeared at a retention time of 13.64 min. (b) HPLC chromatogram of Rosa damascena extract at 365 nm. After acid hydrolysis of 100 mg of the extract (1 h in 2N HCl, at 95°C), the hydrolyzed flavonoids were extracted through ethyl acetate to 5 mL. Then 20 μL of the sample was injected into a Nova-Pak C18, 3.9 × 150 mm (Waters, Milford, MA, USA) using H3PO4 10 mM in water (solvent A) and acetonitrile (solvent B) with gradient elution at a flow rate of 0.8 ml/min. Quercetin peak of the extract appeared at a retention time of 13.48 min. (c): Calibration curve of quercetin using HPLC method and acetonitrile/water as mobile phase with pH adjusted to 2.3 at 365 nm. Using the millennium processing software, the calibration curve was determined by linear regression in the range of 100–1000 μg/mL. The regression equation was y = 86,419x + 7, 94,975
Evaluation of the spatial acquisition phase of the Morris water maze before animal model induction
There were no significant differences in the mean time latency (F (5, 54) =0.041, P = 1 >0.05), mean swimming distance (F (5, 54) =0.128, P = 0.9 >0.05), and mean swimming speed (F (5, 54) =0.426, P = 0.8 >0.05) in blocks 1 and 2 of the spatial acquisition among all groups before application of A-β [Figure 4a–c, blocks 1 and 2], but repeated measure test showed a significant difference in escape latency, swimming distance, and swimming speed (F (1, 54) = 74.96, P < 0.001); (F (1, 54) = 397.22, P < 0.001); (F (1, 54) = 20.14, P < 0.001 respectively) between blocks 1 and block 2 were observed [Figure 4a–c, blocks 1 and 2] to locate the platform across blocks of trials, indicating spatial acquisition performance in all groups improved significantly during the training days and were able to learn the task.
Figure 4.
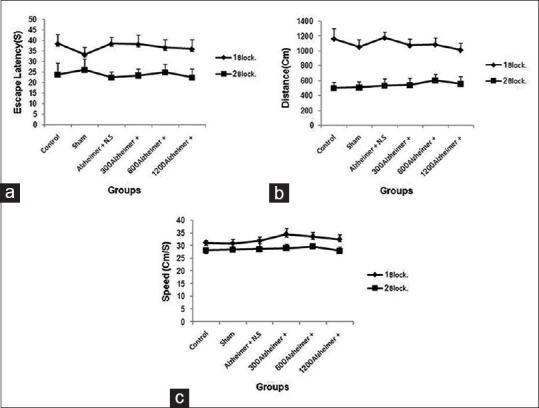
Behavioral assessment. In spatial acquisition phase there were no significant differences in block 1 and 2 among the six groups before application of amyloid-β, but Repeated measure test showed a significant reduction in (a) escape latency (P < 0.001), (b) swimming distance (P < 0.001), and (c) swimming speed (P < 0.001) in 2nd block compared with 1st block to locate the platform across blocks of trials
Evaluation of the training trial of the passive avoidance test before animal model induction
One-way ANOVA showed that the step-through latency did not differ in the training trial among the six groups before operation (F (5, 54) =0.069, P = 0.9 > 0.05) [Figure 5].
Figure 5.
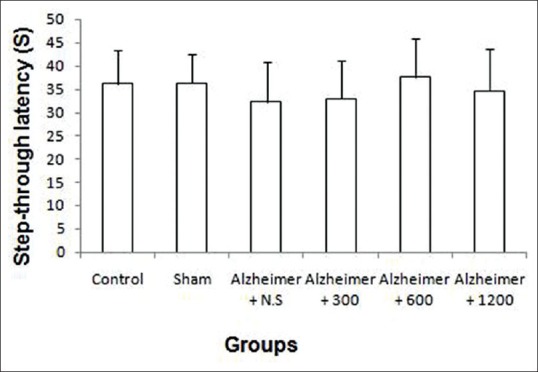
In training trial of the Passive avoidance test, there were no significant differences in step-through latency among the six groups (P = 0.9)
R. damascena extract improved the spatial learning and memory parameters in a rat model of Alzheimer's disease
Multivariate ANOVA analysis showed significant differences in 1st probe trial among groups (P < 0.001) [Figure 6]. Post hoc Tukey's HSD analysis showed that, the mean percentage of time spent in target quadrant in A-β-injected groups (3rd to 6th) after 21 days surgery was significantly decreased in comparison to control and sham groups (P < 0.001), but no significant differences were demonstrated in mean percentage of time spent in target quadrant between control and sham groups, (P = 0.9 > 0.05). These results showed that A-β–induced learning and memory impairment in the rats. Multivariate ANOVA analysis showed that mean percentage of time spent in target quadrant in 2nd probe trial after one month R. damascena extract treatment was significantly increased among groups (P < 0.001) [Figure 6]. Post hoc Tukey's HSD analysis showed that, mean percentage of time spent in target quadrant was significantly increased in extract-treated groups (5th and 6th groups) relative to 3rd group, which had received normal saline (P = 0.006 < 0.05, P < 0.001, respectively), but mean percentage of time in target quadrant in 4th group did not significantly increase relative to 3rd group (P = 0.3 > 0.05). Also mean percentage of time in target quadrant in 6th group was significantly increased in comparison to 4th (P = 0.002 < 0.05). Taken together, these results showed that R. damascena extract improved spatial learning and memory in a dose-dependent manner.
Figure 6.
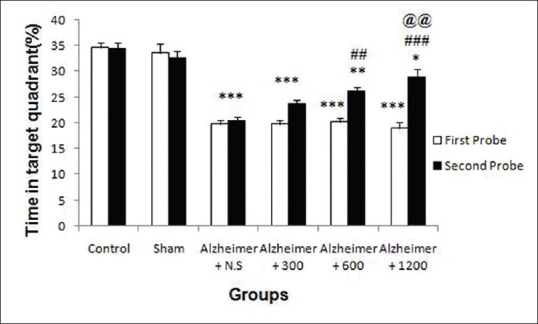
Effects of the amyloid-β and Rosa damascena extract on spatial memory during the first (A, white color) and second (A, black color) probe trials, respectively, as measured by mean percentage time in target quadrant. **P < 0.01; ***P < 0.001 different from the control and sham groups. ##P < 0.01, ###P < 0.001 different from the Aβ-injected group (Alz + NS), @P < 0.05, @@@P < 0.001 different from the Aβ-injected group (Alz + 300)
R. damascena extract improved the long-term memory parameters in a rat model of Alzheimer's disease
One-way ANOVA showed that significant differences in mean step-through latency among groups were observed following surgery in the 1st probe trial (F (5, 54) =275.94, P < 0.001) [Figure 7]. Post hoc Tukey's HSD analysis showed that mean step-through latency reduced in Aβ-treated groups (3rd to 6th) compared with control and sham groups (F (5, 54)=119.24, P < 0.001). One-way ANOVA demonstrated that following extract treatment, step-through latency significantly increased among groups (P < 0.001) [Figure 7]. The mean step-through latency in groups 5 and 6 was significantly increased in comparison to 3rd group (P < 0.001). Mean step-through latency in 4th group did not significantly increase relative to 3rd group (P = 0.07). This parameter in 5th and 6th groups significantly increased when compared with 4th group (P < 0.001). All together these data show that R. damascena extract in medium (600 mg/kg) and high dose (1200 mg/kg) improved long-term memory in a dose-dependent manner.
Figure 7.
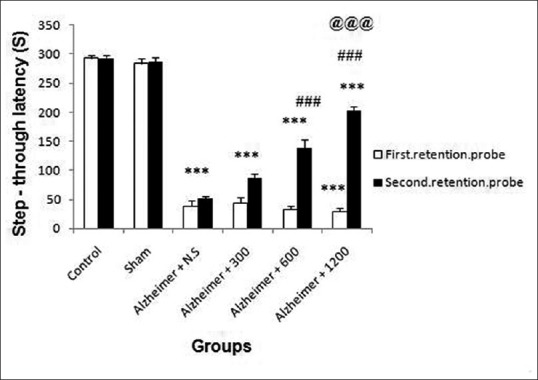
Effects of the amyloid-β and Rosa damascena extract on long-term memory during the first (B, white color) and second (B, black color) probe trials, respectively, as measured by mean time step-through latency. **P < 0.01; ***P < 0.001 different from the control and sham groups. ##P < 0.01, ###P < 0.001 different from the Aβ-injected group (Alz + NS), @P < 0.05, @@@P < 0.001 different from the Aβ-injected group (Alz + 300)
DISCUSSION
The goodness of usual anti-AD drugs, is just a temporary relief of clinical symptoms and signs of dementia, but the efficacy of these cholinesterase inhibitors in AD treatment is not supported by any high-quality and long-lasting clinical trials.[9] These medications cannot cure Alzheimer's dementia or stop the disease progression.[31,32] Because AD is a multifactorial disease, using multifunctions and multitargeted agents have been considered extensively.[33,34] Enormous medical combination therapies have been proposed to cure Alzheimer's disease.[35] Herbal medicines as a result of multifunction, multitarget characteristics have potential of optimum pharmaceuticals and nutraceuticals effects on AD patients.[10] Preclinical and epidemiological studies suggest that herbal drugs might be effective at reversing neurodegenerative pathology and age-related declines in neurocognitive performance.[36] Herbal drugs may act to protect the brain in a number of ways, including by protection of vulnerable neurons, promotion of peripheral and cerebral vascular system, enhancement of existing neuronal function or by stimulating neuronal regeneration and inhibition of neuroinflammation.[37] Moreover, medicinal herbs–derived drugs are natural and hence safer than synthetic drugs, and that a complex mixture of herbs can effectively treat complex diseases. These advantages may account for the sudden increase in herbal use in the last decade.[38] In addition, some of the experimental reports suggest that some herbs may have neuroprotective effects against amyloid-β.[39,40]
The present study examined the effects of a methanolic extract of R. damascene on behavioral deficits in a rat model of amyloid-β (Aβ1-42)–induced Alzheimer's disease. Morris water maze and passive avoidance tests were used for examination of behavioral changes caused by amyloid-β and possible effect of R. damascena extract on learning, spatial memory, and long-term memories.[29,30,41,42] The memory impairment induced by intra-CA1 injection of Aβ in our study was in agreement with Yamada et al.[43] One month after R. damascena extract treatment, spatial memory and long-term memory were improved, whereas cognitive decline was reversed in a dose-dependent manner. The neuroprotective effect of R. damascena against cytotoxicity of A-β was investigated by Awale et al. in vitro. They showed that R. damascena extract significantly causes neurite outgrowth and suppresses the Aβ - induced atrophy and cell death. Moreover, the length of dendrite in the cells treated with R. damascena extract was similar to those of nerve growth factor (NGF)–treated cells.[14] Significant effect of R. damascena on Aβ-injected groups in our study could be because of its multifactorial effects of bioactive flavonoids, polyphenols, and secondary active metabolites. Kumar et al. had detected and quantified some of polyphenols including gallic acid, rutin, quercitrin, myricetin, quercetin, and kaempferol, in methanolic extract of R. damascena by using improved HPLC analysis method.[17] In agreement with Kumar et al., our HPLC analysis showed that quercetin as bioactive compound exists in the R. damascena extract. Flavonoids comprise the most common group of polyphenolic compounds in the human diet and are found ubiquitously in plants.[44,45] The neuroprotective effects of R. damascena were mediated by flavonoids that induce memory and cognitive improvement function via scavenging free radicals, and releasing neurotransmitter (acetylcholine). Other multifunctional effects of flavonoids include antioxidant, antiapoptotic, anti-inflammatory, anticholinesterase, antidepressant, and modulation of signaling pathway cascade[44,45,46,47,48] and cerebrovascular blood flow improvement.[49] Cellular and molecular mechanisms of flavonoids effects may carry out via protein kinase activation and phosphatase inhibition that could lead to activation of cyclic AMP-response element-binding protein (CREB). CREB could increase blood flow, angiogenesis, neurogenesis, dendritic spine growth, neuronal communication, and synaptic plasticity via production and activation of neurotrophic factors such as brain-derived growth factor (BDNF), nerve growth factor (NGF), vascular endothelial growth factor (VGEF), and transform growth factor-β (TGF-β). Flavonoids prevent neurodegeneration and brain aging via reduction of induced nitric oxide synthase (iNOS), nitric oxide releasing, suppression inflammatory cytokines, such as TNF-α, IL-1β.[50,51] Quercetin as a flavonoid which is present in R. damascena attenuates microglia- and/or astrocyte-mediated neuroinflammation.[52] Many neurodegenerative diseases pathogenesis including Alzheimer's disease correlate to oxidative stress enhancement in brain.[16] Brain biological functions of flavonoids attribute to their antioxidant functions.[53] Phenolic compounds are effective hydrogen donors, and because of this they are known as good antioxidants.[54] Their high antioxidant potential is attributed to their capacity of scavenging reactive oxygen species (ROS), inhibiting lipid peroxidation, chelating metal ions, and other free radicals, which are originated from various cellular activities and lead to oxidative stress.[55] The cholinergic system is involved in many physiological processes, including synaptic plasticity and learning and memory.[56,57] Quercetin and rutin, natural compounds widely found in R. damascena and diet have anticholinesterase inhibition effects without severe side effects.[58] Recent studies have focused on amyloid precursor protein (APP) proteolysis and Aβ-generation as potential targets for AD therapy.[59] Aβ is generated from the APP by beta-site APP cleaving enzyme-1 (BACE-1, β- secretase) and γ-secretases through pro-amyloidogenic processing pathway, but α-secretase via nonamyloidogenic processing pathway inhibits Aβ production and promote Aβ degradation.[60] Myricetin, one of the natural flavonoids has been found in R. damascena is a strong antioxidant and has a dual neuroprotective effect by activation of α-secretase and direct inhibition of β-secretase (BACE-1). As myricetin-bound BACE-1 does not hydrate APP at the β-cleavage site, Aβ production would be reduced. It has three hydrogen-binding groups, which stabilize BACE-1 binding for concerning AD drug therapy.[60,61] Myricetin binds also to Aβ through its hydroxyl arms and traps Aβ hydrogen bond, which is necessary to form β-structures or oligomers.[62] Myricetin via this mechanism prevents Aβ structural alteration from a random coil to β-sheet and inhibits Aβ fibril generation. The β-structure of Aβ forms soluble oligomers that are considered to be AD cytotoxic component.[7,63] It is demonstrated that quercetin also disrupts the β-sheet structure to form random coils.[61,64] Therefore R. damascena with high concentration of quercetin, myricetin, and other flavonoids decreases level of Aβ (1–42) in brain and finally improves learning and memory.
CONCLUSION
In summary, R. damascena reversed the Aβ-associated memory abnormalities in a rat model of amyloid-β-induced Alzheimer's disease. Taken together, the diverse actions of individual natural components of the R. damascena on learning and memory represent a new promising way for generation of memory-enhancing drugs, although complementary experiments are still to be down for better characterizing this valuable effect and its underlying mechanisms, in order to provide a basis for proper clinical trials.
ACKNOWLEDGMENTS
This work was supported by Isfahan University of Medical Sciences. The authors thank Dr. Parham Reisi of Department of Physiology for his excellent technical assistance. None of the authors claim any conflict of interest.
Footnotes
Source of Support: This study was supported by Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991;6:487. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 2.Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae JS. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer's disease mice by modulation of immune responses. Stem Cells. 2010;28:329–43. doi: 10.1002/stem.277. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Alzheimer's disease: Genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 4.Rogers J, Webster S, Lue LF, Brachova L, Civin WH, Emmerling M, et al. Inflammation and Alzheimer's disease pathogenesis. Neurobiol Aging. 1996;17:681–6. doi: 10.1016/0197-4580(96)00115-7. [DOI] [PubMed] [Google Scholar]
- 5.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–8. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 6.Huang HC, Jiang ZF. Accumulated amyloid-beta peptide and hyperphosphorylated tau protein: Relationship and links in Alzheimer's disease. J Alzheimers Dis. 2009;16:15–27. doi: 10.3233/JAD-2009-0960. [DOI] [PubMed] [Google Scholar]
- 7.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 8.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–44. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 9.Salloway S, Ferris S, Kluger A, Goldman R, Griesing T, Kumar D, et al. Efficacy of donepezil in mild cognitive impairment: A randomized placebo-controlled trial. Neurology. 2004;63:651–7. doi: 10.1212/01.wnl.0000134664.80320.92. [DOI] [PubMed] [Google Scholar]
- 10.Kim HG, Oh MS. Herbal medicines for the prevention and treatment of Alzheimer's disease. Curr Pharm Des. 2012;18:57–75. [PubMed] [Google Scholar]
- 11.Kalim MD, Bhattacharyya D, Banerjee A, Chattopadhyay S. Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Complement Altern Med. 2010;10:77. doi: 10.1186/1472-6882-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiber A, Mihalev K, Berardini N, Mollov P, Carle R. Flavonol glycosides from distilled petals of Rosa damascena Mill. Z Naturforsch C. 2005;60:379–84. doi: 10.1515/znc-2005-5-602. [DOI] [PubMed] [Google Scholar]
- 13.Ramezani R, Moghimi A, Rakhshandeh H, Ejtehadi H, Kheirabadi M. The effect of Rosa damascena essential oil on the amygdala electrical kindling seizures in rat. Pak J Biol Sci. 2008;11:746–51. doi: 10.3923/pjbs.2008.746.751. [DOI] [PubMed] [Google Scholar]
- 14.Awale S, Tohda C, Tezuka Y, Miyazaki M, Kadota S. Protective Effects of Rosa damascena and its Active Constituent on A {beta} (25-35)-induced Neuritic Atrophy. Evid Based Complement Alternat Med. 2011;2011:131042. doi: 10.1093/ecam/nep149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hongratanaworakit T. Relaxing effect of rose oil on humans. Nat Prod Commun. 2009;4:291–6. [PubMed] [Google Scholar]
- 16.Spencer JP. The impact of fruit flavonoids on memory and cognition. Br J Nutr. 2010;104:S40–7. doi: 10.1017/S0007114510003934. [DOI] [PubMed] [Google Scholar]
- 17.Kumar N, Bhandari P, Singh B, Gupta AP, Kaul VK. Reversed phase-HPLC for rapid determination of polyphenols in flowers of rose species. J Sep Sci. 2008;31:262–7. doi: 10.1002/jssc.200700372. [DOI] [PubMed] [Google Scholar]
- 18.Yu KH, Nation RL, Dooley MJ. Multiplicity of medication safety terms, definitions and functional meanings: When is enough enough? Qual Saf Health Care. 2005;14:358–63. doi: 10.1136/qshc.2005.014159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akhila J. Shetty, Deepa Shyamjith, Alwar M. C. Acute toxicity studies and determination of median lethal dose. Curr Sci. 2007;93:917–20. [Google Scholar]
- 20.Poole AF, Leslie GB. Cambridge, England: Cambridge University Press; 1989. A practical approach to toxicological investigations. [Google Scholar]
- 21.Javan M, Ahmadiani A, Semnanian S, Kamalinejad M. Antinociceptive effects of Trigonella foenum-graecum leaves extract. J Ethnopharmacol. 1997;58:125–9. doi: 10.1016/s0378-8741(97)00089-5. [DOI] [PubMed] [Google Scholar]
- 22.Pant J, Deshpande SB. Acute toxicity of Bisphenol A in rats. Indian J Exp Biol. 2012;50:425–9. [PubMed] [Google Scholar]
- 23.Jagetia G, Baliga M, Malagi K, Sethukumar Kamath M. The evaluation of the radioprotective effect of Triphala (an ayurvedic rejuvenating drug) in the mice exposed to γ-radiation. Phytomedicine. 2002;9:99–108. doi: 10.1078/0944-7113-00095. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G, Watson C. United States: Academic press; 2007. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition. [Google Scholar]
- 25.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 26.de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–90. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 27.Frick KM, Kim JJ, Baxter MG. Effects of complete immunotoxin lesions of the cholinergic basal forebrain on fear conditioning and spatial learning. Hippocampus. 2004;14:244–54. doi: 10.1002/hipo.10169. [DOI] [PubMed] [Google Scholar]
- 28.Venault P, Chapouthier G, de Carvalho LP, Simiand J, Morre M, Dodd RH, et al. Benzodiazepine impairs and beta-carboline enhances performance in learning and memory tasks. Nature. 1986;321:864–6. doi: 10.1038/321864a0. [DOI] [PubMed] [Google Scholar]
- 29.Mazzola C, Micale V, Drago F. Amnesia induced by beta-amyloid fragments is counteracted by cannabinoid CB1 receptor blockade. Eur J Pharmacol. 2003;477:219–25. doi: 10.1016/j.ejphar.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Abdel-Aal RA, Assi AA, Kostandy BB. Rivastigmine reverses aluminum-induced behavioral changes in rats. Eur J Pharmacol. 2011;659:169–76. doi: 10.1016/j.ejphar.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Scarpini E, Scheltens P, Feldman H. Treatment of Alzheimer's disease: Current status and new perspectives. Lancet Neurol. 2003;2:539–47. doi: 10.1016/s1474-4422(03)00502-7. [DOI] [PubMed] [Google Scholar]
- 32.Cummings JL. Alzheimer's disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 33.Youdim MB, Buccafusco JJ. Multi-functional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends Pharmacol Sci. 2005;26:27–35. doi: 10.1016/j.tips.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Pimplikar SW. Reassessing the amyloid cascade hypothesis of Alzheimer's disease. Int J Biochem Cell Biol. 2009;41:1261–8. doi: 10.1016/j.biocel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klafki HW, Staufenbiel M, Kornhuber J, Wiltfang J. Therapeutic approaches to Alzheimer's disease. Brain. 2006;129:2840–55. doi: 10.1093/brain/awl280. [DOI] [PubMed] [Google Scholar]
- 36.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: Food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 37.Youdim KA, Joseph JA. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: A multiplicity of effects. Free Radic Biol Med. 2001;30:583–94. doi: 10.1016/s0891-5849(00)00510-4. [DOI] [PubMed] [Google Scholar]
- 38.Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002;20:522–31. doi: 10.1016/s0167-7799(02)02080-2. [DOI] [PubMed] [Google Scholar]
- 39.Kim DS, Kim JY, Han YS. Alzheimer's disease drug discovery from herbs: Neuroprotectivity from beta-amyloid (1-42) insult. J Altern Complement Med. 2007;13:333–40. doi: 10.1089/acm.2006.6107. [DOI] [PubMed] [Google Scholar]
- 40.Park SY, Kim DS. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: A drug discovery effort against Alzheimer's disease. J Nat Prod. 2002;65:1227–31. doi: 10.1021/np010039x. [DOI] [PubMed] [Google Scholar]
- 41.Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- 42.Jhoo JH, Kim HC, Nabeshima T, Yamada K, Shin EJ, Jhoo WK, et al. Beta-amyloid (1-42)-induced learning and memory deficits in mice: Involvement of oxidative burdens in the hippocampus and cerebral cortex. Behav Brain Res. 2004;155:185–96. doi: 10.1016/j.bbr.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Yamada K, Takayanagi M, Kamei H, Nagai T, Dohniwa M, Kobayashi K, et al. Effects of memantine and donepezil on amyloid beta-induced memory impairment in a delayed-matching to position task in rats. Behav Brain Res. 2005;162:191–9. doi: 10.1016/j.bbr.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 44.Joseph JA, Shukitt-Hale B, Denisova NA, Prior RL, Cao G, Martin A, et al. Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive behavioral deficits. J Neurosci. 1998;18:8047–55. doi: 10.1523/JNEUROSCI.18-19-08047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, et al. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–21. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casadesus G, Shukitt-Hale B, Stellwagen HM, Zhu X, Lee HG, Smith MA, et al. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr Neurosci. 2004;7:309–16. doi: 10.1080/10284150400020482. [DOI] [PubMed] [Google Scholar]
- 47.Goyarzu P, Malin DH, Lau FC, Taglialatela G, Moon WD, Jennings R, et al. Blueberry supplemented diet: Effects on object recognition memory and nuclear factor-kappa B levels in aged rats. Nutr Neurosci. 2004;7:75–83. doi: 10.1080/10284150410001710410. [DOI] [PubMed] [Google Scholar]
- 48.Joseph JA, Shukitt-Hale B, Casadesus G. Reversing the deleterious effects of aging on neuronal communication and behavior: Beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr. 2005;81:313S–6. doi: 10.1093/ajcn/81.1.313S. [DOI] [PubMed] [Google Scholar]
- 49.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, et al. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006;103:1024–9. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spencer JP. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007;2:257–73. doi: 10.1007/s12263-007-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spencer JP. The impact of flavonoids on memory: Physiological and molecular considerations. Chem Soc Rev. 2009;38:1152–61. doi: 10.1039/b800422f. [DOI] [PubMed] [Google Scholar]
- 52.Chen JC, Ho FM, Pei-Dawn Lee C, Chen CP, Jeng KC, Hsu HB, et al. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol. 2005;521:9–20. doi: 10.1016/j.ejphar.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–56. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 54.Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995;22:375–83. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 55.Bors W, Heller W, Michel C, Saran M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990;186:343–55. doi: 10.1016/0076-6879(90)86128-i. [DOI] [PubMed] [Google Scholar]
- 56.Flood JF, Landry DW, Jarvik ME. Cholinergic receptor interactions and their effects on long-term memory processing. Brain Res. 1981;215:177–85. doi: 10.1016/0006-8993(81)90500-x. [DOI] [PubMed] [Google Scholar]
- 57.Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiol Learn Mem. 2003;80:178–93. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 58.Ahmed T, Gilani AH. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer's disease. Pharmacol Biochem Behav. 2009;91:554–9. doi: 10.1016/j.pbb.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 59.Haass C. Take five-BACE and the gamma-secretase quartet conduct Alzheimer's amyloid beta-peptide generation. EMBO J. 2004;23:483–8. doi: 10.1038/sj.emboj.7600061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo T, Hobbs DW. Development of BACE1 inhibitors for Alzheimer's disease. Curr Med Chem. 2006;13:1811–29. doi: 10.2174/092986706777452489. [DOI] [PubMed] [Google Scholar]
- 61.Shimmyo Y, Kihara T, Akaike A, Niidome T, Sugimoto H. Multifunction of myricetin on A beta: Neuroprotection via a conformational change of A beta and reduction of A beta via the interference of secretases. J Neurosci Res. 2008;86:368–77. doi: 10.1002/jnr.21476. [DOI] [PubMed] [Google Scholar]
- 62.McLaurin J, Kierstead ME, Brown ME, Hawkes CA, Lambermon MH, Phinney AL, et al. Cyclohexanehexol inhibitors of Abeta aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat Med. 2006;12:801–8. doi: 10.1038/nm1423. [DOI] [PubMed] [Google Scholar]
- 63.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–9. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shimmyo Y, Kihara T, Akaike A, Niidome T, Sugimoto H. Flavonols and flavones as BACE-1 inhibitors: Structure-activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim Biophys Acta. 2008;1780:819–25. doi: 10.1016/j.bbagen.2008.01.017. [DOI] [PubMed] [Google Scholar]