Abstract
We present a case of an elderly non-smoking gentleman who, since 2005, had been admitted multiple times for recurrent episodes of shortness of breath, wheeze, cough and sputum. The patient was treated as exacerbations of chronic obstructive pulmonary disease (COPD) and/or lower respiratory tract infections. Bronchoscopy was done which revealed multiple hard nodules in the trachea and bronchi with posterior tracheal wall sparing. Biopsies confirmed this as tracheopathia osteochondroplastica (TO). He had increasing frequency of admission due to methicillin-resistant Staphylococcus aureus and pseudomonas infections, which failed to clear despite intravenous, prolonged oral and nebulised antibiotics. The patient developed increasing respiratory distress and respiratory failure. The patient died peacefully in 2012. This case report highlights the typical pathological and radiological findings of TO and the pitfalls of misdiagnosing patients with recurrent chest infections as COPD.
Background
This case highlights a rare disease, with detailed images, that has only been described in case reports. We believe this is the first reported case in the Republic of Ireland. It also highlights the importance of reconsidering the diagnosis and initiating further investigations in patients who represents on multiple occasions with the same clinical problem.
Case presentation
An elderly gentleman had multiple admissions since 2005 with increasing shortness of breath, wheeze, cough and sputum. He had a diagnosis of presumed chronic obstructive pulmonary disease (COPD) and lower respiratory tract infections (LRTI) although he never smoked. He also had atrial fibrillation and hypertension. He was a retired carpenter. Sputum grew Haemophilus influenza, later methicillin-resistant Staphylococcus aureus in 2007, and from 2009 persistant pseudomonas aeruginosa. As he had five admissions in year 2007, further investigations were performed.
Investigations
A CT thorax was done in 2007 which revealed emphysematous change in the left upper lobe and irregular opacities of unknown origin in the right upper lobe. There were no comments on the trachea. Bronchoscopy was performed a week later which revealed multiple hard nodules in the trachea and left main bronchus that were difficult to biopsy. There was sparing of the posterior wall of the trachea. There were thick infected-looking secretions in the left lower lobe. Repeat bronchoscopy in 2009 showed worsening of appearances with nodules extending into both main bronchi (figures 1 and 2), as did repeat CT thorax scan in 2009 (figure 3). An axial view comparing the CT thorax scan in 2007 and 2009 is shown in figure 4. Histology showed inflamed tracheal mucosa with ossification within the lamina propria, consistent with tracheopathia osteochondroplastica (TO; figure 5). Bronchioalveolar lavage showed a low yield of pseudomonas aeruginosa. Pulmonary function tests (PFT) in 2009 showed obstructive airways disease with forced expiratory volume over 1 second/forced vital capacity (FEV1/FVC)=66.4%. Flow volume loops revealed greater expiratory versus inspiratory obstruction with maximum inspiratory flow at 50% FVC/maximum expiratory flow at 50% FVC (MIF50/MEF50=49.7%). This might be explained by the fact that the lesions extended from the extrathoracic trachea into the intrathoracic bronchi.
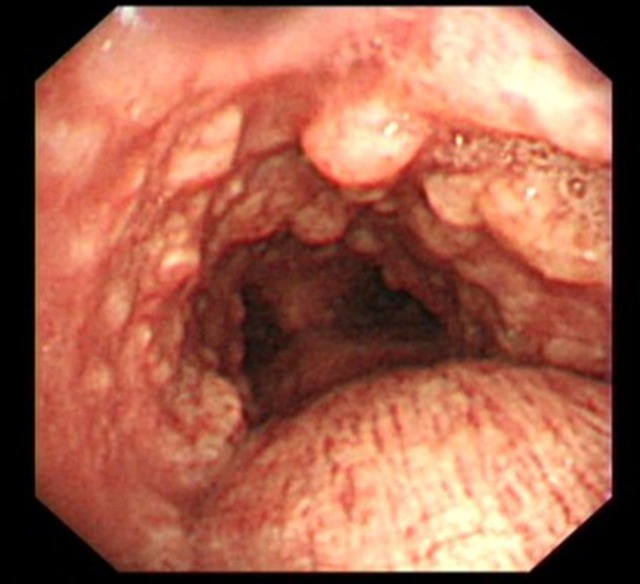
Figure 1.
Bronchoscopy—mid-tracheal view showing multiple hard nodules protruding into the lumen, with sparing of posterior wall of the trachea.
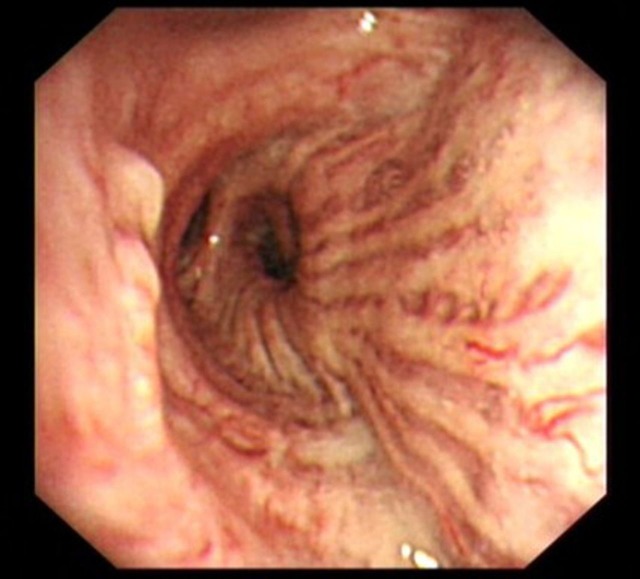
Figure 2.
Bronchoscopy showing nodule extending into right bronchus intermedius.
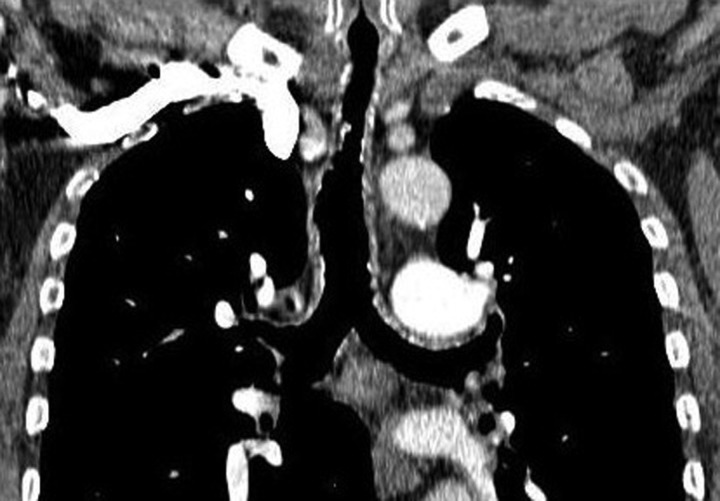
Figure 3.
CT thorax coronal view showing abnormal areas of calcification/ossification throughout the trachea, extending to the main bronchi.
Figure 4.
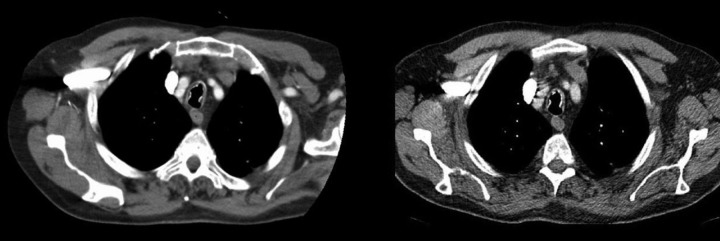
Comparison of the CT thorax scan in 2007 (left) and 2009 (right) taken at the level above the arch of aorta showing the progression of the ossified nodules in the trachea.
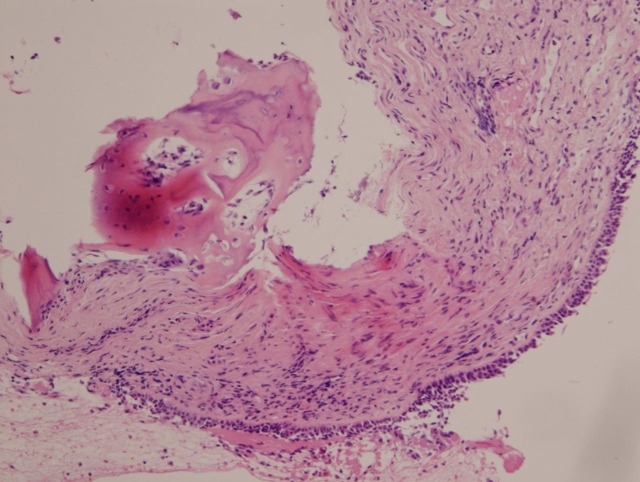
Figure 5.
Ciliated respiratory epithelium with subepithelial bone formation (metaplasia).
Differential diagnosis
Initially the patient was treated as recurring LRTI/COPD. Asthma was excluded on the basis of the PFTs showing non-reversibility of the airways obstruction. Later, primary tracheal diseases were considered, such as amyloidosis, Wegener's granulomatosis and relapsing polychondritis. These were excluded on the basis of the pathognomonic nodules sparing the posterior wall of the trachea and TO was confirmed by typical biopsy appearances. Antineutrophil cytoplasmic antibodies test was negative.
Treatment
The patient continued to re-present with recurrent LRTI and increasing respiratory distress, responding partially to intravenous antibiotics and steroids. He was maintained on long-term ciprofloxacin and later azithromycin without benefit, and failed trials of nebulised colomycin and tobramycin in an effort to eradicate his persistent pseudomonas infection.
Outcome and follow-up
The patient's lung function continued to deteriorate and stridor became more prominent. He was referred to the palliative care services. The patient died peacefully in 2012.
Discussion
Tracheopathia/tracheobronchopathia osteoplastica/osteochondroplastica is a rare, benign chronic disease characterised by the presence of subepithelial osteocartilaginous nodules projecting into the tracheobronchial lumen.1 It was first described by Rokitansky in 1855, Luschka in 1856 and Wilks in 1857.1 2 We believe that this is the first reported case from the Republic of Ireland. Recently, Toth2 further subdivided the disease (according to the type of tracheal ossification and nodule formation) into:
Tracheopathia osoteoplastica tuberosa—degenerative changes with nodule formation and ossification.
Tracheopathia osteoplastica peripherica—diffuse degeneration of the tracheal cartilage with ossification of the outer third of the cartilaginous rings, which is almost exclusively only found during autopsy.
The patient presented in our case likely had the former.
Although many theories have been proposed, including chronic irritation3 and metabolic disorder,4 the aetiology is still unclear. With regard to pathogenesis, the first hypotheses proposed by Virchow5 (that tracheobronchial enchondromas form and subsequently develop ossification and calcium deposition) seems to be supported by Toth2 whose team recently performed a pathological study on 20 cases. They further postulate that the degenerative process begins at the tracheal cartilage tissues, which subsequently form advanced lesions that protrude into the tracheal lumen.2 This might explain why the disease spares the posterior membranous wall of the trachea; a finding that separates it from other airway disease like amyloidosis or relapsing polychondritis. This however cannot be confirmed by a major control study because of the rarity of the disease.
This disease is usually seen in men over the age of 50 (as in this case), but it has also been described in women,6 children7 and one case described in a dog.8 Interestingly, there are recently more reports in younger adults.9 10 This is likely due to the increasing use of bronchoscopy in young adults, which is the ‘gold standard’ for diagnosis of luminal changes in the tracheobronchial tree, leading to the early detection of the disease rather than the disease presenting earlier in life.
Clinical manifestations of the disease occur when obstructive or infective complications occur. Infections mainly occur due to the disturbance in the normal mucociliary clearance of the trachea and bronchi. There have been suggestions that chronic irritation and infection may play a role in the disease process;3 11–14 however, it is difficult to determine if this is a cause or a consequence of the disease. Nevertheless, we continuously aimed to eradicate the pseudomonas infection that was colonising the trachea in this case, albeit unsuccessfully.
The treatment of this disease is unknown. If there is localised disease, possible resection of the affected area may be attempted. Tracheal stenting may not be useful/possible due to the nature of the hard protrusions that make securing the stent position difficult.
This case highlights the frequent overdiagnosis of COPD. Often, it appears that every patient who is hospitalised with recurrent chest infections is labelled as ‘COPD’, without thought given to the correct underlying diagnosis. This is particularly relevant in this case, as he was a life-long non-smoker. The problem of misdiagnosis of TO has been highlighted by others,15 16 thereby showing the importance of having a low threshold for further investigations in a patient with recurrent chest infections.
It was also noted that the report of the initial CT scan of the thorax in 2007 did not mention any abnormality in the trachea. However, on retrospective review, abnormalities could be seen in the trachea even in the 2007 CT scan. This would not be the first case of this as Kanat et al17 have also mentioned the missed findings on the CT scan of their case, which was found on retrospective review of the scans after bronchoscopic diagnosis.
Learning points
Not all presumed chronic obstructive pulmonary disease (COPD) is COPD, especially in non-smokers.
Look for cause of recurrent chest infections—think of the trachea.
Is chronic pseudomonas a cause or effect of the pathological process of tracheopathia osteochondroplastica (TO).
Eradication of pseudomonas may not be possible in TO.
Acknowledgments
Dr Finbarr O'Connell, Consultant Respiratory Physician, St James's Hospital. Dr Wael Hamarneh, Histopathology Registrar, St James's Hospital.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Chroneou A, Zias N, Gonzalez AV, et al. Tracheobronchopathia osteochondroplastica. An underrecognized entity? Monaldi Arch Chest Dis 2008;69:65–9. [DOI] [PubMed] [Google Scholar]
- 2.Toth C. Tracheopathia osteoplastica. A 100-year-old mystery. Pathologe 2012;33:129–34. [DOI] [PubMed] [Google Scholar]
- 3.Smid L, Lavrencak B, Zargi M. Larngo-tracheo-bronchopathia chondro-osteoplastica. J Laryngol Otol 1992;106:845–8. [PubMed] [Google Scholar]
- 4.Hempel KJ, Glaser A. Pathogenesis of chondro-osteoplastic tracheopathy. Virchows Arch 1958;331:36–50. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron D, Cormier Y, Desmeules M. Trancheobronchopathia osterochondroplastica. Am Rev Respir Dis 1976;114:803–6. [DOI] [PubMed] [Google Scholar]
- 6.Huang CC, Kuo CC. Chronic cough: trancheobronchopathia osteochondroplastica. CMAJ 2010;182:E859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simsek PO, Ozcelik U, Demirkazik F, et al. Trancheobronchopathia osteochondroplastica in a 9-year-old girl. Pediatr Pulmonol 2006;41:95–7. [DOI] [PubMed] [Google Scholar]
- 8.Sellon RK, Johnson JL, Leathers CW, et al. Tracheobronchopathia osteochondroplastica in a dog. J Vet Intern Med 2004;18:359–62. [DOI] [PubMed] [Google Scholar]
- 9.Al-Busaidi N, Dhuliya D, Habibullah Z. Tracheaobronchopathia Osteochondroplastica: case report and literature review. Sultan Qaboos Univ Med J 2012;12:109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swamy TL, Hasan A. Tracheopathia osteoplastica presenting with haemoptysis in a young male. Indian J Chest Dis Allied Sci 2010;52:119–21. [PubMed] [Google Scholar]
- 11.Gautam HP. Tracheopathia osteoplastica. Postgrad Med J 1968;44:186–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shih JY, Hsueh PR, Chang YL, et al. Tracheal botryomycosis in a patient with tracheopathia osteochondroplastica. Thorax 1998;53:73–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jepsen O, Sorensen H. Tracheopathia osteoplastica and ozaena. Acta Otolaryngol 1960;51:79–83. [DOI] [PubMed] [Google Scholar]
- 14.Baugnee PE, Delaunois LM. Mycobacterium avium-intracellulare associated with tracheobronchopathia osteochondroplastica. Eur Respir J 1995;8:180–2. [DOI] [PubMed] [Google Scholar]
- 15.Park SS, Shin DH, Lee DH, et al. Tracheopathia osteoplastica simulating asthmatic symptoms: diagnosis by bronchoscopy and computerized tomography. Respiration 1995;62:43–5. [DOI] [PubMed] [Google Scholar]
- 16.Hayes D., Jr Tracheopathia osteoplastica misdiagnosed as asthma. J Asthma 2007;44:253–5. [DOI] [PubMed] [Google Scholar]
- 17.Kanat F, Teke T, Ozer F. Tracheopathia osteoplastica associated with iron deficiency anaemia. Indian J Chest Dis Allied Sci 2005;47:47–51. [PubMed] [Google Scholar]