Abstract
Glomus tumours are a rare type of subepithelial mesenchymal tumours that present in deep visceral organs such as the stomach, which are difficult to diagnose. We report a case of a 44-year-old woman with diabetes who presented with anaemia, abdominal pain and melena diagnosed preoperatively with a gastric glomus tumour initially misdiagnosed as a gastric ulcer located at the lesser curvature. Upon referral to our centre a repeat endoscopy and biopsy were performed. A partial gastrectomy was performed with no complications. Histopathological analysis of the tumour reported clear margins and immunostaining was positive for smooth muscle actin and collagen IV. The patient remains asymptomatic at 3-month follow-up.
Case presentation
A 44-year-old female patient with diabetes was referred to our hospital with a 2-month history of fatigue, dyspnoea and melena following a motor vehicle accident that caused fracture of the left humerus for which she was managed with immobilisation and ketorolac. She was seen at another hospital where she was found to be anaemic with haemoglobin (Hb) of 3.7 g/dl.
Investigations
An upper digestive endoscopy reported a Forrest IIIB gastric ulcer at the lesser curvature. She was started on omeprazole 40 mg four times a day and was transfused several times before arrival at our Hospital. At presentation her laboratory diagnosis showed: Hb 12.3 g/dl, haematocrit 36.9%, platelet 291×103, white blood cell count 5.9×103, glucose 263 mg/dl, blood urea nitrogen 6.0 mg/dl, creatinine 0.3 mg/dl and total cholesterol 174 mg/dl. Physical examination was unremarkable. A repeat upper endoscopy was performed where a 2.5 cm polipoid lesion covered with fibrin was found in the lesser curvature. Histological analysis and immunohistochemistry reported a C-Kit negative tumour compatible with a gastric glomus (GG).
Differential diagnosis
▸ Gastrointestinal stromal tumour.
Treatment
The patient was taken to surgery after imaging studies were negative for metastasis. A partial gastrectomy without lymphadenectomy was performed with no complications. The patient was discharged 4 days after surgery. Histopathological analysis reported ulcerated lesion forming nodules separated by hemangiopericytoma-like vascular spaces with clear margins. Immunohistochemistry was positive for SMA, Ki-67 and negative for CD34, C-Kit (CD117), PS100, chromogranin and synaptohysina (figure 1).
Figure 1.
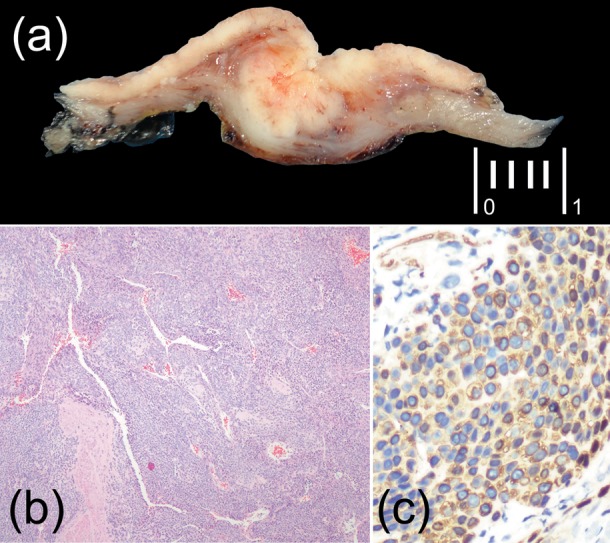
(A) Gross appearance and cut surface, the lesion shows nodular edges, infiltrated into the muscular layer. (B) The lesion grows forming nodules separated by haemangiopericytoma-like vascular spaces (H&E ×5). (C) Positive immunostaining for smooth muscle actin.
Outcome and follow-up
The patient continues to be asymptomatic at last follow-up 3 months after the surgery.
Discussion
Gastric submucosal tumours (SMT) include benign and malignant neoplasm arising from the submucosa or muscularis propia of the gastric wall. The most common malignant type of SMT are gastrointestinal stromal tumours (GIST). GG are a rare entity estimated to be 1% of GISTs with a slight predominance in women in the fifth or sixth decades of life.1 In contrast to typical GIST, GG are C-Kit negative and usually behave in a non-malignant form. They were first described in 1951 by Key et al.2 Glomus tumours of the stomach are benign mesenchymal neoplasms arising from the neuromyoarterial glomus, composed of smooth muscle cells representing the neoplastic complement to perivascular glomus bodies which help regulate arteriolar blood flow.3 They are usually benign; however, malignant behaviour cannot be excluded. GG tumours usually present as a solitary submucosal nodule found in the antrum or prepyloric areas, most commonly on the greater curvature arising in the intramuscular layer. Most patients present with gastrointestinal bleeding and a variety of symptoms like epigastric discomfort, nausea and vomiting. According to the WHO Classification of Tumours of Soft Tissue and Bone, glomus tumours should be considered malignant when their size is>2 cm and are located at subfascia or viscera, with atypical mitotic figures or marked nuclear atypia and mitotic activity.4 GG tumours grossly appear as red-blue nodules that originate from the muscularis propria.5 6 Preoperative diagnosis of glomus neoplasms is difficult due to intramural location and lack of characteristic radiological features. They are usually found accidentally on endoscopy as a well-circumscribed subepithelial mass. In barium studies, they appear as smooth submucosal masses with or without ulceration. On multidetector CT, they manifest as well-circumscribed submucosal masses with homogeneous density on unenhanced study and may contain tiny flecks of calcifications. Although endoscopic ultrasound (EUS) findings are insufficient to establish a diagnosis of glomus tumour, this imaging technique helps identify the layer of origin of the tumour (usually a well-circumscribed hypoechoic mass located in the third and/or fourth layer). It is important to note that EUS features suggestive of malignancy in GIST (irregular border, necrotic or cystic areas and echogenic foci) can be present in benign glomus tumours.7
Owing to their hypervascular nature these tumours show strong enhancement on arterial phase and persistent enhancement on portal venous phase after contrast medium administration.8–10 Although these methods may be helpful for preoperative evaluation, a high index of suspicion is needed and glomus tumours are commonly diagnosed histologically after surgical resection. Glomus tumours stain positive for ɑ-smooth muscle actin, calponin and vimentin, however, unlike GISTs they are negative for CD117 (C-KIT).11 Operative intervention should be carefully planned. Lymph node metastases are uncommon. As GG tumours are mesenchymal tumours with potential malignant behaviour, wedge resection with negative margins is the treatment of choice.12 Enucleation is not recommended due to high recurrence rates.13 GG tumours should always be included in the differential diagnosis of submucosal gastric lesions.
Learning points.
Submucosal gastric tumours other than gastrointestinal stromal tumours can present with bleeding.
Rare tumours need to be included in the differential diagnosis of persistent upper gastrointestinal bleeding.
Gastric glomus tumours are rarely suspected preoperatively.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Miettinen M, Paal E, Lasota J, et al. Gastrointestinal glomus tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol 2002;26:301–11. [DOI] [PubMed] [Google Scholar]
- 2.Key S, Callaahn WP, Murray MR. Glomus tumor of the stomach. Cancer 1951;4:726–36. [DOI] [PubMed] [Google Scholar]
- 3.Xu XD, Lu XH, Ye GX, et al. Immunohistochemical analysis and biological behaviour of gastric glomus tumours: a case report and review of the literature. J Int Med Res 2010;38:1539–46. [DOI] [PubMed] [Google Scholar]
- 4.Folpe AL. Glomus tumours. In: Fletcher CDM, Unni KK, Mertens F, (eds). World Health Organization classification of tumours: pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press; 2002:136–7. [Google Scholar]
- 5.Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol 2001;25:1–12. [DOI] [PubMed] [Google Scholar]
- 6.Tsai TL, Changchien CS, Hu TH, et al. Demonstration of gastric submucosal lesions by high-resolution transabdominal sonography. J Clin Ultrasound 2000;28:125–32. [DOI] [PubMed] [Google Scholar]
- 7.Kang G, Park HJ, Kim JY, et al. Glomus tumor of the stomach: a clinicopathologic analysis of 10 cases and review of the literature. Gut Liver 2012;6:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan SL, Yeh YH, Chen CH, et al. Gastric glomus tumor: a hypervascular submucosal tumor on power Doppler endosonography. J Clin Ultrasound 2007;35:164–8. [DOI] [PubMed] [Google Scholar]
- 9.Imamura A, Tochihara M, Natsui K, et al. Glomus tumor of the stomach: endoscopic ultrasonographic findings. Am J Gastroenterol 1994;89:271–2. [PubMed] [Google Scholar]
- 10.Agawa H, Matsushita M, Nishio A, et al. Gastric glomus tumor. Gastrointest Endosc 2002;56:903. [DOI] [PubMed] [Google Scholar]
- 11.Kay S, Callahan WP, Murray HT, et al. Glomus tumors of the stomach. Cancer 1951;4:726. [DOI] [PubMed] [Google Scholar]
- 12.Pidhorecky I, Cheney RT, Kraybill WG, et al. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol 2000;7:705–12. [DOI] [PubMed] [Google Scholar]
- 13.Park YS, Park SW, Kim TI, et al. Endoscopic enucleation of upper-GI submucosal tumors by using an insulated-tip electrosurgical knife. Gastrointest Endosc 2004;59:409–15. [DOI] [PubMed] [Google Scholar]


