Abstract
Purpose
Develop a paradigm to map binocular perceptual visual distortions in adult amblyopes and visually normal controls, measure their stability over time, and determine the relationship between strength of binocular single vision and distortion magnitude.
Methods
Perceptual visual distortions were measured in 24 strabismic, anisometropic, or microtropic amblyopes (interocular acuity difference ≥ 0.200 logMAR or history of amblyopia treatment) and 10 controls (mean age 27.13 ± 10.20 years). The task was mouse-based target alignment on a stereoscopic liquid crystal display monitor, measured binocularly five times during viewing dichoptically through active shutter glasses, amblyopic eye viewing cross-hairs, fellow eye viewing single target dots (16 locations within central 5°), and five times nondichoptically, with all stimuli visible to either eye. Measurements were repeated over time (1 week, 1 month) in eight amblyopic subjects, evaluating test–retest reliability. Measurements were also correlated against logMAR visual acuity, horizontal prism motor fusion range, Frisby/Preschool Randot stereoacuity, and heterophoria/heterotropia prism cover test measurement.
Results
Sixty-seven percent (16/24) of amblyopes had significant perceptual visual distortions under dichoptic viewing conditions compared to nondichoptic viewing conditions and dichoptic control group performance. Distortions correlated with the strength of motor fusion (r = −0.417, P = 0.043) and log stereoacuity (r = 0.492, P = 0.015), as well as near angle of heterotropic/heterophoric deviation (r = 0.740, P < 0.001), and, marginally, amblyopia depth (r = 0.405, P = 0.049). Global distortion index (GDI, mean displacement) remained, overall, consistent over time (median change in GDI between baseline and 1 week = −0.03°, 1 month = −0.08°; x-axis Z = 4.4256, P < 0.001; y-axis Z = 5.0547, P < 0.001).
Conclusions
Perceptual visual distortions are stable over time and associated with poorer binocular function, greater amblyopia depth, and larger angles of ocular deviation. Assessment of distortions may be relevant for recent perceptual learning paradigms specifically targeting binocular vision.
Keywords: distortion, amblyopia, binocular vision, strabismus
The study aimed to pilot a dichoptic measuring paradigm for quantifying severity of perceptual visual distortion in adult amblyopes. Distortion severity was found to be related to strength of binocular vision, strabismus/heterophoria size, and visual acuity, remaining consistent over the time period of a month.
In clinical practice, conventional amblyopia treatments focus on improvement of monocular visual acuity as the primary treatment outcome, with improvement of binocular vision not a primary consideration.1 However, it has been suggested that certain visual processing deficits in amblyopia are a consequence of decorrelated binocular input.2,3 The findings of McKee et al.4 even suggest that binocular status is a better predictor of the pattern of visual function loss than the subtype of amblyopia assessed. Decorrelated binocular vision is now thought to play an important role in amblyogenesis,5 and it is suggested that periods of binocular stimulation can facilitate amblyopia recovery.6
Another visual processing deficit occurring among amblyopic individuals is perceptual visual distortions, first identified in the literature by Pugh,7,8 who described the abnormal spacing, fragmentation, and warping of Snellen letters experienced by her amblyopic patients. Perceptual visual distortions have been subsequently profiled in adults with both treated and untreated amblyopia9–21 through use of a variety of measures, including dichoptic or monocular shape reconstructions,14,15,17–20,22–25 sketching suprathreshold gratings,10,19–22 hyperacuity/Vernier alignment,9,11–16,26,27 and matching portions of the visual space.11,14,28 Studies using hyperacuity-type tasks have identified a correlation between amblyopic visual acuity deficits and severity of perceptual visual distortions,12,14,15,17,26 likely due to the strong links between Vernier acuity and optotype acuity.29 However, a range of distortion severities can be associated with a given level of amblyopic eye visual acuity15 even when using a hyperacuity-type task measure. It is therefore possible for an amblyopic individual with a substantial visual acuity deficit (and therefore Vernier deficit) to not have significant measurable distortions. Overall, these perceptual visual distortions are considered to be independent of the low-level deficits in strabismic or anisometropic amblyopia.9,10,13,16,18,19,21,27
It has been suggested that the existence and severity of perceptual distortions may have a potential role in reducing the correlation of binocular images and disrupting disparity processing, thereby acting as a mechanism for the decorrelation of binocular vision.30 Despite this suggested relationship, the majority of studies in this area have been conducted monocularly in strabismic amblyopes with no binocular vision, although Sireteanu and colleagues17,19,22,31 did study a small number of microtropic amblyopes, who frequently have subnormal binocular vision in association with their abnormal retinal correspondence. The literature suggests a dichotomy in the nature of perceptual visual distortions between strabismic amblyopes and anisometropic amblyopes; anisometropic amblyopes, who usually have normal binocular function,32 frequently have little or no distortion.11,14,15,21,26 It would therefore be of clinical interest to investigate the relationship between severity of perceptual visual distortions and strength of binocular function in amblyopic individuals; if severe perceptual distortions could act as a barrier to good binocular vision, then improving them could potentially be a part of future amblyopia treatment approaches.
Decorrelated binocular vision can have a wide-ranging impact upon aspects of visual function, including depth judgments,33–36 visually guided reaching/grasping kinematics,34,37–40 obstacle negotiation,41 and fine visuomotor tasks.35,36,42–46 Prior to this only a small number of studies have attempted to use binocular methods of measuring perceptual visual distortions.17,23 Thus, part of our exploration of the relationship between binocularity and perceptual visual distortions involved the development and piloting of a dichoptic measurement paradigm.
The aim of this study was to pilot a dichoptic alignment paradigm in adult amblyopes for the assessment of perceptual visual distortions and to identify possible relationships between strength of binocular function and perceptual visual distortion severity.
Methods
Participants
Twenty-four amblyopic and 10 visually normal individuals aged 18 and over (mean age 27.13 ± 10.20 years, upper limit 51 years) were recruited from the staff and student population at Glasgow Caledonian University. The study was approved by the Glasgow Caledonian University Research Ethics Committee and followed the tenets of the Declaration of Helsinki. Informed consent was obtained, and all participants wore their up-to-date refractive correction, as determined from history taking. If refractive correction was more than 2 years old and visual acuity was worse than 0.200 logMAR in either eye, then a refraction and new spectacles were provided free of charge, through an optometrist at Glasgow Caledonian University Eye Clinic. Participants also underwent a routine orthoptic assessment consisting of a cover test, ocular motility assessment, assessment of convergence, measurement of binocular single vision (horizontal phasic prism fusion range to assess motor fusion, Frisby and Preschool Randot tests to assess stereoacuity), and a prism cover test to measure the size of heterophoria/heterotropia.
Exclusion criteria for amblyopic subjects were coexisting ophthalmic pathology other than refractive error, or an interocular acuity difference ≤ 0.200 logMAR with no history of occlusion or atropine penalization treatment. Exclusion criteria for visually normal controls were coexisting ophthalmic pathology other than corrected refractive error, best-corrected interocular acuity difference > 0.200 logMAR, heterophoria > 10 prism diopters, presence of microtropia or ocular motility defect, abnormal motor fusion amplitudes (<12 prism diopters base in [BI], <20 prism diopters base out [BO]), or abnormal stereoacuity (>40″ arc on Frisby stereotesting, >60″ arc on Preschool Randot stereotesting43). Ocular dominance in visually normal participants was established using the Porta test,47 involving the participant's aligning his or her vertically extended index finger with a distant vertical object. The examiner can observe by standing next to the object which eye the index finger is in front of, thereby determining the dominant eye. In cases in which the participant's index finger was positioned between the two eyes and no bias toward either eye could be determined, the participant's right eye was arbitrarily assigned as the dominant eye for testing purposes. Clinical characteristics of the amblyopic participants are shown in the Supplementary Table available online.
Mapping of Perceptual Visual Distortions
All stimuli were presented using MATLAB 2012b (Mathworks, Natick, MA, USA) running PsychToolbox 3.0 on Windows 7 (Microsoft, Redmond, WA, USA). Viewing distance was 87 cm. Participants viewed a central fixation target (0.228-diameter circle) presented binocularly to both eyes through NVIDIA 3DVision active shutter glasses (NVIDIA, Santa Clara, CA, USA). Central fixation was monitored using a calibrated Eyelink 1000 eye tracker (SR Research, Kanata, ON, Canada) running in remote mode at 500 Hz. The central fixation dot was colored green if fixation was central, and changed color to red if fixation strayed more than 1.15° away from it, in which case no data were recorded and points repeated. To the amblyopic/nondominant eye, a mouse cursor was presented in a cross-hair shape (arms 0.58° × 0.05° with 1.73° gap centrally to minimize binocular rivalry against the target stimulus). To the fellow/dominant eye, a target stimulus (0.22°-diameter circle) was presented at one of 16 target locations forming two nested rectangles covering the central 5° of the visual field.
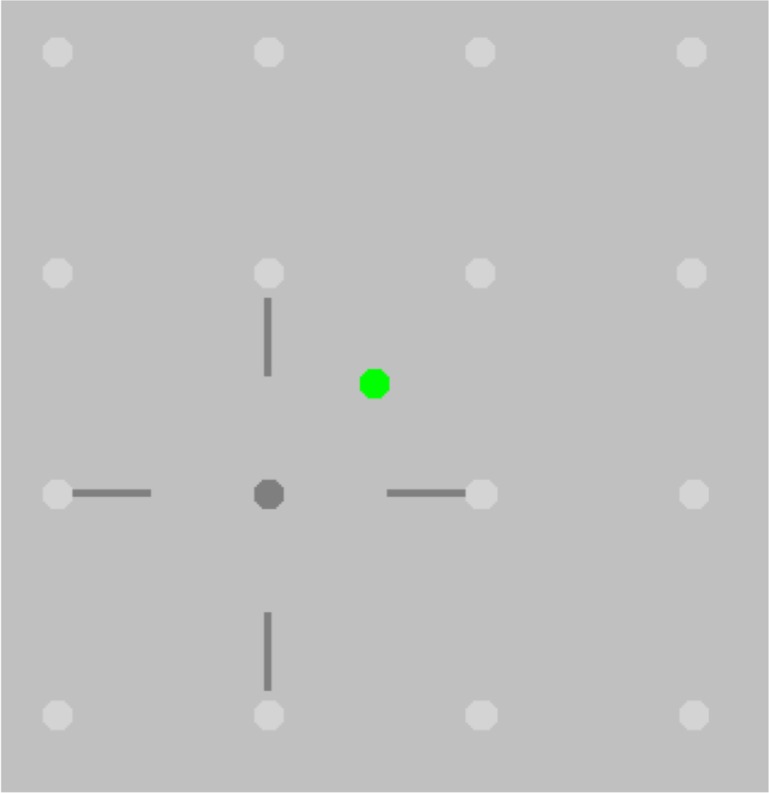
A pink noise background on a gamma-corrected monitor (1920 × 1080 resolution LG FlatTron; Yeouido-dong, Seoul, South Korea) was used, with 75% contrast, a suprathreshold contrast level at which amblyopic contrast perception is unimpaired.48 The background also masked the 1% cross-talk, measured with a SpectraScan 6500 photometer (Photo Research, Chatsworth, CA, USA) for a fully white screen in one eye and a fully black screen in the other eye. A screen view schematic, without the pink noise background, is shown in Figure 1.
Figure 1.
Schematic showing the stimuli used in the dichoptic mapping paradigm. Cross-hair: Mouse-controlled cursor, presented only to the amblyopic eye. Dark gray dot: Target dot presented singly, only to the fellow eye. The observer's task was to use the mouse to align the center of the cross-hairs on the target dot and then to click a mouse button. Light gray dots: Target dot locations, creating two nested rectangles spanning the central 5° of the visual field. The observer does not see these on the screen; they are shown here to illustrate the target positions. Green dot: Central fixation target, presented to both eyes; changes color to red when Eyelink 1000 detects that participant is not fixating centrally.
Subjects were instructed to fixate centrally and manipulate the cross-hair position using a wireless mouse, placing the cross-hair over the target dot as accurately as possible, lining both horizontal and vertical arms of the cross-hair with target dot center. Upon clicking of the mouse, mouse x and y pixel coordinates relative to the top left corner of the screen were recorded. The target dot then moved to another of the 16 locations in random order. This method has been employed in other studies,17,19,20 and participants must make a judgment of cross-hair alignment and centering to enable accurate localization of the target stimulus. However, this is not strictly a hyperacuity forced-choice task, since the adjustment method used in this study has been designed for future use with children with amblyopia and therefore has been simplified to remove the memorized target locations employed by other studies.17,19,20
Participants were tested five times without the shutter glasses being worn; under these viewing conditions (nondichoptic), all stimuli are viewable with either eye and completion of the paradigm provides a measure of mouse click accuracy. This was then repeated five times dichoptically with the shutter glasses in place to measure perceptual visual distortions. Comparison between dichoptic and nondichoptic measures ensures that any spatial displacements or uncertainty detected using the paradigm are not a simple consequence of poor mouse-clicking accuracy—if this were the case, there would be no significant difference between nondichoptic and dichoptic results.
Assessment of the Consistency of Perceptual Visual Distortions Over Time
A subset of amblyopic participants from the previous experiment with confirmed visual distortions who were happy to participate in further research (n = 8; 3 strabismic, 3 microtropic, 2 anisometropic) had their perceptual visual distortions measured using the methodology described above at baseline, and again 1 week (±2 days) and 1 month (±6 days) after the baseline visit.
Statistical Analysis
Distortion Mapping Task.
For both dichoptic and nondichoptic measurements, the local distortion for each of the 16 points was calculated as an x-y vector. A global distortion index (GDI) was calculated as the mean of the 16 local distortion absolute values obtained from each run of the experiment, which was repeated five times, generating five GDI measures per participant. The global uncertainty index (GUI) was calculated as the standard deviation of the GDI. For all participants, heterophoric/heterotropic angle of deviation was accounted for by calculating the mean horizontal and vertical local displacement value for each of the 16 points across the five runs of the experiment and subtracting this value from the results prior to calculating the global distortion value. Fixation distance was set and a suprathreshold acuity fixation target used, resulting in the same amount of accommodative convergence occurring during each trial.
All statistical analyses were conducted using SPSS (IBM, Armonk, NY, USA). Distortion data were found to not be normally distributed even with transformations applied, and so nonparametric statistics were used during analysis. For each participant, the five measures of dichoptic and nondichoptic GDI were compared by Mann-Whitney U test. If the dichoptic GDI significantly exceeded the nondichoptic GDI, and also exceeded the dichoptic GDI of the control group (who had no significant difference between their dichoptic and nondichoptic GDIs), the participant was classified as having perceptual visual distortions. This method ensures that perceptual visual distortions are differentiated from poor mouse-clicking accuracy in-task. The GDI and GUI were compared between amblyopic and visually normal subjects by Mann-Whitney U test. Within amblyopes, they were compared between amblyopia types (discussed below).
Amblyopia types and diagnostic criteria were strabismic (n = 7, manifest deviation on cover test), anisometropic (n = 5, ≥2.00 diopter sphere or ≥1.00 diopter cylinder anisometropia), and microtropic (n = 12, 4 prism diopter prism not overcome by amblyopic eye viewing foveal target). Four subjects in the strabismic category had mixed strabismic and anisometropic amblyopia, but there were insufficient subjects to have separate categories for purely strabismic and mixed strabismic/anisometropic amblyopia. Spearman's rank correlations were performed to identify relationships between GDI and the clinical parameters of fellow eye/amblyopic eye visual acuity and refractive error, interocular acuity difference, positive and negative fusional amplitudes, stereoacuity, and near angle of deviation.
Consistency of Perceptual Visual Distortions Over Time.
The GDIs from each visit were not found to be normally distributed by Shapiro-Wilk testing, and thus nonparametric analysis procedures were applied. The n-1 analysis technique adapted from Peters et al.49 and Dorr et al.50 was then applied to these GDIs. The n-1 analysis technique was selected due to the heterogeneity of global distortion within the population of amblyopes studied here and is adapted from the normalized scanpath saliency method used in gaze analysis.49,50
The technique allows the comparison of two distribution curves: one generated by summing cross-products of the (mean-centered) GDIs between participants (e.g., participant 001 visit 1 × participant 002 visit 1), and another generated from summing cross-products of these indices within participants, across the three visits (e.g., participant 001 visit 1 × participant 001 visit 2). If distortion values were similar across visits, correlation values would be high within participants compared to between participants and thus produce larger cross-product values. Thus, a significant separation between the between- and within-participant distribution curves would indicate that the distortions do not change substantially over time. The summed cross-product values from between participants and within participants were tested for normality, and a rank sum test was applied to compare the distributions to determine whether the separation of the curves was statistically significant.
Results
Identification of Perceptual Visual Distortions
Sixteen of the 24 (67%) amblyopic subjects were found to have perceptual visual distortions under dichoptic viewing conditions exceeding those measured under nondichoptic conditions. Table 1 shows the average clinical attributes of the amblyopic subjects with and without perceptual visual distortions, alongside the control subjects.
Table 1.
Clinical Attributes of Amblyopic Participants With and Without Perceptual Visual Distortion (n = 24) Compared to Visually Normal Controls (n = 10)
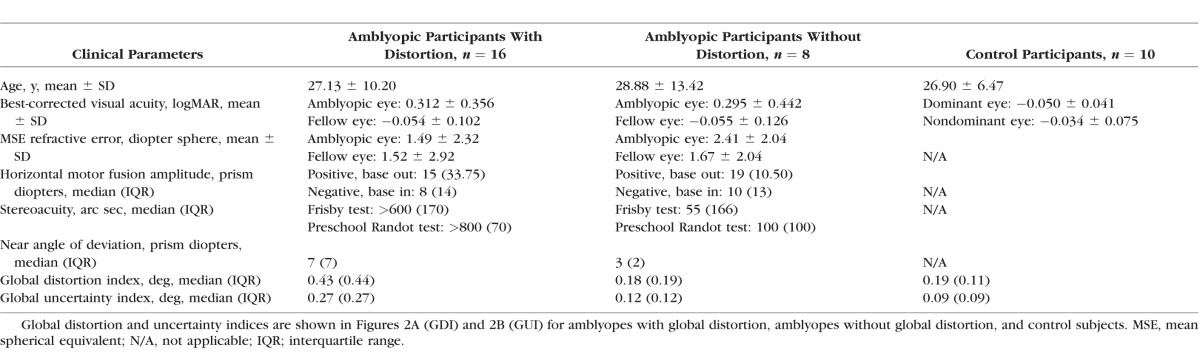
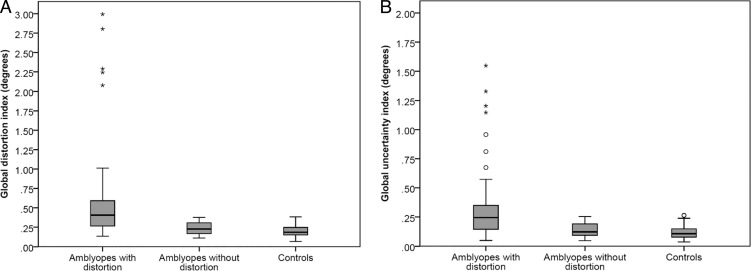
Amblyopes without significant global distortion had median global distortion and uncertainty indices similar to those of the control group (Figs. 2A, 2B), in contrast to the amblyopes with significant global distortion (difference in median GDI = 0.24°, Z = −6.85, P < 0.001; difference in median GUI = 0.18°, Z = −6.32, P < 0.001).
Figure 2.
Global distortion (A) and uncertainty (B) indices in amblyopes with distortion (left), amblyopes without distortion (center), and control subjects (right). ○Outlier less than 1.5 × interquartile range (IQR); *outlier exceeding 1.5 × IQR; error bars: smallest/largest recorded sample value; box bounds: upper/lower quartile; horizontal bar within box bounds: median.
Effect of Amblyopia Type Upon Perceptual Visual Distortions
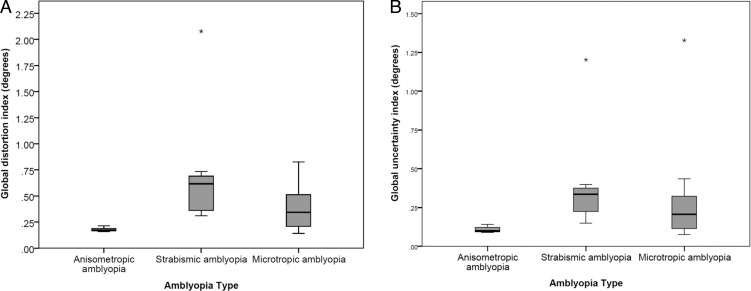
When analyzing the whole cohort of amblyopes regardless of presence/absence of perceptual visual distortions, which enables analysis of the anisometropic amblyopes (n = 5; only 2 had significant global distortion/uncertainty), a significant effect of amblyopia type was found (H = 10.06, P = 0.007), which is demonstrated in Figures 3A (GDI) and 3B (GUI).
Figure 3.
Global distortion (A) and uncertainty (B) indices by amblyopia type. *Outlier exceeding 1.5 × interquartile range (IQR); error bars: smallest/largest recorded sample value; box bounds: upper/lower quartile; horizontal bar within box bounds: median.
Strabismic amblyopes (n = 7, 6 with significant global distortion/uncertainty) were found to have more global distortion and uncertainty than anisometropic amblyopes (GDI median difference = 0.44°, Z = −2.84, P = 0.003; GUI median difference = 0.24°, Z = −2.84, P = 0.003). Microtropic amblyopes (n = 12, 8 with significant global distortion/uncertainty) also had more global distortion than the anisometropic participants, but this was not statistically significant following Bonferroni correction for multiple comparisons (Bonferroni corrected α value = 0.017, GDI median difference = 0.17°, Z = −2.32, P = 0.019). These findings highlight the greater incidence of distortions in strabismic (74%) compared to nonstrabismic (40%) amblyopia types, although the significance of this difference is limited by the smaller number of anisometropic amblyopes in the sample. No significant difference was found in global distortion or uncertainty indices between the strabismic and microtropic participants.
Correlation Between Perceptual Visual Distortions and Clinical Features of Amblyopia
Due to the small number of individuals in the cohort of amblyopes who did not have perceptual visual distortions (n = 8), comparison of clinical features between amblyopes with significant GDI/GUI and amblyopes without allows only limited conclusions to be drawn. Instead, clinical features were correlated against global distortion/uncertainty indices for the whole group of amblyopes to identify any relationships.
A moderate negative correlation was found between the GDI and negative fusional amplitudes (ρ = −0.417, P = 0.043); thus poorer negative fusional amplitudes were associated with an increased GDI. Moderate positive correlations were found for stereoacuity measures (Frisby ρ = 0.492, P = 0.015; Preschool Randot ρ = 0.542, P = 0.006) and a strong positive correlation was found for the near angle of deviation (ρ = 0.740, P < 0.001); thus poorer binocular functions and/or a larger angle of deviation were associated with a higher GDI. Visual acuity in the amblyopic eye was also positively correlated with GDI, albeit more weakly than other visual function measures and with borderline significance (ρ = 0.405, P = 0.049). Thus strength of binocular functions, near angle of deviation, and visual acuity in the amblyopic eye appear associated with severity of the perceptual visual distortions identified. However, moderate to strong correlations were also identified between the near angle of deviation and negative fusional reserves (ρ = −0.527, P = 0.008), along with Frisby (ρ = 0.599, P = 0.002) and Preschool Randot stereoacuities (ρ = 0.637, P = 0.001). Thus the key correlates for global distortion also interact with each other, making it difficult to draw conclusions about the relative contributions made by these factors to global distortion in isolation from each other. For GUI, the measure of perceptual uncertainty, near angle of deviation was the only statistically significant correlate (ρ = 0.578, P = 0.019), indicating that higher uncertainty was associated with a larger near angle of deviation.
Consistency of Perceptual Visual Distortions Over Time
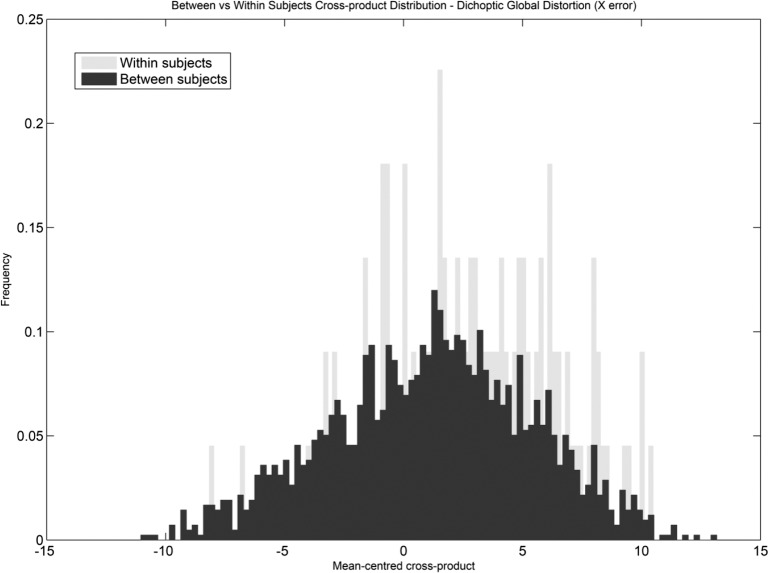
For amblyopic participants, the distribution of the between-participants global distortion cross-products against within-participants cross-products is shown in Figure 4 for the horizontal meridian (X error). A positive shift from zero can be seen for the between-subjects cross-product distribution curve, indicating that between subjects there is some similarity in the GDIs measured. The within-subjects curve, however, shows a prominent positive shift and increased kurtosis, indicating an increase in the number of positive values; therefore distortion values between visits, within subjects, are highly correlated and are not significantly changed over time.
Figure 4.
Cross-product distribution between and within participants for the dichoptic horizontal global distortion. The within-participants curve is shifted to the right with a higher kurtosis, indicating a high correlation for global distortion indices between visits and therefore no significant change in global distortion over time.
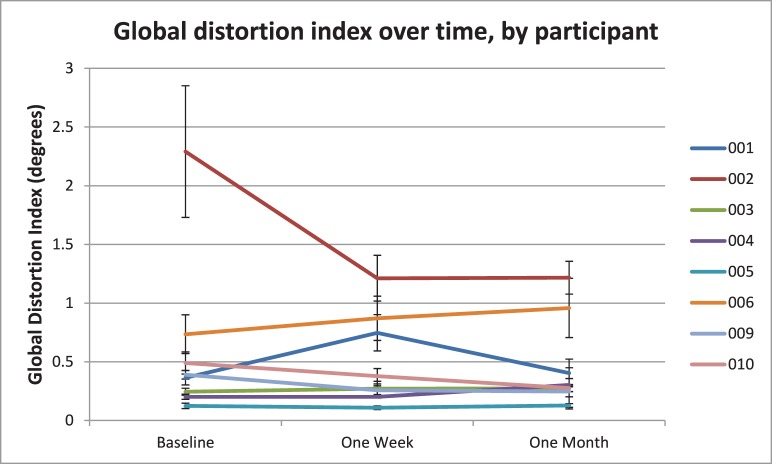
The median change in GDI over time was −0.03° (interquartile range, 0.18°) at 1 week from baseline and −0.08° (interquartile range, 0.30°) at 1 month from baseline, and a visual representation of this stability over time for each participant can be seen in Figure 5, a graph of GDI over time. In this graph, it can be seen that seven of the eight participants had no significant change in their global distortion over time.
Figure 5.
Change in global distortion index over time, by participant. Seven of eight subjects have changes in global distortion over time that do not exceed their interquartile range; thus error bars overlap. Subject 002 shows a reduction in distortion between baseline and 1 week, due to strabismic alternation during baseline testing generating artifactually large global distortion values. Global distortion indices for this subject on a test 7 months prior to conducting this study were a median of 1.22°. Line graph points represent median global distortion index; error bars represent the interquartile range.
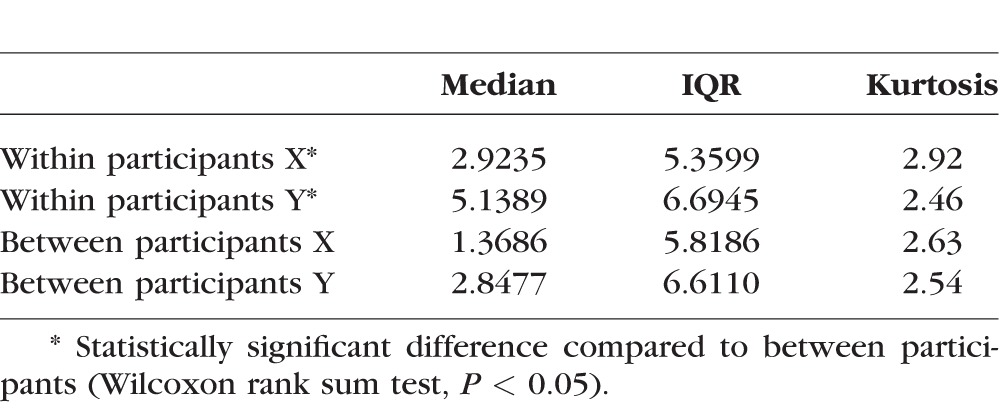
Table 2 shows the median cross-product for X and Y error between and within amblyopic participants, along with the interquartile range and kurtosis. The median cross-product was larger for the within-participants comparison for both X and Y error values, which was statistically significant at the 5% level, demonstrating the positive shift observed in Figure 4. Global distortion levels across repeat visits are therefore well correlated despite the increased amount of global uncertainty in the strabismic and microtropic amblyopes noted in the previous experiment. The sample as a whole showed no significant change over time, although some individual subjects did have some change shown by instances of a negative value in Figure 4—such values occur where a global distortion value has significantly increased or decreased between one visit and another.
Table 2.
Median Cross-Product, Interquartile Range, and Kurtosis for X and Y Error Between and Within Amblyopic Participants
Discussion
Exploration of Global Distortion and Uncertainty in Amblyopia
The distortion mapping paradigm piloted appears effective in identifying amblyopic individuals who experience spatial displacement of perceived targets and spatial uncertainty in perceived target location when localizing dichoptic targets across the central visual field. Not every amblyope tested experienced perceptual visual distortions, showing that this is not a universal occurrence in amblyopia. Along with extensive individual differences in the pattern of perceptual visual distortions between subjects, this phenomenon has also been identified in other studies.10,15,17–19,22,26,31 Perceptual visual distortions were found in this study to be more severe in participants with a strabismic component to their amblyopia than in those with pure anisometropia, as found in other studies.11,14,15,21,26 However, there were only five anisometropic amblyopes to make comparisons against in our pilot study; therefore further investigation of this potential dichotomy in a larger group of amblyopes would be helpful.
Strength of binocular function and the near angle of deviation both appear to be strong correlates with the magnitude of perceptual visual distortions, while depth of amblyopia has a smaller association. The significance of the relationship between perceptual visual distortions and amblyopic eye visual acuity may have been limited by the fact that a range of visual acuities occurred at any given level of distortion and predictions of the level of distortion could not always be made based on the amblyopic eye acuity—for example, the participant with the most severe perceptual visual distortions (subject 002 with a global distortion of 2.48°) had a visual acuity of 0.100 logMAR in the amblyopic eye. This is in keeping with the findings of previous studies.15,19
Study findings regarding the relationship of binocularity to perceptual visual distortions could provide further justification for the focus on treatment methodologies avoiding binocular decorrelation of the eyes during amblyopia treatment, such as recent binocular perceptual learning paradigms51,52 and the use of atropine penalization, determined as effective as occlusion for amblyopia treatment.1 However, it is also important to consider the possibility that individuals manifesting these perceptual visual distortions under such dichoptic testing conditions, where the amblyopic eye contributes to portions of the visual percept, may actually struggle with perceptual learning techniques that utilize similarly presented stimuli to encourage binocular cooperation. For example, in a dichoptic visual training game, the perceived positions of game elements presented to the amblyopic eye could potentially be affected by the existence of perceptual visual distortions such as those identified in this study, even after having corrected for the static angle of deviation via nonius line control, which could affect game performance.
Larger angles of deviation are associated with poorer binocular functions due to the amount of fusional vergence required to maintain binocularity. Although the current study has identified the predicted association between these clinical factors and perceptual visual distortions, the correlated nature of these two factors as demonstrated in our study renders it difficult to tease out the relative contributions of binocularity and angle of deviation to distortion severity. A larger study would be required to conclusively identify whether they are a consequence or cause of perceptual visual distortions, for the lack of a perfect correlation would seem to indicate that severe perceptual visual distortions are not associated in all cases with poor binocular function or a large angle of deviation. This could possibly indicate additional factors contributing to the distortions measured, such as subject error (although the disparity between dichoptic and nondichoptic distortion measures would seem to rule this out as a factor) or compounding of perceptual visual distortions in cascade along the visual pathway, similar to the extrastriate visual deficit that occurs in amblyopia.53 If the latter were the case, one would expect there to be a relationship between severity of perceptual visual distortions and the level of extrastriate deficit in global motion or orientation processing, which is an area for further research.
Historically the undersampling29 and neural disarray21 theories were proposed to explain the visual deficit in amblyopia, but more recently elevated internal noise within the amblyopic visual system has been suggested to be a key factor in the poorer performance of amblyopic individuals for many behavioral tasks.54–56 Such elevated levels of internal noise could also be influencing the pattern of distortions measured, although this idea is still being explored in the current literature and the exact location where the noise arises in the visual pathway is still yet to be established.54–56 Our findings seem to indicate that there is a prominent retinotopic aspect to the measured distortions, as for many subjects, linking the mean local distortion indices for each of the 16 test points produces a polygon that remains consistent in shape between visits, suggesting geotopic stability for the distortion patterns. However, other factors such as those discussed above may be contributing or even compounding the perceptual visual distortions identified in our cohort.
If this were the case, eye position, including fixation disparity, may have an impact on the measured amount of distortion. The mean centering performed during data analysis eliminates retinotopic shifts associated with a fixed angle of strabismus or binocular fixation disparity. However, if there were variations in the angle of strabismus during testing, or changes in the amount of fixation disparity in binocular individuals, this would cause an elevated standard deviation (i.e., elevated GUI) and reduce the efficacy of the correction employed during analysis. Global distortion index was correlated strongly with GUI (Spearman's ρ = 0.92, P < 0.001), and it is therefore important to consider the possibility that strabismic and microtropic amblyopes may experience greater between-test variability due to these factors, subsequently limiting the test–retest reliability of our paradigm assessing these types of amblyopia.
One example of this can be seen in the Figure 5: Participant 002 has an obvious reduction in global distortion between the baseline and 1-week assessments. However, rather than this being a natural reduction in distortion over time, this change is an artifact of an inflated baseline distortion measurement. This participant had been measured by us previously, but on the occasion of the baseline visit inexplicably had trouble holding fixation with the fellow eye, resulting in a large amount of global distortion related to the perceived cross-hair position swapping sides according to alternation of the deviation. On a previous test 7 months earlier, the participant's median GDI was 1.22°, which is more consistent with the distortion levels recorded for this participant at 1 week and 1 month on this graph, at which visits the participant found it much easier to maintain fixation preference. This demonstrates how the paradigm can be subject to artifacts when large variations in the strabismic angle occur, such as, in this case, alternation of the strabismus between eyes.
In some participants, variability between tests was primarily on the horizontal meridian, and thus test–retest variability could explain the pattern of distortions in these cases. However, other amblyopic participants showed variability in both the horizontal and vertical plane (despite having only a horizontal deviation) and more for some test points than others, and therefore this explanation doesn't hold for all cases. Positional uncertainty is frequently reported in conjunction with spatial displacement distortion,11,16,17,23 and such variability could possibly be an inherent aspect of amblyopic perception. This emphasizes the importance of validating our paradigm by assessing for the consistency of these measured distortions over time.
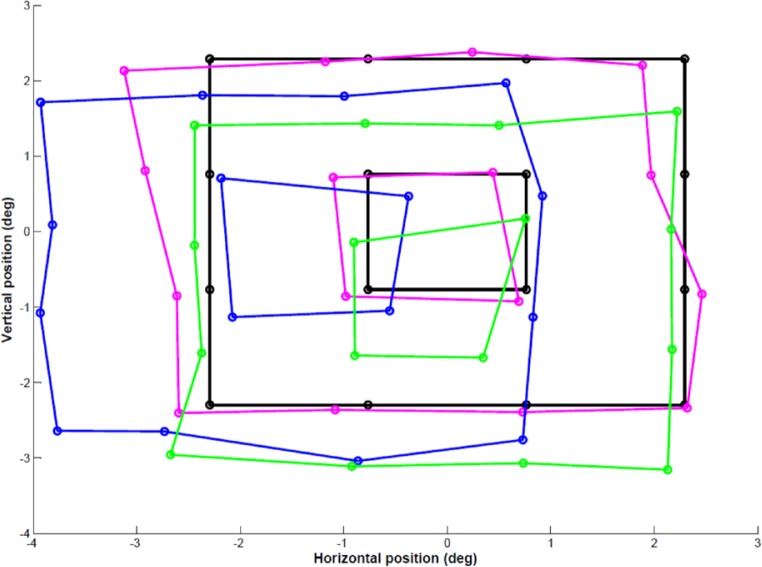
The current study found perceptual visual distortions in most subjects (seven of eight) to be stable over a time period of a month, which would not be expected if variation in the strabismic angle or fixation disparity amount were a significant factor in every individual whose distortions were measured. Participant 002, discussed above, is a demonstration of the kind of findings that could be expected under such circumstances, and it is reasonable to conclude on this basis that the paradigm would not be suitable for measurement of distortion in freely alternating strabismic individuals. Post hoc, one of our strabismic subjects (see 003 in the Supplementary Table for the clinical characteristics) was retested while also recording binocular eye position during each test point. Figure 6 shows the polygon distortion maps created based on mean amblyopic eye position (green) and mean fellow eye position (blue), in comparison to the mean global distortion mapped using our paradigm (magenta). The position of neither eye can be used to accurately predict the pattern of distortion, supporting the idea suggested above that a cortical element may be contributing.
Figure 6.
Predicted and actual mean perceived location of target stimuli relative to central fixation for strabismic participant 003. Black: Veridical location of target stimulus; magenta: mean perceived location of target stimulus; green: mean predicted perceived location of target stimulus based on amblyopic left eye position; blue: mean predicted perceived location of target stimulus based on fellow right eye position.
The extent of the contribution made by some of these factors could be determined with the development of an equivalent monocular paradigm to compare dichoptic against monocular distortion patterns, as monocularly the strabismic eye would be forced to take up fixation and binocular cooperation would not be necessary.
Conclusions
The current study has identified a quantitative correlation between certain clinical parameters and the severity of perceptual visual distortions—more severe perceptual visual distortions are associated with poorer visual acuity, poorer binocular function (motor fusion and stereoacuity), and/or a larger angle of heterophoric/heterotropic deviation. Our data are correlational, so it is not possible to determine whether decorrelated binocular function is caused by or is the cause of perceptual visual distortions, nor can they tease out the relative roles in or contributions of visual acuity, quality of binocular single vision, and near angle of deviation to the distorted amblyopic percept. These questions are the focus of our ongoing work.
Supplementary Material
Acknowledgments
The authors thank Hannah Oldfield for her assistance with data collection for the test–retest reliability component of the study.
Supported by a Fight for Sight PhD studentship (MEFP), in part by Chief Scientist Office Grant ETM\375 (AJS), and in part by National Institutes of Health Grant R01 EY021553 (PJB).
Disclosure: M.E.F. Piano, None; P.J. Bex, None; A.J. Simmers, None
References
- 1. Li T,, Shotton K. Conventional occlusion versus pharmacologic penalization for amblyopia. Cochrane Database Syst Rev. 2009: CD006460. [DOI] [PMC free article] [PubMed]
- 2. Baker DH,, Meese TS,, Mansouri B,, Hess RF. Binocular summation of contrast remains intact in strabismic amblyopia. Invest Ophthalmol Vis Sci. 2007; 48: 5332–5338. [DOI] [PubMed] [Google Scholar]
- 3. Mansouri B,, Thompson B,, Hess RF. Measurement of suprathreshold binocular interactions in amblyopia. Vision Res. 2008; 48: 2775–2784. [DOI] [PubMed] [Google Scholar]
- 4. McKee SP,, Levi DM,, Movshon JA. The pattern of visual deficits in amblyopia. J Vis. 2003; 3 (5): 380–405. [DOI] [PubMed] [Google Scholar]
- 5. Birch EE. Amblyopia and binocular vision. Prog Retin Eye Res. 2013; 33: 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitchell DE. A special role for binocular visual input during development and as a component of occlusion therapy for treatment of amblyopia. Restor Neurol Neurosci. 2008; 26: 425–434. [PubMed] [Google Scholar]
- 7. Pugh M. Visual distortion in amblyopia. Br J Ophthalmol. 1958; 42: 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pugh M. Amblyopia and the retina. Br J Ophthalmol. 1962; 46: 193–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barbeito R,, Bedell HE,, Flom MC. Does impaired contrast sensitivity explain the spatial uncertainty of amblyopes? Invest Ophthalmol Vis Sci. 1988; 29: 323–326. [PubMed] [Google Scholar]
- 10. Barrett BT,, Pacey IE,, Bradley A,, Thibos LN,, Morrill P. Nonveridical visual perception in human amblyopia. Invest Ophthalmol Vis Sci. 2003; 44: 1555–1567. [DOI] [PubMed] [Google Scholar]
- 11. Bedell HE,, Flom MC. Monocular spatial distortion in strabismic amblyopia. Invest Ophthalmol Vis Sci. 1981; 20: 263–268. [PubMed] [Google Scholar]
- 12. Bedell HE,, Flom MC,, Barbeito R. Spatial aberrations and acuity in strabismus and amblyopia. Invest Ophthalmol Vis Sci. 1985; 26: 909–916. [PubMed] [Google Scholar]
- 13. Demanins R,, Hess RF. Positional loss in strabismic amblyopia: inter-relationship of alignment threshold, bias, spatial scale and eccentricity. Vision Res. 1996; 36: 2771–2794. [DOI] [PubMed] [Google Scholar]
- 14. Flom MC,, Bedell HE,, Barbeito R. Spatial mechanisms for visual acuity deficits in strabismic and anisometropic amblyopia - developmental failure or adaptation? : Keller EL,, Zee DS, Adaptive Processes in Visual and Oculomotor Systems. Pacific Grove, CA: Pergamon Press; 1985: 45–51. [Google Scholar]
- 15. Fronius M,, Sireteanu R. Monocular geometry is selectively distorted in the central visual field of strabismic amblyopes. Invest Ophthalmol Vis Sci. 1989; 30: 2034–2044. [PubMed] [Google Scholar]
- 16. Hess RF,, Holliday IE. The spatial localization deficit in amblyopia. Vision Res. 1992; 32: 1319–1339. [DOI] [PubMed] [Google Scholar]
- 17. Lagreze WD,, Sireteanu R. Two-dimensional spatial distortions in human strabismic amblyopia. Vision Res. 1991; 31: 1271–1288. [DOI] [PubMed] [Google Scholar]
- 18. Mansouri B,, Hansen BC,, Hess RF. Disrupted retinotopic maps in amblyopia. Invest Ophthalmol Vis Sci. 2009; 50: 3218–3225. [DOI] [PubMed] [Google Scholar]
- 19. Sireteanu R,, Baumer CC,, Iftime A. Temporal instability in amblyopic vision: relationship to a displacement map of visual space. Invest Ophthalmol Vis Sci. 2008; 49: 3940–3954. [DOI] [PubMed] [Google Scholar]
- 20. Sireteanu R,, Lagreze WD,, Constantinescu DH. Distortions in two-dimensional visual space perception in strabismic observers. Vision Res. 1993; 33: 677–690. [DOI] [PubMed] [Google Scholar]
- 21. Hess RF,, Campbell FW,, Greenhalgh T. On the nature of the neural abnormality in human amblyopia; neural aberrations and neural sensitivity loss. Pflugers Arch. 1978; 377: 201–207. [DOI] [PubMed] [Google Scholar]
- 22. Sireteanu R,, Baumer CC,, Sarbu C,, Iftime A. Spatial and temporal misperceptions in amblyopic vision. Strabismus. 2007; 15: 45–54. [DOI] [PubMed] [Google Scholar]
- 23. Mansouri B,, Hansen BC,, Hess RF. Disrupted retinotopic maps in amblyopia. Invest Ophthalmol Vis Sci. 2009; 50: 3218–3225. [DOI] [PubMed] [Google Scholar]
- 24. Sireteanu R,, Lagreze W,, Constantinescu DH. Distortions in two-dimensional visual space perception in strabismic observers. Vision Res. 1993; 33: 677–690. [DOI] [PubMed] [Google Scholar]
- 25. Sireteanu R,, Baumer CC,, Iftime A. Temporal instability in amblyopic vision: relationship to a displacement map of visual space. Invest Ophthalmol Vis Sci. 2008; 49: 3940–3954. [DOI] [PubMed] [Google Scholar]
- 26. Fronius M,, Sireteanu R,, Zubcov A. Deficits of spatial localization in children with strabismic amblyopia. Graefes Arch Clin Exp Ophthalmol. 2004; 242: 827–839. [DOI] [PubMed] [Google Scholar]
- 27. Gingras G,, Mitchell DE,, Hess RF. Haphazard neural connections underlie the visual deficits of cats with strabismic or deprivation amblyopia. Eur J Neurosci. 2005; 22: 119–124. [DOI] [PubMed] [Google Scholar]
- 28. Levi DM,, Klein SA. Spatial localization in normal and amblyopic vision. Vision Res. 1983; 23: 1005–1017. [DOI] [PubMed] [Google Scholar]
- 29. Levi DM,, Klein SA. Vernier acuity, crowding and amblyopia. Vision Res. 1985; 25: 979–991. [DOI] [PubMed] [Google Scholar]
- 30. Hess RF. Is amblyopia an impediment to binocular function? Eye (Lond). 1996; 10 (pt 2): 245–249. [DOI] [PubMed] [Google Scholar]
- 31. Baumer C,, Sireteanu R. Temporal instability in the perception of strabismic amblyopia. Strabismus. 2006; 14: 59–64. [DOI] [PubMed] [Google Scholar]
- 32. Ansons A,, Davis H. Diagnosis and Management of Ocular Motility Disorders. Oxford: Blackwell; 2005: 220–221. [Google Scholar]
- 33. Brown KC,, Buckley D. Do we really need binocular single vision? Br Ir Orthopt J. 2004; 1: 46–51. [Google Scholar]
- 34. Niechwiej-Szwedo E,, Goltz HC,, Chandrakumar M,, Hirji Z,, Crawford JD,, Wong AM. Effects of anisometropic amblyopia on visuomotor behavior, part 2: visually guided reaching. Invest Ophthalmol Vis Sci 2011; 52: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O'Connor AR,, Birch EE,, Anderson S,, Draper H. Relationship between binocular vision, visual acuity, and fine motor skills. Optom Vis Sci. 2010; 87: 942–947. [DOI] [PubMed] [Google Scholar]
- 36. O'Connor AR,, Birch EE,, Anderson S,, Draper H. The functional significance of stereopsis. Invest Ophthalmol Vis Sci. 2010; 51: 2019–2023. [DOI] [PubMed] [Google Scholar]
- 37. Grant S,, Suttle C,, Melmoth DR,, Conway MC,, Sloper JJ. Age- and stereovision-dependent eye-hand coordination deficits in children with amblyopia and abnormal binocularity. Invest Ophthalmol Vis Sci. 2014; 55: 5687–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Melmoth DR,, Finlay AL,, Morgan MJ,, Grant S. Grasping deficits and adaptations in adults with stereo vision losses. Invest Ophthalmol Vis Sci. 2009; 50: 3711–3720. [DOI] [PubMed] [Google Scholar]
- 39. Niechwiej-Szwedo E,, Goltz HC,, Chandrakumar M,, Wong AM. Effects of strabismic amblyopia on visuomotor behavior: part II. Visually guided reaching. Invest Ophthalmol Vis Sci. 2014; 55: 3857–3865. [DOI] [PubMed] [Google Scholar]
- 40. Suttle CM,, Melmoth DR,, Finlay AL,, Sloper JJ,, Grant S. Eye-hand coordination skills in children with and without amblyopia. Invest Ophthalmol Vis Sci. 2011; 52: 1851–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buckley JG,, Panesar GK,, MacLellan MJ,, Pacey IE,, Barrett BT. Changes to control of adaptive gait in individuals with long-standing reduced stereoacuity. Invest Ophthalmol Vis Sci. 2010; 51: 2487–2495. [DOI] [PubMed] [Google Scholar]
- 42. Joy S,, Davis H,, Buckley D. Is stereopsis linked to hand-eye coordination? Br Orthopt J. 2001; 58: 38–41. [Google Scholar]
- 43. Piano ME,, O'Connor AR. The effect of degrading binocular single vision on fine visuomotor skill task performance. Invest Ophthalmol Vis Sci. 2013; 54: 8204–8213. [DOI] [PubMed] [Google Scholar]
- 44. Sheedy JE,, Bailey IL,, Buri M,, Bass E. Binocular vs. monocular task performance. Am J Optom Physiol Opt. 1986; 63: 839–846. [DOI] [PubMed] [Google Scholar]
- 45. Webber AL,, Wood JM,, Gole GA,, Brown B. The effect of amblyopia on fine motor skills in children. Invest Ophthalmol Vis Sci. 2008; 49: 594–603. [DOI] [PubMed] [Google Scholar]
- 46. Hrisos S,, Clarke MP,, Kelly T,, Henderson J,, Wright CM. Unilateral visual impairment and neurodevelopmental performance in preschool children. Br J Ophthalmol. 2006; 90: 836–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Porac C,, Coren S. The dominant eye. Psychol Bull. 1976; 83: 880–897. [PubMed] [Google Scholar]
- 48. Hess RF,, Bradley A. Contrast perception above threshold is only minimally impaired in human amblyopia. Nature. 1980; 287: 463–464. [DOI] [PubMed] [Google Scholar]
- 49. Peters RJ,, Iyer A,, Itti L,, Koch C. Components of bottom-up gaze allocation in natural images. Vision Res. 2005; 45: 2397–2416. [DOI] [PubMed] [Google Scholar]
- 50. Dorr M,, Martinetz T,, Gegenfurtner KR,, Barth E. Variability of eye movements when viewing dynamic natural scenes. J Vis. 2010; 10 (10): 28. [DOI] [PubMed] [Google Scholar]
- 51. Knox PJ,, Simmers AJ,, Gray LS,, Cleary M. An exploratory study: prolonged periods of binocular stimulation can provide an effective treatment for childhood amblyopia. Invest Ophthalmol Vis Sci. 2012; 53: 817–824. [DOI] [PubMed] [Google Scholar]
- 52. Xi J,, Jia WL,, Feng LX,, Lu ZL,, Huang CB. Perceptual learning improves stereoacuity in amblyopia. Invest Ophthalmol Vis Sci. 2014; 55: 2384–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ho CS,, Giaschi DE. Low- and high-level motion perception deficits in anisometropic and strabismic amblyopia: evidence from fMRI. Vision Res. 2009; 49: 2891–2901. [DOI] [PubMed] [Google Scholar]
- 54. Levi DM,, Klein SA. Noise provides some new signals about the spatial vision of amblyopes. J Neurosci. 2003; 23: 2522–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Levi DM,, Klein SA,, Chen I. The response of the amblyopic visual system to noise. Vision Res. 2007; 47: 2531–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Levi DM,, Klein SA,, Chen I. What limits performance in the amblyopic visual system: seeing signals in noise with an amblyopic brain. J Vis. 2008; 8 (4): 1–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.