Abstract
Sarcoidosis is a multisystem, non-infectious, granulomatous disease of unknown cause, characterised by histological evidence of non-caseating granulomas. Gastrointestinal (GI) involvement is uncommon, reported in <1% of patients with the disease. Herein, we present a rare case of isolated gastric sarcoidosis in a patient with latent pulmonary sarcoidosis and unexplained manifestations of GI disease, illustrating that clinical disease expression is variable; may be organ-specific; and, known disease latency confined to one organ does not exclude the possibility of active disease in another organ system. In patients with organ-specific sarcoidosis, whether active or in remission, presenting with GI symptoms, the possibility of gastric sarcoidosis should be considered. Oesophagogastroduodenoscopy and biopsy, when indicated, should be considered for definitive diagnosis.
Background
Sarcoidosis is a multisystem disease characterised by non-caseating granulomas.1 Among patients with sarcoidosis the disease course is highly variable, and thus patients may present with a wide array of clinical manifestations. Gastrointestinal (GI) tract involvement is rare, with an incidence <1%.1 These cases are often clinically silent, causing symptoms in 0.9% of patients.2 Symptomatic gastric sarcoidosis has been reported; however, few reports describing biopsy-proven disease exist, and isolated GI tract involvement, in patients with organ-specific sarcoidosis in remission is not well described.3 4 Herein, we report a case of gastric sarcoidosis in a patient with pulmonary sarcoidosis in remission, and highlight the importance of oesophagastroduodenoscopy (EGD) and biopsy to confirm the diagnosis.
Case presentation
A 49-year-old African-American man with pulmonary sarcoidosis in remission presented with right upper quadrant abdominal pain. Twenty years prior to presentation he developed exertional dyspnoea. Hilar lymph node biopsy at that time revealed noncaseating granulomas. The diagnosis of pulmonary sarcoidosis was made. His symptoms resolved with a 12-month course of systemic corticosteroids. Over the prior 2 weeks, 20 years after his initial diagnosis of sarcoid was made, he developed intermittent abdominal pain exacerbated by food, and associated with non-bilious vomiting. He denied dyspnoea, chest pain, visual changes, change in weight and bloody stools. Physical examination was notable for a normal blood pressure (123/88 mm Hg), heart rate (54 beats/min), respiratory rate (18 breaths a minute) and temperature (97°F). His oxygen saturation was 100% in ambient air. His skin was anicteric. Abdominal examination revealed tenderness elicited with moderate palpation over the right upper quadrant, with no guarding, rebound tenderness or costovertebral angle tenderness. A digital rectal exam was negative for occult blood.
Investigations
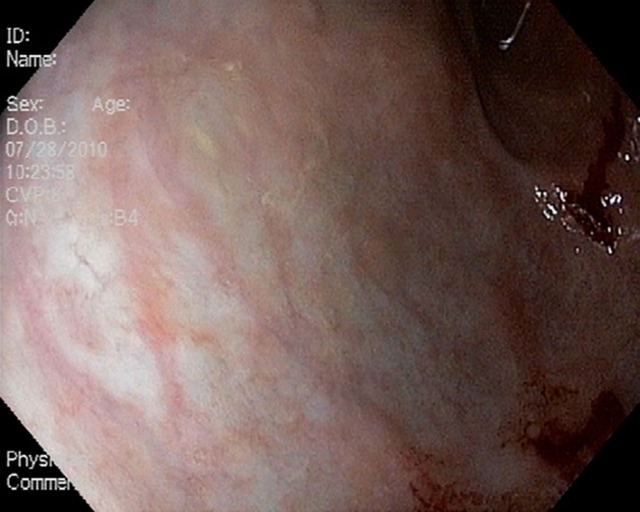
Laboratory assessment demonstrated normal blood cell counts, electrolytes, liver function tests, iron studies, pancreatic enzymes and a rapid urea test were negative. C-reactive protein was elevated slightly (11 mg/l). A chest radiograph demonstrated no evidence of hilar lymphadenopathy. Right upper quadrant ultrasound demonstrated a normal gallbladder and common bile duct. A cat scan performed with oral contrast of the abdomen and pelvis showed an appendix dilated at 1 cm with no inflammatory changes. A hepatobilliary scan and MRI of the abdomen were unrevealing. A trial of proton-pump inhibitors provided no symptom relief. EGD was performed, demonstrating atrophic mucosa within the body and antrum of the stomach (figure 1). Biopsies from these sites did not reveal acid-fast bacilli and a Gömöri methenamine silver stain was negative for fungal organisms. Histopathology from the gastric antrum revealed granulomatous gastritis with multiple non-caseating granulomas (figure 2A,B). The serum angiotensin-converting enzyme level was 72 U/l. A diagnosis of gastric sarcoidosis was made.
Figure 1.
Oesophagogastroduodenoscopy demonstrating atrophic mucosa within the gastric body and antrum of the stomach.
Figure 2.
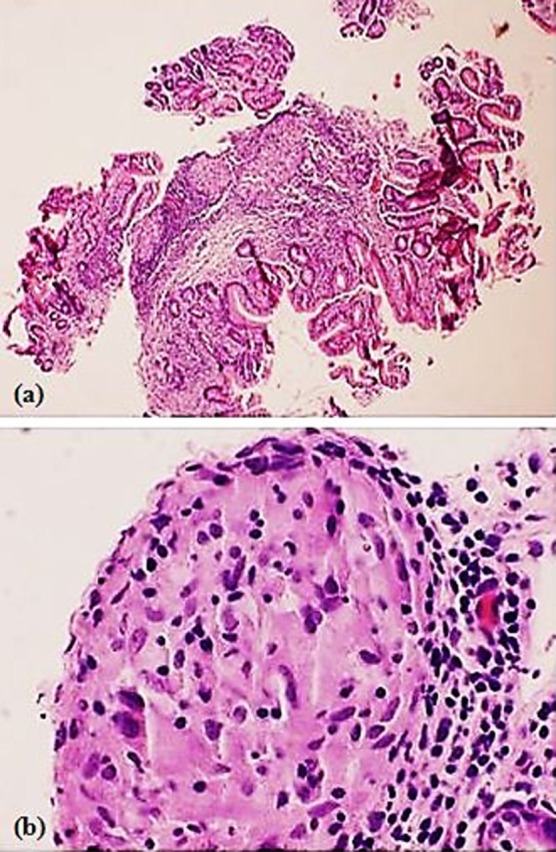
Histopathology of the gastric antrum from an oesophagogastroduodenoscopy specimen. There is (A) granulomatous gastritis with multiple, well-formed, non-necrotising granulomas and focal minimal active inflammation (H&E stain × 40) and (B) granuloma composed of epithelioid histiocytes, surrounded by lymphocytes (H&E stain × 200).
Outcome and follow-up
A 6-month course of a systemic corticosteroid led to complete resolution of his abdominal pain. One year later, he remains free of symptoms and continues an active lifestyle.
Discussion
The diagnosis of sarcoidosis depends on clinical manifestations of the disease and, when possible, histology demonstrating non-caseating granulomas, in the absence of other diseases capable of producing a similar histological or clinical picture.1 The disease course in sarcoidosis is highly variable, with fewer than 7% of patients developing extra pulmonary disease.5 6 Among these patients, heart, lymphatic system, eyes and skin are the most frequently affected organ systems.5 GI tract involvement is uncommon, reported in <1% of patients with the disease.1 Within the GI tract the stomach is most frequently involved; however, sarcoidosis of the oesophagus, gallbladder, liver,7 pancreas,8 appendix, intestines9 and rectum10 have been described.
GI sarcoidosis can mimic many other disease processes. Common symptoms among patients with sarcoid involving the GI tract include abdominal pain, haematemesis and hematochezia. Chinitz et al11 reviewed 20 cases of symptomatic gastric sarcoidosis. In this review, abdominal pain and GI tract bleeding occurred in 75% and 25% of patients, respectively. Two-thirds of the symptomatic patients improved on oral corticosteroids. The remaining patients required surgery. The authors concluded that clinical manifestations are variable, thus endoscopic biopsy is essential in establishing the diagnosis. In another analysis of 44 reports of biopsy-proven gastric sarcoidosis, epigastric pain and emesis occurred in 70% and 33% of cases, respectively.12 Of the patients with abdominal pain, 50% had normal mucosa on endoscopy. Similar to Chinitz et al, the authors of this analysis concluded that diagnosis depend on a compatible history and the demonstration of non-caseating granulomas. Our patient's symptoms were not specific and closely resembled peptic ulcer disease, hence our initial trial of a proton pump inhibitor. His suboptimal response to this therapy raised suspicion for an alternate disease process. Subsequent EGD and biopsy confirmed the diagnosis of sarcoidosis and helped to guide therapy.
Optimal treatment for GI sarcoidosis is not well defined. Compared to sarcoidosis of the heart, nervous system and eye, where a clear role for systemic corticosteroids in the management of the disease exists,1 the role of corticosteroids in treating sarcoid involving other organ systems is less obvious.13 In our patient, systemic corticosteroids ameliorated symptoms and induced clinical disease remission at 1 year clinical follow-up, demonstrating the usefulness of the drug in treating patients with sarcoidosis of the stomach. Still, further studies aimed at assessing the efficacy of corticosteroids in treating patients with GI sarcoidosis are needed.
Our case report serves as a reminder that individuals with organ-specific sarcoidosis in remission are still at risk for other, unrelated organ manifestations of the disease. In patients with a history of sarcoidosis, whether active or in remission, presenting with GI symptoms such as postprandial epigastric pain and vomiting, the possibility of gastric sarcoidosis should be considered. EGD and biopsy, when indicated, should be considered for definitive diagnosis.
Learning points.
Organ-specific sarcoidosis in remission does not preclude unrelated manifestations of the disease in another organ system.
GI sarcoidosis may mimic other disease processes.
Among patients with sarcoidosis, active or in remission, presenting with GI symptoms, the possibility of gastric sarcoid should be considered.
The diagnosis of sarcoidosis depends on clinical manifestations of the disease and, when possible, histology demonstrating non-caseating granulomas, in the absence of other diseases capable of producing a similar histological or clinical picture.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736–55. [DOI] [PubMed] [Google Scholar]
- 2.Liang D, Price BJC, Ahmed H, et al. Gastric sarcoidosis: case report and literature review. J Natl Med Assoc 2010;102:348–51. [DOI] [PubMed] [Google Scholar]
- 3.Beniwal RS, Cummings OW, Cho WK. Symptomatic gastrointestinal sarcoidosis: case report and review of the literature. Dig Dis Sci 2003;48:174–8. [DOI] [PubMed] [Google Scholar]
- 4.Panella VS, Katz S, Kahn E, et al. Isolated gastric sarcoidosis. Unique remnant of disseminated disease. J Clin Gastroenterol 1988;10:327–31. [PubMed] [Google Scholar]
- 5.Takada K, Ina Y, Noda M, et al. The clinical course and prognosis of patients with severe, moderate or mild sarcoidosis. J Clin Epidemiol 1993;46:359–66. [DOI] [PubMed] [Google Scholar]
- 6.Scadding JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Br Med J 1961;2:1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts JC, Rang MC. Sarcoidosis of liver and spleen. Lancet 1958;2:296–9. [DOI] [PubMed] [Google Scholar]
- 8.Garcia C, Kumar V, Sharma OP. Pancreatic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1996;13:28–32. [PubMed] [Google Scholar]
- 9.Tsujino T, Ito Y, Yoshida H, et al. Duodenal mass in a patient with weight loss and liver dysfunction. Duodenal and liver sarcoidosis. Gut 2011;60:1659–60, 1677. [DOI] [PubMed] [Google Scholar]
- 10.Konda J, Ruth M, Sassaris M, et al. Sarcoidosis of the stomach and rectum. Am J Gastroenterol 1980;73:516–18. [PubMed] [Google Scholar]
- 11.Chinitz MA, Brandt LJ, Frank MS, et al. Symptomatic sarcoidosis of the stomach. Dig Dis Sci 1985;30:682–8. [DOI] [PubMed] [Google Scholar]
- 12.Afshar K, BoydKing A, Sharma OP, et al. Gastric sarcoidosis and review of the literature. J Natl Med Assoc 2010;102:419–22. [DOI] [PubMed] [Google Scholar]
- 13.Martin WJ, II, Iannuzzi MC, Gail DB, et al. Future directions in sarcoidosis research: summary of an NHLBI working group. Am J Respir Crit Care Med 2004;170:567–71. [DOI] [PubMed] [Google Scholar]