Abstract
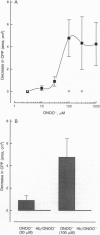
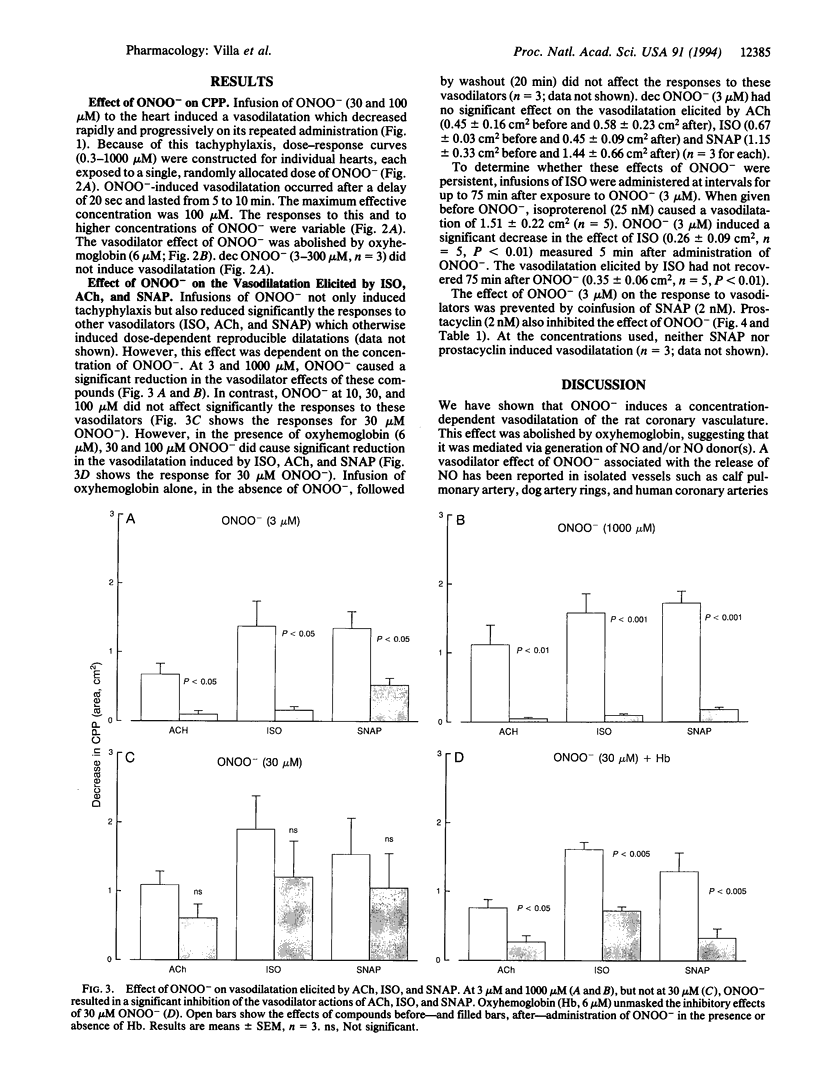
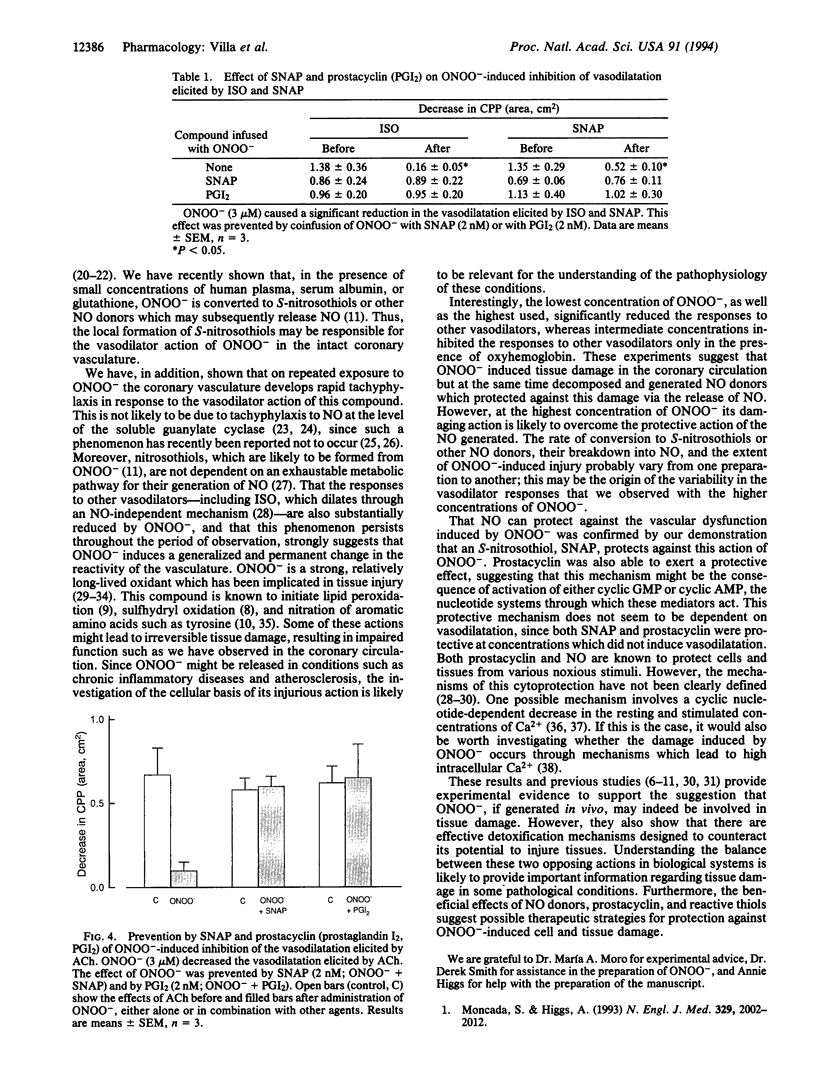
The effects of the oxidant species peroxynitrite (ONOO-) on coronary perfusion pressure and vasodilatation elicited by acetylcholine, isoproterenol, and S-nitroso-N-acetyl-DL-penicillamine were investigated in the isolated perfused rat heart. ONOO- (0.3-1000 microM) caused a concentration-dependent vasodilatation of the coronary vasculature. This dilator response was inhibited by oxyhemoglobin, indicating that it was due to the generation of nitric oxide. Tachyphylaxis to ONOO- developed rapidly, so that the response disappeared after three or four applications of this compound. ONOO- not only induced tachyphylaxis but also inhibited the vasodilatation induced by the three vasodilators studied. This latter effect of ONOO- was critically dependent on its concentration, since it occurred at 3 microM, which was subthreshold as a dilator, and at 1000 microM, which was supramaximal, but not at 30 and 100 microM. These latter concentrations inhibited the responses to vasodilators only in the presence of oxyhemoglobin. Thus, a wide range of concentrations of ONOO- induce a vascular dysfunction, as evidenced by the tachyphylaxis to its own vasodilator actions and the long-lasting impairment of the responses to other vasodilators. However, at the same time ONOO- generates nitric oxide, which at certain concentrations of ONOO- is sufficient to counteract its deleterious action. Coinfusion of S-nitroso-N-acetyl-DL-penicillamine or prostacyclin at low concentrations that did not produce vasodilatation also protected against ONOO(-)-induced vascular dysfunction: these compounds may be protective through a common mechanism, as yet undefined.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amezcua J. L., Dusting G. J., Palmer R. M., Moncada S. Acetylcholine induces vasodilatation in the rabbit isolated heart through the release of nitric oxide, the endogenous nitrovasodilator. Br J Pharmacol. 1988 Nov;95(3):830–834. doi: 10.1111/j.1476-5381.1988.tb11711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amezcua J. L., Palmer R. M., de Souza B. M., Moncada S. Nitric oxide synthesized from L-arginine regulates vascular tone in the coronary circulation of the rabbit. Br J Pharmacol. 1989 Aug;97(4):1119–1124. doi: 10.1111/j.1476-5381.1989.tb12569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson K. L., Andersson R. G. Tolerance towards nitroglycerin, induced in vivo, is correlated to a reduced cGMP response and an alteration in cGMP turnover. Eur J Pharmacol. 1983 Mar 18;88(1):71–79. doi: 10.1016/0014-2999(83)90393-x. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S., Crow J. P. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem Soc Trans. 1993 May;21(2):330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- Beckman J. S. The double-edged role of nitric oxide in brain function and superoxide-mediated injury. J Dev Physiol. 1991 Jan;15(1):53–59. [PubMed] [Google Scholar]
- Beckmann J. S., Ye Y. Z., Anderson P. G., Chen J., Accavitti M. A., Tarpey M. M., White C. R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994 Feb;375(2):81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- Berti F., Magni F., Rossoni G., De Angelis L., Galli G. Production and biologic interactions of prostacyclin and platelet-activating factor in acute myocardial ischemia in the perfused rabbit heart. J Cardiovasc Pharmacol. 1990 Nov;16(5):727–732. doi: 10.1097/00005344-199011000-00006. [DOI] [PubMed] [Google Scholar]
- Drexler H., Zeiher A. M., Wollschläger H., Meinertz T., Just H., Bonzel T. Flow-dependent coronary artery dilatation in humans. Circulation. 1989 Sep;80(3):466–474. doi: 10.1161/01.cir.80.3.466. [DOI] [PubMed] [Google Scholar]
- Ely D., Dunphy G., Dollwet H., Richter H., Sellke F., Azodi M. Maintenance of left ventricular function (90%) after twenty-four-hour heart preservation with deferoxamine. Free Radic Biol Med. 1992;12(6):479–485. doi: 10.1016/0891-5849(92)90101-l. [DOI] [PubMed] [Google Scholar]
- Graham A., Hogg N., Kalyanaraman B., O'Leary V., Darley-Usmar V., Moncada S. Peroxynitrite modification of low-density lipoprotein leads to recognition by the macrophage scavenger receptor. FEBS Lett. 1993 Sep 13;330(2):181–185. doi: 10.1016/0014-5793(93)80269-z. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hogg N., Darley-Usmar V. M., Wilson M. T., Moncada S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J. 1992 Jan 15;281(Pt 2):419–424. doi: 10.1042/bj2810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiff R. J., Moss D. W., Moncada S. Effect of nitric oxide gas on the generation of nitric oxide by isolated blood vessels: implications for inhalation therapy. Br J Pharmacol. 1994 Oct;113(2):496–498. doi: 10.1111/j.1476-5381.1994.tb17016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käser-Glanzmann R., Jakäbovä M., George J. N., Lüscher E. F. Stimulation of calcium uptake in platelet membrane vesicles by adenosine 3',5'-cyclic monophosphate and protein kinase. Biochim Biophys Acta. 1977 May 2;466(3):429–440. doi: 10.1016/0005-2736(77)90336-4. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Lefer D. J. Endothelial dysfunction in myocardial ischemia and reperfusion: role of oxygen-derived free radicals. Basic Res Cardiol. 1991;86 (Suppl 2):109–116. doi: 10.1007/978-3-642-72461-9_12. [DOI] [PubMed] [Google Scholar]
- Lefroy D. C., Crake T., Uren N. G., Davies G. J., Maseri A. Effect of inhibition of nitric oxide synthesis on epicardial coronary artery caliber and coronary blood flow in humans. Circulation. 1993 Jul;88(1):43–54. doi: 10.1161/01.cir.88.1.43. [DOI] [PubMed] [Google Scholar]
- Lepoivre M., Flaman J. M., Henry Y. Early loss of the tyrosyl radical in ribonucleotide reductase of adenocarcinoma cells producing nitric oxide. J Biol Chem. 1992 Nov 15;267(32):22994–23000. [PubMed] [Google Scholar]
- Liu S., Beckman J. S., Ku D. D. Peroxynitrite, a product of superoxide and nitric oxide, produces coronary vasorelaxation in dogs. J Pharmacol Exp Ther. 1994 Mar;268(3):1114–1121. [PubMed] [Google Scholar]
- Matheis G., Sherman M. P., Buckberg G. D., Haybron D. M., Young H. H., Ignarro L. J. Role of L-arginine-nitric oxide pathway in myocardial reoxygenation injury. Am J Physiol. 1992 Feb;262(2 Pt 2):H616–H620. doi: 10.1152/ajpheart.1992.262.2.H616. [DOI] [PubMed] [Google Scholar]
- McCall T. B., Boughton-Smith N. K., Palmer R. M., Whittle B. J., Moncada S. Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem J. 1989 Jul 1;261(1):293–296. doi: 10.1042/bj2610293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Moro M. A., Darley-Usmar V. M., Goodwin D. A., Read N. G., Zamora-Pino R., Feelisch M., Radomski M. W., Moncada S. Paradoxical fate and biological action of peroxynitrite on human platelets. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6702–6706. doi: 10.1073/pnas.91.14.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima S., Tohmatsu T., Hattori H., Okano Y., Nozawa Y. Inhibitory action of cyclic GMP on secretion, polyphosphoinositide hydrolysis and calcium mobilization in thrombin-stimulated human platelets. Biochem Biophys Res Commun. 1986 Mar 28;135(3):1099–1104. doi: 10.1016/0006-291x(86)91041-7. [DOI] [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991 Mar 5;266(7):4244–4250. [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991 Aug 1;288(2):481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- Rossaint R., Falke K. J., López F., Slama K., Pison U., Zapol W. M. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993 Feb 11;328(6):399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- Shaul P. W., Muntz K. H., Buja L. M. Comparison of beta adrenergic receptor binding characteristics and coupling to adenylate cyclase in rat pulmonary artery versus aorta. J Pharmacol Exp Ther. 1990 Jan;252(1):86–92. [PubMed] [Google Scholar]
- Waldman S. A., Rapoport R. M., Ginsburg R., Murad F. Desensitization to nitroglycerin in vascular smooth muscle from rat and human. Biochem Pharmacol. 1986 Oct 15;35(20):3525–3531. doi: 10.1016/0006-2952(86)90622-2. [DOI] [PubMed] [Google Scholar]
- Yu L., Gengaro P. E., Niederberger M., Burke T. J., Schrier R. W. Nitric oxide: a mediator in rat tubular hypoxia/reoxygenation injury. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1691–1695. doi: 10.1073/pnas.91.5.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet A., O'Neill C. A., Halliwell B., Cross C. E., Kaur H. Aromatic hydroxylation and nitration of phenylalanine and tyrosine by peroxynitrite. Evidence for hydroxyl radical production from peroxynitrite. FEBS Lett. 1994 Feb 14;339(1-2):89–92. doi: 10.1016/0014-5793(94)80391-9. [DOI] [PubMed] [Google Scholar]