Abstract
Japanese encephalitis (JE) is an epidemic encephalitis characterised by altered sensorium, convulsions, headache, brainstem signs with pyramidal and extrapyramidal features. Immune-mediated manifestation as acute transverse myelitis (ATM) has not been previously reported in JE. We describe a 40-year-old man who presented with an acute onset quadriparesis with urinary retention, which was preceded by fever and headache 3 weeks prior. He had elevated IgM titres against JE virus in serum and cerebrospinal fluid. MRI of cervico-thoracic spine demonstrated signal intensity alterations extending from C1 to D10 spinal segments. The patient was treated with intravenous methyl prednisolone for 5 days. He regained normal power at 6 months follow-up and repeat MRI study demonstrated complete resolution of the lesion. We conclude that in a case of JE, one should be vigilant for early diagnosis of possible complication as ATM, in which an early institution of immunomodulator therapy prevents adverse consequences.
Background
Japanese encephalitis (JE) is an important cause of epidemic encephalitis in southeast Asian countries. A huge population living in the endemic regions makes this disease a global health concern. JE is caused by Japanese encephalitis virus (JEV), a mosquito-borne virus, belonging to the genus Flavivirus (family Flaviviridae). WHO estimated that approximately 67 900 JE cases typically occur annually in the 24 JE-endemic countries, for an incidence of 1.8 per 100 000 overall.1 In India, many outbreaks of JE have been reported since 1955. During recent years, majority of cases during this epidemic came from eastern Uttar Pradesh (Gorakhpur and adjoining areas).2–4 The mortality of JE ranges between 20% and 40%.5 The reported clinical presentation of JE include altered sensorium, convulsions, headache, hyperkinetic movements and brain stem involvement features as opsoclonus, gaze palsies and pupillary changes.3 However, immune-mediated demyelinating neurological manifestation as acute transverse myelitis (ATM) has not been reported previously in JE. We describe a patient who developed ATM following an infection with JE virus. This case report has its importance in view of potential therapeutic implications of this complication of JE, which was not expected previously.
Case presentation
A 40-year-old man presented with complaints of weakness in both lower limbs which started 3 days prior and progressed to weakness of both upper limbs the next day. He also had urinary retention for 3 days. The weakness in upper limbs was mild and distal, compared to lower limbs in which he had a complete paralysis. He had fever and headache about 3 weeks prior to the onset of weakness. There was no history of trauma, vaccination or similar attack previously.
Examination showed normal vital parameters and higher mental functions. Cranial nerves examination was normal. Muscle tone was normal in upper limbs and reduced in lower limbs. The power was MRC (Medical Research Council) grade 3/5 at shoulder, elbow and wrist and hand grip was weak bilaterally. In the lower limb the power was grade 0/5 at all joints. Deep tendon reflexes were present in upper limbs but absent in lower limbs. The abdominal and cremasteric reflexes were absent. Planters were bilaterally extensors. There was a sensory loss below C5 for all modalities of sensation.
Investigations
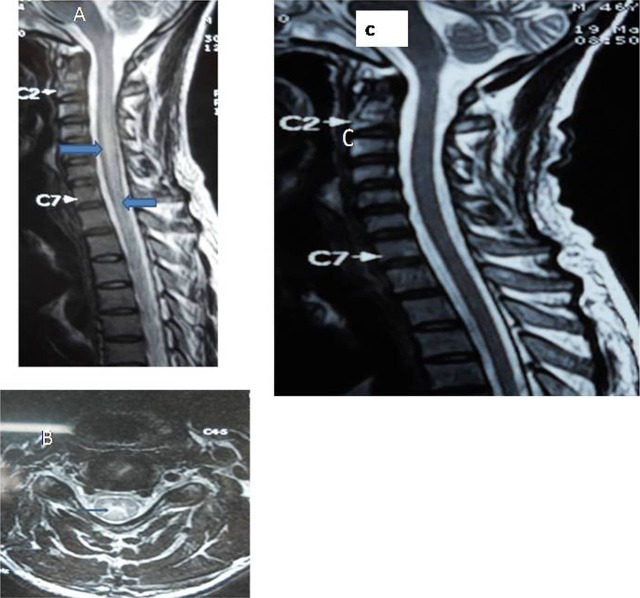
He had normal haematological, biochemical and thyroid parameters. Cerebrospinal fluid (CSF) analysis depicted a cell count of 35/mm3 (all lymphocytes), protein—101.4 mg%, sugar—93.2 mg% using Gram-negative and acid-fast bacilli stains. The serology for Dengue virus, Chikangunia virus, measles, mumps, Hepatitis virus, HIV, Epstein-Barr virus and cytomegalovirus was negative. The serum and CSF ELISA for JE carried out with JE IgM COMBO ELISA (Panbio, Australia) showed elevated IgM antibody titres in serum and CSF (serum-23.01PBU (Panbio units) against 11 PBU as the upper limit of normal, CSF-27.1 PBU against 11 PBU as the upper limit). Serum aquaporin-E antibody for neuro myelitis optica (NMO) was negative. MRI of cervico-thoracic spine demonstrated signal intensity alterations, hyperintense on T2-weighted image (figure 1A) and hypointense on T1-weighted image, extending from C1 to D10 spinal segments without any obvious postcontrast enhancement. Changes were also well depicted in axial images (figure 1B). MRI cranium did not reveal altered signals.
Figure 1.
(A) MRI, cervicothoracic spine T2-weighted sagittal image showed hyperintense signals, extending from cervical first till thoracic second segments with swollen cord. (B) T2-weighted axial image depicted hyperintensities at cervical area. (C) Repeat T2-weighted sagittal image showed complete resolution of the lesion.
Treatment
A diagnosis of ATM was made and the patient was treated with intravenous methyl prednisolone 1 g daily for 5 days. The patient received physiotherapy followed by gait training on recovery of power for rehabilitation.
Outcome and follow-up
The patient did not show any improvement in power during the first week. Towards the seventh day, the patient had atrophy of thigh and calf muscles and hypotonia persisted. Suspecting anterior horn cell involvement, an electromyography (EMG) was done, which showed no spontaneous activity. The patient regained normal power and other myelopathic features were reversed at 6 months follow-up. The repeat MRI study demonstrated complete resolution of the lesion (figure 1C).
Discussion
The important presenting features of JE in a previous large case series were altered sensorium, convulsions, headache, hyperkinetic movement disorders, features of brain stem involvement as opsoclonus, gaze palsies and pupillary changes, dystonia, decerebrate rigidity, paralysis and seizures.3 Misra et al5 reported that patients with JE present with altered sensorium, anterior horn cell involvement and hyperkinetic movement disorders. Unusual features of JE described in the literature include respiratory paralysis,6 oromandibular dystonia,7 acute flaccid paralysis8 and hemiplegia with dysarthria.9 However, there are no previous case reports of immune-mediated demyelinating neurological syndrome as ATM following JE.
Transverse myelitis results from inflammation of complete thickness of the spinal cord leading to loss of motor, sensory and autonomic functions below the involved spinal cord segment. The diagnosis of ATM in our patient was made on the basis of a typical clinical and radiological picture. Preceding JE virus infection was mild, manifested by fever and headache 3 weeks prior to the onset of weakness. It was confirmed by positive IgM antibody in the serum and the CSF to JE virus which has very high sensitivity and specificity.10 The aetiology of ATM is varied, including viral infections, autoimmune disorders such as systemic lupus erythematosus, vasculitis, multiple sclerosis and following vaccination. Various viral infections associated with ATM include herpes simplex virus, Varicella zoster virus, Epstein-Barr virus, cytomegalovirus, enteroviruses, HIV, influenza and rabies virus.11 ATM associated with viral infection is mostly the result of immunological injury to spinal cord rather than direct viral invasion.
The phenomenon of acute flaccid paralysis has been identified in JE patients. This manifests as asymmetric weakness, predominantly involving the lower limbs. This was described to be due to anterior horn cell lesion. Anterior horn cell involvement in JE has been proved on the basis of prominent fibrillation and neurogenic changes in the wasted muscles on EMG.12 However, our patient did not have any such evidence of anterior horn cell involvement on EMG. Thus, his weakness cannot be explained by anterior horn cell involvement and was purely due to ATM. As JE infection has not been previously reported to cause ATM, it is difficult to provide a probable explanation for this complication on the basis of current literature.
Pathological changes observed in humans and animals with JE include (1) neuronal and glial damage directly caused by viral replication; (2) inflammation, including perivascular infiltration of small lymphocytes, plasma cells and macrophages; (3) cellular nodule formation composed of activated microglia and mononuclear cells and (4) cerebral interstitial oedema.13 14 It is likely that the neuronal damage and disruption of blood−neuron barrier secondary to inflammation lead to exposure of neuronal antigen to immune responsive elements, further mounting an antineuronal autoimmune response, which leads to neuronal damage manifesting as ATM. Furthermore, a delay of 3 weeks between JE virus infection and ATM favours an immune-mediated insult to the spinal cord. However, further studies into the pathogenesis of neurological manifestations in JE are needed to explain such presentation.
To conclude, our case report suggests that in a case of JE, one should be vigilant for early diagnosis of possible immune-mediated demyelinating syndrome such as ATM, for consideration of an early institution of immunomodulator therapy to prevent adverse consequences.
Learning points.
Acute transverse myelitis, a previously unreported complication is also possible in Japanese encephalitis virus infection.
It is probably an immune-mediated phenomenon and responds well to corticosteroid therapy.
A high index of suspicion is needed for an early diagnosis and treatment of this complication.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Campbell GL, Hills SL, Fischer M, et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ 2011;89:766–74, 774A–E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saxena SK, Mishra N, Saxena R, et al. Trend of Japanese encephalitis in North India: evidence from thirty-eight acute encephalitis cases and appraisal of niceties. J Infect Dev Ctries 2009;3:517–30. [DOI] [PubMed] [Google Scholar]
- 3.Sarkari NB, Thacker AK, Barthwal SP, et al. Japanese encephalitis (JE). Part I: clinical profile of 1282 adult acute cases of four epidemics . J Neurol 2012;259:47–57. [DOI] [PubMed] [Google Scholar]
- 4.Sarkari NB, Thacker AK, Barthwal SP, et al. Japanese encephalitis (JE) part II: 14 years’ follow-up of survivors. J Neurol 2012;259:58–69. [DOI] [PubMed] [Google Scholar]
- 5.Misra UK, Kalita J, Goel D, et al. Clinical, radiological and neurophysiological spectrum of JEV encephalitis and other non-specific encephalitis during post-monsoon period in India. Neurol India 2003;51:55–9. [PubMed] [Google Scholar]
- 6.Tzeng SS. Respiratory paralysis as a presenting symptom in Japanese encephalitis—a case report. Zhonghua Yi Xue Za Zhi (Taipei) 1989;43:208–12. [PubMed] [Google Scholar]
- 7.Kalita J, Misra UK, Pradhan PK. Oromandibular dystonia in encephalitis. J Neurol Sci 2011;304:107–10. [DOI] [PubMed] [Google Scholar]
- 8.Chung CC, Lee SS, Chen YS, et al. Acute flaccid paralysis as an unusual presenting symptom of Japanese encephalitis: a case report and review of the literature. Infection 2007;35:30–2. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Shrivastava AK, Singh DK, et al. Hemiplegia with dysarthria: an initial manifestation of Japanese encephalitis in a 4-year-old child. J Vector Borne Dis 2008;45:328–30. [PubMed] [Google Scholar]
- 10.Zhang MJ, Wang MX, Jiang SZ, et al. A rapid IgM antibody capture ELISA and its employ in the early diagnosis of Japanese encephalitis. Virology 1989;3:251–5. [Google Scholar]
- 11.Scott TF. Nosology of idiopathic transverse myelitis syndromes. Acta Neurol Scand 2007;115:371–6. [DOI] [PubMed] [Google Scholar]
- 12.Misra UK, Kalita J. Anterior horn cells are also involved in Japanese encephalitis. Acta Neurol Scand 1997;96:114–17. [DOI] [PubMed] [Google Scholar]
- 13.Hase T, Summers PL, Dubois DR. Ultrastructural changes of mouse brain neurons infected with Japanese encephalitis virus. Int J Exp Pathol 1990;71:493–505. [PMC free article] [PubMed] [Google Scholar]
- 14.Hase T. Virus−neuron interactions in the mouse brain infected with Japanese encephalitis virus. Virchows Arch B Cell Pathol Incl Mol Pathol 1993;64:161–70. [DOI] [PubMed] [Google Scholar]