Abstract
We describe a 55-year-old man presenting to our institution with a gastrointestinal bleed. He was found to have a 5 cm pancreatic extra-gastrointestinal stromal tumours (EGISTs) eroding into the duodenum and ampulla of Vater. Pancreaticoduodenectomy was performed and the tumour was noted to be positive for CD117 and CD34 with six mitotic figures per 50/high-powered field. At 5 months postoperatively he is receiving treatment with imatinib and doing well. To the best of our knowledge, our patient is only the 18th case reported in the literature to date.
Background
Within the last 10 years, gastrointestinal stromal tumour (GIST) has become a buzz-word in the surgical community, largely due to the advent of imatinib (Gleevec). Not recognised as a particular entity until the late 1990s, GISTs have since become a hot-bed for multiple areas of research.1 Gastrointestinal stromal tumours are actually the most common mesenchymal neoplasm of the alimentary tract, presenting anywhere from oesophagus to anus.2 3 Most commonly, they arise from the stomach (50–60%) and the small intestine (20–30%).3 GISTs occur due to neoplastic transformation of the interstitial cells of Cajal (ICCs), which normally act as pacemakers of intestinal motility.2–4Histologically they are composed of spindle cells, and have an estimated incidence of 10–20 per million.5
In contrast to the wealth of knowledge we have accrued about GISTs, we still have a lot to learn about primary extra-gastrointestinal stromal tumours (EGISTs). EGISTs are essentially identical to their gastrointestinal counterparts in terms of morphology, molecular profile, and immunohistochemistry, but arise from the soft tissues of the abdomen without a visceral connection. They have been reported to occur in such locations as the gallbladder, omentum, mesentery, retroperitoneum, prostate, bladder and liV.2 3 5–19 EGISTs are extremely rare (only 5% of all GISTs), and, among those, only 17 cases of stromal tumours arising in the pancreas have been reported in the literature to date.3 19 20 Our case, to the best of our knowledge, is the only documented occurrence of a pancreatic EGIST presenting as a gastrointestinal haemorrhage. Here, we present the summary of our case, as well as a review of the current literature available related to pancreatic EGISTs.
Case presentation
Our patient is a healthy 55-year-old man who transferred to our institution with a 12 h history of haematemesis and haematochezia. Upon initial evaluation, he was fairly asymptomatic, had a benign abdominal exam and haemoglobin of 12. He was placed on a Nexium drip and an emergent oesophagogastroduodenoscopy was performed. Endoscopy revealed a large ulcerated mass within the duodenum with a necrotic centre and adherent clot which was not amenable to endoscopic therapy due to its friable nature and risk for further bleeding (figure 1). A CT scan of the abdomen and pelvis was obtained to better characterise the lesion. This revealed a 4.4×4.5×4.6 cm cystic mass originating from the pancreatic head and eroding into the duodenum and ampulla of Vater (figures 2 and 3). Unfortunately, due to the size of the tumour, its friability, and its involvement of the ampulla of Vater, it was clear that the only surgical option would be a pancreaticoduodenectomy (Whipple) procedure. A biopsy was not possible without the risk of further bleeding. Given the lack of diagnosis and the patient's unstable condition, we did not believe an emergent Whipple was in his best interest. The patient was taken to the angiography suite, where bleeding vessels in the posterior pancreaticoduodenal arcade were identified and coiled. After embolisation, the patient quickly stabilised and was able to be discharged home 4 days later.
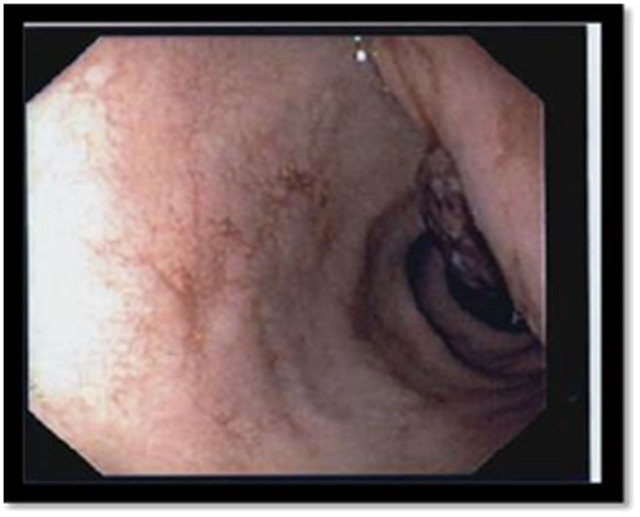
Figure 1.
Necrotic duodenal mass visualised by ECG.
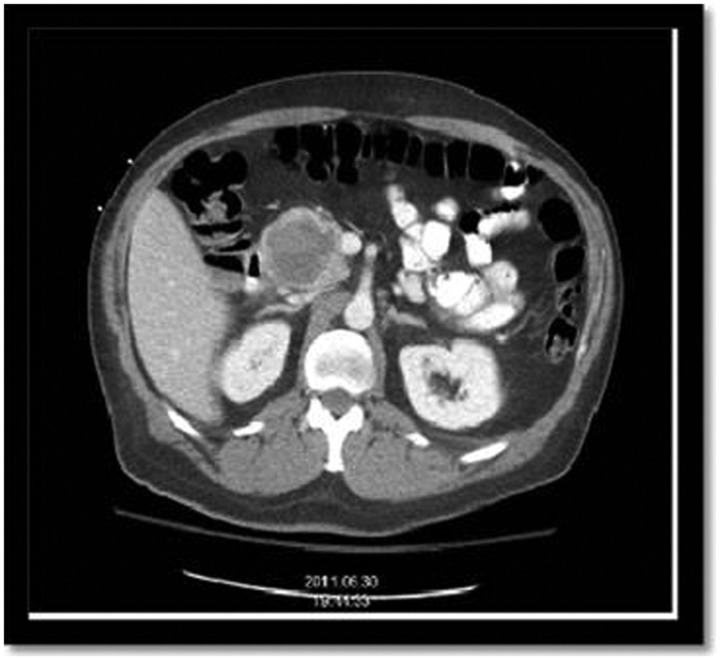
Figure 2.
A CT scan of the abdomen and pelvis showing cystic pancreatic mass.
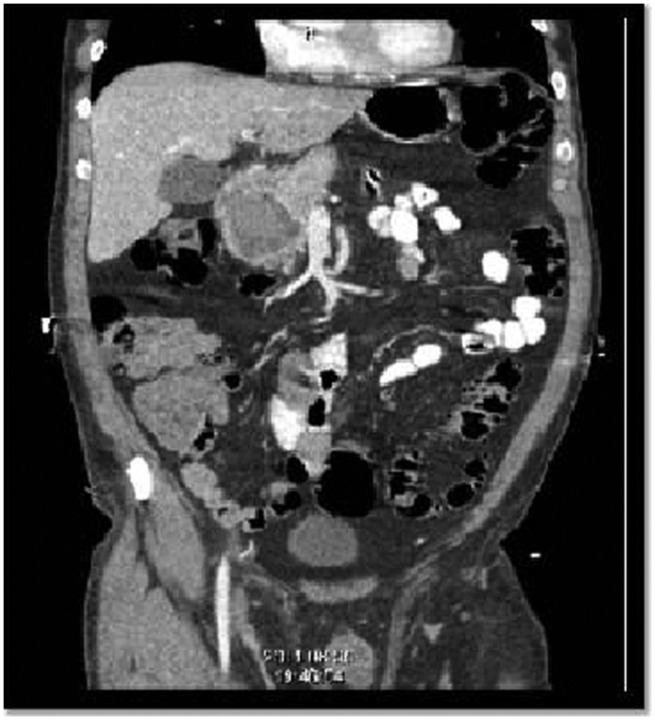
Figure 3.
Coronal view of cystic pancreatic mass.
Investigations
Liver enzymes, CA19-9 and carcinoembrionic antigen were obtained and all noted to be normal. Magnetic resonance cholangiopancreatography (MRCP) confirmed the presence of a 5 cm complex cystic mass in the head of the pancreas with erosion into the duodenum. Involvement of the pancreatic duct was difficult to ascertain due to mass effect and motion artefact.
Differential diagnosis
Differential diagnosis included intraductal papillary mucinous neoplasm, mucinous cystic tumour, or cystic adenocarcinoma of the pancreas.
Treatment
The patient was scheduled for endoscopic ultrasound with biopsies, after which surgical intervention was to proceed. However, on the morning of the follow-up appointment (10 days postdischarge), the patient suffered another episode of haematochezia. On laboratory evaluation, his haemoglobin had dropped to 5.7 (from 8 at time of discharge). He was directly admitted to the hospital and resuscitated. Colonoscopy showed diverticulosis and a small adenomatous polyp with no other abnormalities or source of bleeding. After several days of optimisation, pancreaticoduodenectomy was performed with choledochojejunostomy, pancreaticojejunostomy and gastrojejunostomy.
Outcome and follow-up
At gross exam, a large, firm, 4.5×4.2 cm pancreatic mass was identified infiltrating into the duodenum with no signs of metastasis. On closer evaluation, it was a solid, cavitated lesion, surrounded by a 0.5 cm-thick fibrous capsule, intimately in contact with the muscularis propria of the ampulla of vater. Final pathology was consistent with a pancreatic gastrointestinal stromal tumour with clear surgical margins. The tumour was composed of spindled cells with pale eosinophilic cytoplasm, and there were six mitotic figures per 50/high-powered field (HPF). Immunohistochemistry was positive for CD117/ c-kit, DOG-1 and CD34, and negative for actin, desmin and S100 (figure 4 and 5). Seven lymph nodes were identified, all of which were negative for metastatic disease. Based on these findings, the mass was classified as intermediate risk for tumour progression.
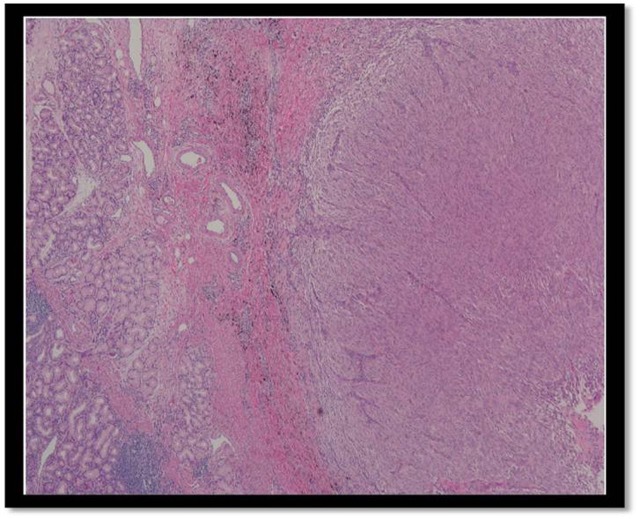
Figure 4.
H&E stain of tumour composed of multiple spindle cells.
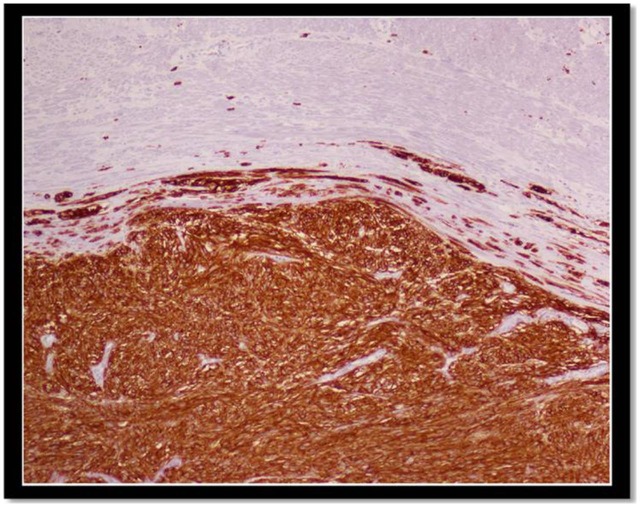
Figure 5.
The tumour showed positive staining for CD117 (c-kit).
Postoperatively, the patient developed a leak which required revision of his gastrojejunostomy via roux-en-y configuration. Additionally, he developed a low-output pancreatic fistula initially controlled with interventional radiology drainage and octrotide. Given the intermediate risk classification of his tumour, per current guidelines, adjuvant therapy with imatinib (Gleevec; Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA) was recommended. Currently, his fistula has closed, with complete resection of his tumour, and no signs of recurrence. He is currently receiving therapy with imatinib and continues to do well.
Discussion
It has now been well established that the ICCs from which GISTs are derived express the receptor tyrosine kinase c-kit.2 CD117 is a proto-oncogene protein of c-kit.2 3 In all, 95% of GISTs express this antigen, making it their defining feature. Thus, demonstrating positivity for CD117 via immunohistochemical analysis is the gold standard for GIST diagnosis.2 5 Diagnosis can be further solidified by positivity for CD34, (60–70% expression) smooth muscle actin (30–40%) and S100 (5%).2 3 5 6 Currently, rather than being designated ‘benign’ or ‘malignant’, GISTs are assigned to a ‘risk category’ based on their propensity for aggressive behaviour and/or metastasis. Originally proposed by Fletcher in 2002, tumours are categorised according to size and mitotic rate per 50 HPFs (see table 1).1
Table 1.
Classification of primary gastrointestinal tumours by risk of metastasis
| Risk category | Size | Mitotic count |
|---|---|---|
| Very low | <2 cm | <5 per 50 HPFs |
| Low | 2–5 cm | <5 per 50 HPFs |
| Intermediate | <5 cm | 6–10 per HPFs |
| 5–10 cm | <5 per 50 HPFs | |
| High | >5 cm | >5 per 50 HPFs |
| >10 cm | Any mitotic rate | |
| Any size | >10 per 50 HPFs |
HPF, high-powered field.
Adapted from Bertagnolli.4
Tumours larger than 5 cm with greater than 5 mitoses per 50 HPF are considered ‘high risk’, as well as lesions >10 cm, regardless of histology.6 13 Recent studies have shown that gastric and proximal small bowel GISTs are actually less aggressive than more distal small bowel and colonic tumours.1 10 As a result, risk stratification was updated in the 2007 National Comprehensive Cancer Network (NCCN) guidelines to include anatomic tumour location.1 Categorising the tumour helps predict prognosis, as well as the need for neo-adjuvant or adjuvant therapy with imatinib.3
Imatinib is a tyrosine kinase receptor inhibitor approved by the Food and Drug Administration for the treatment of metastatic and unresectable GISTs.10 It is also recommended for neo-adjuvant and/or adjuvant therapy of intermediate-risk to high-risk tumours and recurrent disease, though a standardised treatment algorithm for these lesions does not yet exist.1 Sunitinib (Sutent) is also available for patients who are resistant to or cannot tolerate Gleevec.10 Though GISTs are very responsive to Gleevec, complete surgical resection with negative margins is still the standard of care and provides the best long-term prognosis. Patients with metastatic disease also have better results with debulking, as it increases the effectiveness of imatinib on the tumour.3 4
In contrast to GISTs, the origin of EGISTs still remains controversial. Some propose that these tumours may actually be the result of extramural growth of a primary GIST so extensive that they completely lose contact with the muscularis propria of the adjacent structure.2 19 Others suggest that both GISTs and EGISTs arise from a common precursor to both the ICCs and smooth muscle cells. This seems more likely, given that recent studies have confirmed the existence of c-kit positive ICC's within extra-intestinal organs and vessels. Furthermore, these very same cells have also been documented within the exocrine pancreas, further explaining the origin of pancreatic EGISTs.2 16 21 Additionally, these pancreatic cells have been shown to respond to Imatinib.22 Studies demonstrate that EGISTs have a histologically similar presentation to GISTs, but probably behave differently in terms of prognosis and malignant potential. Thus, they may require different risk stratification. Trends seem to show that, in fact, EGISTs behave similar to GISTs in the distal GI tract, in that they may be more aggressive with a greater propensity to metastasise.2 10 Other studies have shown high recurrence rates despite adequate resection and appropriate adjuvant therapy.20
Pancreatic EGISTs, on the other hand, seem to remain fairly asymptomatic and treatable with favourable survival and low malignant potential. However, given the small number of cases available, it is difficult to determine statistically significant trends.10 17 Definitive treatment remains complete surgical resection with negative margins, and neo-adjuvant and/or adjuvant therapy with imatinib according to current guidelines. This seems to be particularly important/effective in those pancreatic tumours with high mitotic counts.17 There have been several cases of recurrence despite Gleevec, as well as some success reported with re-resection.17
Pancreatic EGISTs are extremely rare. To the best of our knowledge, our patient is only the 18th case reported in the literature to date. In general, these tumours seem to have a varied clinical presentation, largely dependent on the size of the lesion at diagnosis. It is well known that EGISTs tend to grow larger than GISTs due to their deeper intra-abdominal location.2 10 Fittingly, due to their lack of mucosal involvement, pancreatic EGISTs often remain asymptomatic until large enough to cause significant mass effect.2 18 In analysing the data from the 18 available cases, there are some clinically useful patterns which begin to emerge (complete information was not reported for all patients). These findings are summarised in table 2.
Table 2.
Characteristics of pancreatic gastrointestinal stromal tumours: a summary of 18 cases
| Age (years) | Range: 31−74 | Mean: 52 | Median: 53 |
|---|---|---|---|
| Gender | Female: 55% (10/18) | Male: 45% (8/18) | |
| Presentation | Asymptomatic | Weakness | Weight loss |
| Abdominal pain | Fatigue | Nausea | |
| Emesis | Gastrointestinal bleed | Back pain | |
| Palpable mass | Anaemia (33%, 6/18) | ||
| Location | Pancreatic head: 50% (9/18) | Pancreatic body/tail: 50% (9/18) | |
| Tumour size (cm) | Range: 2–30 | Mean: 12 | Median: 10.5 |
| Mitoses (per 50 HPF) | Range: 1–120 | Mean: 14.8 | Median: 7 |
| Risk assessment | High: 88% (15/17) | Intermediate: 12% (2/17) | |
| Histology | CD117: 100% (17/17) | CD34: 77% (10/13) | |
| Preoperative dx | 22% (4/18) | ||
| Surgical therapy | Whipple: 53% (8/15) | Distal pan: 40% (6/15) | Cystej: 7% (1/15) |
| Medical therapy | Imatinib: 54% (7/13) | Sunatinib: 8% (1/13) | |
| Outcome | Follow-up: 5 month–5 year Recurrence: 17% (2/12 mets: 25% (3/12) | ||
Overall, the tumours tend to present in middle-aged patients without a predilection for gender or location within the pancreas. Surgical therapy was tailored according to tumour location. As expected, all tumours (except for one case where results of immunohistochemistry were not reported) were positive for CD117 and 77% (of the 13 cases with available data) were positive for CD34.
As already mentioned, pancreatic EGISTs tend to be quite large at presentation. As a result, almost all pancreatic EGISTs are considered high risk due to size alone, though most also exhibit high mitotic rates. However, actual behaviour (as discussed above) is quite discordant with this traditional risk stratification. Most patients had been followed up for a number of years by the time of publication; with a recurrence rate of only 17% and metastasis of only 25% (six cases did not report follow-up data). Even more interesting is the fact that these rates are so low despite the fact that only half of the patients received therapy with imatinib. Many of these patients were treated before Gleevec became standard therapy for intermediate to high-risk GISTs, whereas today, nearly all of these patients would have qualified for medical therapy. Of the three patients with metastases, all were reported in the liver, two were able to be resected, and all three did well with medical therapy. All these data support the favourable nature of these tumours. It is likely that we will eventually need to adjust the current risk assessment and treatment paradigm in relation to pancreatic EGISTs, but a much larger sample size will be required in order to truly determine these characteristics.
Our case is the only reported occurrence of a pancreatic EGIST presenting as a gastrointestinal haemorrhage. However, Saif et al15 also presented an anaemic patient with a tumour eroding into the duodenum with friable mucosa unable to be biopsied. In fact, at least 6 of the patients (33%) did manifest with significant anaemia/subclinical bleeding with haemoglobin in the 4–5 range (not all cases had laboratory values available). Therefore, the finding of considerable anaemia and/or bleeding coupled with the discovery of a pancreatic mass should prompt a higher suspicion for the diagnosis of EGIST and may tailor work-up and treatment. Once again, more cases are clearly needed to appropriately define this relationship, however.
Given that GISTs were only regularly recognised within the last decade, and that the first case of a pancreatic EGIST was published in 2004, it is likely that multiple pancreatic EGISTs have gone un-recognised. Four of the more recent reports (22%) have shown that the utilisation of endoscopic ultrasound-fine needle aspiration (EUS-FNA) and ultrasound (US)-FNA in preoperative diagnosis of these tumours seems quite promising.18 In particular, EUS is the most successful at differentiating tumour origin between the duodenum and pancreas, as it can accurately distinguish the different layers of the gastrointestinal tract.11 12 17 23 Thus, it seems as though pre-operative EUS-FNA or US-FNA may be an efficacious addition to the diagnostic and treatment algorithm of pancreatic tumours. Unfortunately, we were unable to biopsy our patient's tumour due to its friable nature and the risk for haemorrhage. This left us without a diagnosis, and the location of the tumour forced our hand in performing a radical surgical resection. However, in other cases, a preoperative diagnosis of EGIST coupled with amenable anatomic location might allow for a more limited surgical resection as long as negative margins were able to be obtained. Additionally, a proper diagnosis with large extensive tumours would allow for neo-adjuvant therapy with Gleevec, with the hopes of down-staging the tumour and gaining a better surgical result.23–25 Currently, neo-adjuvant therapy is recommended for tumours involving the second portion of the duodenum for these same reasons. However, as with our case, this is often not possible, either due to inability to obtain preoperative diagnosis, or need for emergent resection.25
Additionally, it is important to differentiate between pancreatic and duodenal GISTs, as data show that pancreatic lesions may have a better prognosis. Our patient's tumour actually presented earlier and smaller than most due to its erosive nature and resultant haemorrhage. This is similar to duodenal GISTs which are usually detected earlier than other GISTs due to the very same factors.25
In summary, pancreatic EGISTs are extremely rare. However, the information that we do have available seems to show that these tumours are actually quite treatable with a favourable prognosis. Thus, when faced with a pancreatic mass, the differential should include a diagnosis of EGIST, especially when assessing large cystic masses presenting with anaemia. Additionally, every effort should be made to obtain an appropriate preoperative diagnosis by utilising such tools as EUS and FNA. In the future, treatment and classification of pancreatic EGISTs will likely change to reflect these factors as more data become available.
Learning points.
Pancreatic extra-gastrointestinal stromal tumours (EGISTs) are rare.
It is important to differentiate between pancreatic and duodenal GISTs, as data show that pancreatic lesions may have a better prognosis.
Pancreatic EGISTs are treatable with a favourable prognosis.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Liegel-Atzwanger B, Fletcher JA, Fletcher CDM. Gastrointestinal stromal tumors. Virchows Arch 2010;456:111–27. [DOI] [PubMed] [Google Scholar]
- 2.Vij M, Agrawal V, Pandey R. Malignant extra-gastrointestinal stromal tumor of the pancreas. A case report and review of literature. J Pancreas (Online) 2011;12:200–4. [PubMed] [Google Scholar]
- 3.Cecka F, Jon B, Ferko A, et al. Long-term survival of a patient after resection of a gastrointestinal stromal tumor arising from the pancreas. Hepatobiliary Pancreat Dis Int 2011;10:330–2. [DOI] [PubMed] [Google Scholar]
- 4.Bertagnolli MM. Gastrointestinal stromal tumors. In: Zinner MJ, Ashley SW, eds. Maingot's abdominal operations. 11th edn New York:McGraw-Hill Professional, 2007. [Google Scholar]
- 5.Meng L, Fang SH, Jin M. An unusual case of pancreatic and gastric neoplasms. Eur Radiol 2011;21:663–5. [DOI] [PubMed] [Google Scholar]
- 6.Neto MR, Machuca TN, Pinho RV, et al. Gastrointestinal stromal tumor: report of two unusual cases. Virchows Arch 2004;444:594–6. [DOI] [PubMed] [Google Scholar]
- 7.Yamaura K, Kato K, Miyazawa M, et al. Stromal tumor of the pancreas with expression of c-kit protein: report of a case. J Gastroenterol Hepatol 2004;19:467–70. [DOI] [PubMed] [Google Scholar]
- 8.Krska Z, Peskova M, Povysil C, et al. GIST of pancreas. Prague Med Rep 2005;106:201–8. [PubMed] [Google Scholar]
- 9.Daum O, Klecka J, Ferda J, et al. Gastrointestinal stromal tumor of the pancreas: case report with documentation of KIT gene mutation. Virchows Arch 2005;446:470–2. [DOI] [PubMed] [Google Scholar]
- 10.Showalter SL, Lloyd JM, Glassman DT, et al. Extra-gastrointestinal stromal tumor of the pancreas: case report and a review of the literature. Arch Surg 2008;143:305–8. [DOI] [PubMed] [Google Scholar]
- 11.Yan BM, Pai RK, Dam JV. Diagnosis of pancreatic gastrointestinal stromal tumor by EUS guided FNA. J Pancreas (online) 2008;9:192–6. [PubMed] [Google Scholar]
- 12.Harindhanavudhi T, Tanawuttiwat T, Pyle J, et al. Extra-gastrointestinal stromal tumor presenting as a hemorrhagic pancreatic cyst diagnosed by EUS-FNA. J Pancreas (online) 2009;10:189–91. [PubMed] [Google Scholar]
- 13.Trabelsi A, Yacoub-Abid LB, Mtimet A, et al. Gastrointestinal stromal tumor of the pancreas: a case report and review of the literature. North Am J Med Sci 2009;1:324–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Goh BKP, Kesavan SM, Wong WK. An unusual cause of a pancreatic head tumor. Gastroenterology 2009;137:e5–6. [DOI] [PubMed] [Google Scholar]
- 15.Saif MW, Hotchkiss S, Kaley K. Gastrointestinal stromal tumor of the pancreas. J Pancreas (online) 2010;11:405–6. [PubMed] [Google Scholar]
- 16.Padhi S, Kongara R, Uppin SG, et al. Extragastrointestinal stromal tumor arising in the pancreas: a case report with a review of the literature. J Pancreas (online) 2010;11:244–8. [PubMed] [Google Scholar]
- 17.Yang F, Jin C, Fu D, et al. Extra-gastrointestinal stromal tumor of the pancreas: clinical characteristics, diagnosis, treatment, and outcome. J Surg Oncol 2011;103:739–40. [DOI] [PubMed] [Google Scholar]
- 18.Rao RN, Vij M, Nidhi S, et al. Malignant pancreatic extra-gastrointestinal stromal tumor diagnosed by ultrasound guided fine needle aspiration cytology. A case report with a review of the literature. J Pancreas (Online) 2011;12:283–6. [PubMed] [Google Scholar]
- 19.Agaimy A, Wunsch PH. Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. A review of 200 cases to critically re-evaluate the concept of so-called extra-gastrointestinal stromal tumours. Langenbecks Arch Surg 2006;391:322–9. [DOI] [PubMed] [Google Scholar]
- 20.Barros A, Linhares E, Valadao M, et al. Extragastrointestinal stromal tumors (EGIST): a series of case reports. Hepatogastroenterology 2011;58:865–8. [PubMed] [Google Scholar]
- 21.Popescu LM, Hinescu ME, Ionescu N, et al. Interstitial cells of Cajal in pancreas. J Cell Mol Med 2005;9:169–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasuda A, Sawai H, Takahashi H, et al. The stem cell factor/c-kit receptor pathway enhances proliferation and invasion of pancreatic cancer cells. Mol Cancer 2006;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machado NO, Chopra PJ, Al-Haddabi IH, et al. Large duodenal gastrointestinal stromal tumor presenting with acute bleeding managed by a Whipple resection. A review of surgical options and the prognostic indicators of outcome. J Pancreas (Online) 2011;12:194–9. [PubMed] [Google Scholar]
- 24.Kwon SH, Cha HJ, Jung SW, et al. A gastrointestinal stromal tumor of the duodenum masquerading as a pancreatic head tumor. World J Gastroenterol 2007;13:3396–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamoto Y, Yamamoto J, Takahashi H, et al. Segmental resection of the third portion of the duodenum for a gastrointestinal stromal tumor: a case report. Jpn J Clin Oncol 2003;33:365–6. [DOI] [PubMed] [Google Scholar]