Abstract
Anthelmintics used for intestinal helminthiasis treatment are generally effective; however, their effectiveness in tissue parasitosis (i.e. visceral toxocariasis) is moderate. The aim of this study was to evaluate the in vitroactivity of lapachol, β-lapachone and phenazines in relation to the viability of Toxocara canis larvae. A concentration of 2 mg/mL (in duplicate) of the compounds was tested using microculture plates containing Toxocara canis larvae in an RPMI-1640 environment, incubated at 37 °C in 5% CO2 tension for 48 hours. In the 2 mg/mL concentration, four phenazines, lapachol and three of its derivatives presented a larvicide/larvistatic activity of 100%. Then, the minimum larvicide/larvistatic concentration (MLC) test was conducted. The compounds that presented the best results were nor-lapachol (MLC, 1 mg/mL), lapachol (MLC 0.5 mg/mL), β-lapachone, and β-C-allyl-lawsone (MLC, 0.25 mg/mL). The larvae exposed to the compounds, at best MLC with 100% in vitro activity larvicide, were inoculated into healthy BALB/c mice and were not capable of causing infection, confirming the larvicide potential in vitro of these compounds.
Keywords: Toxocara canis, Quinones, Chemotherapy, Anthelmintics
Abstract
Os anti-helmínticos empregados no tratamento das helmintoses intestinais, de modo geral, são eficazes, porém nas parasitoses teciduais, como é o caso da toxocaríase visceral, a eficácia é moderada. Este estudo teve como objetivo avaliar in vitro a atividade do lapachol, β-lapachona e fenazinas derivadas da β-lapachona sobre a viabilidade de larvas de Toxocara canis. Os compostos foram testados na concentração de 2 mg/mL (em duplicata) em placas de microcultivo, contendo larvas de T. canis em meio RPMI-1640, sendo incubados, a 37 °C, em tensão de CO2 de 5%, por 48 horas. Na concentração de 2 mg/mL, quatro fenazinas, o lapachol e três derivados, apresentaram atividade larvicida/larvostática de 100%. A seguir, foi realizado o teste de concentração larvicida/larvostártica mínima (CLM). Os compostos que apresentaram os melhores resultados foram o nor-lapachol (CLM, 1 mg/mL), lapachol (CLM, 0,5 mg/mL), a β-lapachona e a β-C-alil-lausona (CLM, 0,25 mg/mL). As larvas expostas aos compostos, na melhor CLM 100% in vitro foram inoculadas em camundongos BALB/c saudáveis não sendo capazes de causar infecção, confirmando o potencial larvicida in vitro desses compostos.
INTRODUCTION
Human visceral toxocariasis is a neglected zoonotic infection caused by the larvae of Toxocara canis and, less frequently, Toxocara cati 31. According to recent reports, their prevalence seems to be underestimated mainly because of the difficulties of diagnosis and non-specific symptomatology36. The symptoms of this parasitic disease are characterized by cutaneous reactions, extensive eosinophilia, hepatomegaly, myocarditis, pulmonary infiltrates, and nodules accompanied by cough and fever13 , 18. The severity of symptoms depends on the location of the larvae and the number of larvae housed in tissues, which induces mechanical damage and, in turn, results in an immune-mediated inflammatory response26. Therefore, death is frequently associated with inflammatory granulomatous reactions around the larvae15, which may persist for a long time and, with it, reactivated larval migration into the eye or the brain may occur at any time40. The long-term survival of T. canis larvae has been attributed to molecular strategies evolved by the parasite26.
Generally, the drugs used to treat this disease have limited effectiveness, such as diethylcarbamazine and thiabendazole faced with poor tolerability and the need for prolonged use30. The low water solubility of benzimidazole compounds appears to collaborate with the low bioavailability of compounds in this group, such as albendazole38, the drug of choice in the treatment of visceral toxocariasis9. Nevertheless, albendazole is the drug that crosses the blood brain barrier34 and shows results superior to thiabendazole37 and diethylcarbamazine, because it does not reduce the levels of specific IgE and produces side effects in treated patients25. Therefore, an effective drug for treating human infections caused by T. canis is still needed28.
Among the possibilities of assisting in the treatment of visceral toxocariasis, natural and synthetic products33 stand out. Plant extracts are important sources of biologically active natural products and may be a model for the development of new drugs12 , 32.
Lapachol, an important representative of the quinone group, is isolated from plants of the Bignoniaceae family19. It performs biological activities against several pathogens, especially anti-parasitic activities against Trypanosoma cruzi, Schistosoma mansoni, Leishmania amazonensis and L. braziliensis 7 , 23 , 24.
β-lapachone is an ortho-naphthoquinone, a natural derivative of lapachol, present in small quantities in the woods of Tabebuia spp (Bignoniaceae). β-lapachone is easily synthesized by sulfuric acid treatment of lapachol16 and has a wide range of biological activities, including trypanocidal, antibacterial, anti-inflammatory, and anticancer activity2 , 3 , 4 , 7 , 29.
Several heterocyclic compounds were synthesized from β-lapachone (i.e. , phenazines) and have attracted considerable attention due to their biological activities, including antimalarial5, antimycobacterial2, antitumor, and antiparasitic21 ones. Therefore, the use of this group of compounds as pharmacophores for the development of new drugs has consequently been investigated.
In this study, lapachol, β-lapachone and three of its derivatives, and 17 phenazines synthesized from β-lapachone analogues were tested against T. canis larvae.
MATERIALS AND METHODS
Synthesis: Lapachol was extracted from the heartwood of Tabebuia spp (Tecoma) and purified by recrystallization from ethanol, following a previously described procedure11. Nor-lapachol was synthesized from lapachol through Hooker oxidation14.
β-lapachone, nor-β-lapachone, and β-C-allyl-lawsone were obtained through the cyclisation of the prenyl side chain of lapachol, nor-lapachol and C-allyl-lawsone, respectively. 10 mmol of the naphthoquinone were solubilized in 15mL of sulfuric acid and mixed for several minutes. The reaction was poured over cold water. The red solid was filtered, washed with cold water (3 × 100 mL) and purified by recrystallization using a mixture of acetone/hexane17.
The phenazines were prepared by the reaction of the naphthoquinone (1.00 mmol), o-phenylenediamine (1.10 mmol) and sodium acetate (1.30 mmol) in glacial acetic acid (50 mL). The reaction was maintained under reflux for two hours and monitored by TLC. After the reaction, the mixture was poured over ice and left to incubate overnight. The yellow precipitate was filtered through a Buchner funnel, washed with cold water (3 × 100 mL), and the phenazine was isolated. All phenazines were synthesized with > 95% yield35.
Test compounds: All synthesized compounds were solubilized in DMSO at 2.5% (Sigma®) and in sterile distilled water to obtain a concentration of 2 mg/mL33.
Preparation of T. canis larvae: T. canis eggs were initially collected directly from the uterine tubes of female adult parasites following the treatment of young dogs with pyrantel pamoate (15 mg/kg). Afterwards, the eggs were incubated in a 2% formalin solution at 28 ºC for 30 days in a humidity of > 90%27. By using a 5% sodium hypochlorite solution (Vetec), the eggs' protein cover was dissolved and the hatched T. canis larvae were collected in sterile tubes for cultivation with a (Gibco) RPMI-1640 medium supplemented with (Sigma) 25mM HEPES, 1% glucose, (Gibco) PSF antibiotic-antimycotic solution, and 0.4 µg/mL ofloxacin. Samples were maintained at 37 ºC strain with 5% CO2.
Larvicidal/larvistatic activity test: A microplate was used to measure the activity of substances at a concentration of 2 mg/mL. The tests were conducted in duplicate. 100 T. canis larvae, 200 µL of RPMI-1640 medium, and 100 µL of the test substances were added in each well. The larvae were then maintained at 37 ºC for 48 hours with 5% CO2.
The activity was tested in vitro and after exposure to the test compound the larval mobility was tested by the state of the larvae (i.e. , motile, immobile but not dead, or dead). Cell viability was tested by using a 0.4% trypan blue indicator.
The substances that showed larvicidal activity in 100% of larvae with the in vitro test at concentrations of 2 mg/mL were re-tested at lower concentrations (MLC) (i.e. , 1 mg/mL, 0.5 mg/mL, 0.25 mg/mL, 0.125 mg/mL and 0.05 mg/mL). Afterwards, the substances with larvicidal/larvistatic activity at the lowest concentrations were assessed for their viability of infection in mice. In order to assess their viability, the content of each microplate well was inoculated into 5-week-old BALB/c female mice by intraperitoneal injection. All mice were given food without antibiotics and had free access to water. The mice were kept on a 12 hour light to 12 hour dark cycle at a 22 ºC (± 2 ºC) room temperature.
Furthermore, a control group of live larvae (100 larvae/well) in mice was used to confirm the viability of larvae that were not exposed to the substances. A single mouse was used for each compound and each control. Mice were euthanized after 30 days of inoculation. The animals were examined for larvae by having their carcass, brain, liver, lungs, kidneys, heart, eyes, and spleen digested in a solution of 1% hydrochloric acid and 1% pepsin39.
RESULTS
Lapachol, β-lapachone and three of its derivatives, and 17 phenazines were tested against T. canis larvae.
β-lapachone and β-C-allyl-lawsone showed the highest activity (MLC = 0.25 mg/mL), followed by lapachol (MLC = 0.5 mg/mL) and nor-lapachol (MLC = 1 mg/mL) (Table 1).
Table 1. Larvicide/larvistatic activity, MLC and in vivo viability of the T. canis larvae treated with lapachol and derivatives (n = 5).
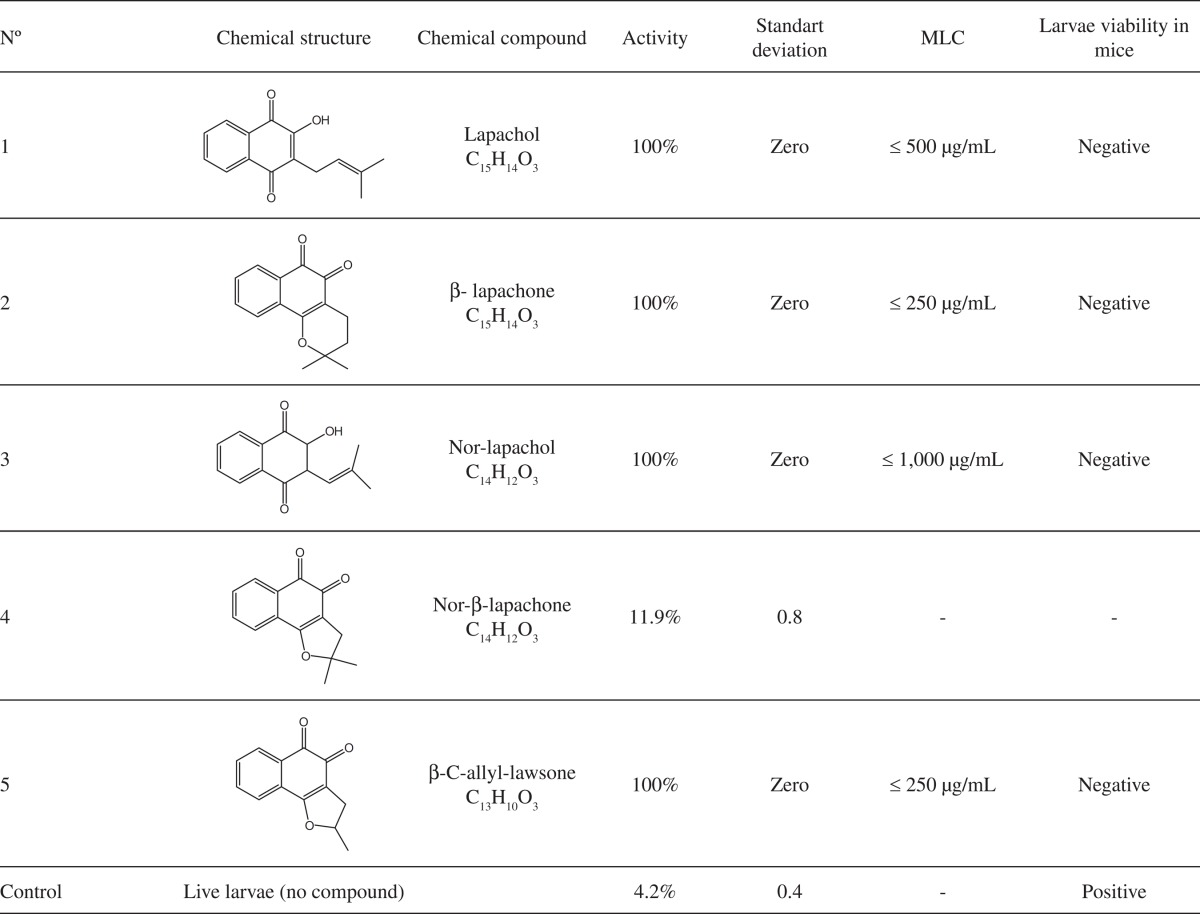
Negative to detection of T. canis larvae in mice tissues; Positive to detection of T. canis larvae in mice tissues.
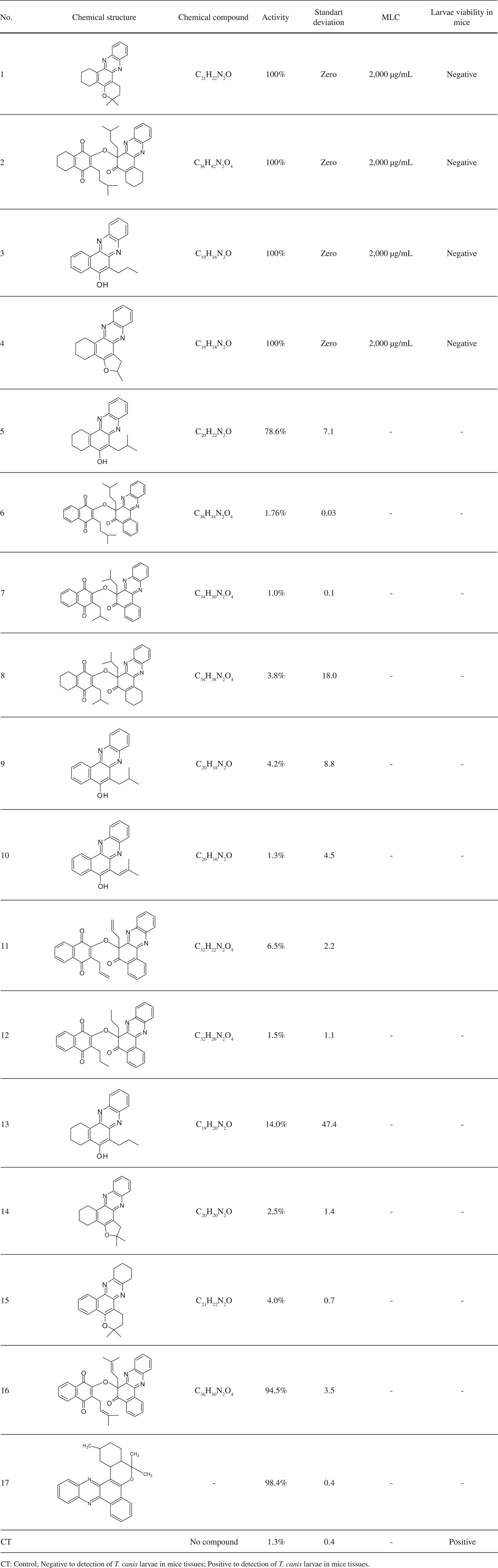
Out of the 17 phenazines tested on T. canis larvae ,four compounds (i.e. , compounds 1, 2, 3, and 4) showed 100% activity at a concentration of 2 mg/mL. Additionally, three compounds (i.e. , compounds 5, 16, and 17) showed a larvicidal activity of 78.6-98.4% at the same concentration. The other phenazines showed < 14% activity (Table 2).
Table 2. Larvicide/larvistatic activity, MLC and in vivo viability of the T. canis larvae treated with phenazines (n = 17).
The larvae exposed to the compounds with 100% activity in vitro were not viable and, therefore, were not able to infect the mice. The control group consisted of live larvae and caused infection when inoculated into the mice, which validates the in vitro evaluation criteria used in this study.
DISCUSSION
The search for new therapeutic prototypes with effectiveness against T. canis larvae housed in human tissues is relevant for the efficacy of visceral toxocariasis treatment. The new drugs should eradicate all larvae housed in the tissues, not only decrease the intensity of infection as it was noted in the administration of albendazole1 , 6 , 32 , 33, ivermectin, mebendazole, and thiabendazole22 in mice.
In this study, the possible effect of lapachol and β-lapachone and its derivatives against T. canis larvae was tested. Among all the synthetic compounds tested, β-lapachone and β-C-allyl-lawsone showed the best anthelmintic activity in vitro. Although these results are relevant, the quinones present significant toxicity, possibly due to the redox potential. This toxicity may cause cell damage due to oxidative stress, which could result in undesirable side effects10.
Nevertheless, due to the presence of larvicidal activity and by the easy access of quinones to natural sources from Brazilian flora7, justify the utilization of these compounds as a pharmacophore to develop heterocyclic derivatives more active and less toxic.
This approach was previously used to synthesize trypanocidal naphthoimidazoles from β-lapachone and to demonstrate that naphthoimidazoles were more active and less toxic than β-lapachone8.
The larvicidal potential of in vitro tests and the capacity to inhibit viability of infection in the mice, demonstrated by quinones, indicated the relevance of studies in this area. Furthermore, motivates realize cytotoxicity studies, for further evidence of the biological activity of these compounds, in preclinical trials in experimental models, aiming the development of prototype compound with anthelmintic activity which could be used in the treatment of visceral toxocariasis.
Four phenazines (i.e. , compounds 1, 2, 3, and 4) out of the 17, showed 100% activity at a concentration of 2 mg/mL. However, these phenazines did not present satisfactory results when exposed to low concentrations; similar results were obtained with the same phenazines against Plasmodium falciparum, P. berghei 5, and Mycobacterium tuberculosis 2. In these studies, the compounds showed 50% antimalarial activity in vitro, and only one-fourth of the phenazines tested against M. tuberculosis demonstrated strong antimycobacterial activity (minimum inhibitory concentration = 0.78 µg/mL). A significant antimalarial activity in vitro was also shown in the other phenazines synthesized from naphthols that were assayed against P. falciparumstrains resistant to chloroquine. However, they are not able to promote an effective cure when tested against P. bergheiin vivo 20.
Structural changes that arose in other phenazines (i.e. , compound 5-17) tested in this study did not increase the specific activity of the molecules. The lower activity of compounds 5-17, compared to compounds 1-4, indicates that new modifications to these molecules are necessary to promote effective action against T. canislarvae.
ACKNOWLEDGEMENTS
The authors are grateful to Antônio Ventura Pinto and Núcleo de Pesquisa de Produtos Naturais (UFRJ) for the compounds.
REFERENCES
- 1.Abo-Shehada MN, Herbert IV. The migration of larval Toxocara canis in mice. II. Post-intestinal migration in primary infections. Vet Parasitol. 1984;17:75–83. doi: 10.1016/0304-4017(84)90066-9. [DOI] [PubMed] [Google Scholar]
- 2.Coelho TS, Silva RS, Pinto AV, Pinto MC, Scaini CJ, Moura KC, et al. Activity of β-lapachone derivatives against rifampicin-susceptible and-resistant strains of Mycobacterium tuberculosis . Tuberculosis (Edinb) 2010;90:293–297. doi: 10.1016/j.tube.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Da Silva MN, Ferreira VF, De Souza MCBV. Um panorama atual da química e da farmacologia de naftoquinonas, com ênfase na β-Lapachona e derivados. Química Nova. 2003;26:407–416. [Google Scholar]
- 4.De Almeida ER, da Silva AA, Filho, dos Santos ER, Lopes CA. Antiinflammatory action of lapachol. J Ethnopharmacol. 1990;29:239–241. doi: 10.1016/0378-8741(90)90061-w. [DOI] [PubMed] [Google Scholar]
- 5.De Andrade-Neto VF, Goulart MOF, da Silva JF, Filho, da Silva MJ, Pinto MC, Pinto AV, et al. Antimalarial activity of phenazines from lapachol, beta-lapachone and its derivatives against Plasmodium falciparumin vitro and Plasmodium bergheiin vivo . Bioorg Med Chem Lett. 2004;14:1145–1149. doi: 10.1016/j.bmcl.2003.12.069. [DOI] [PubMed] [Google Scholar]
- 6.Delgado O, Botto C, Mattei R, Escalante A. Effect of albendazole in experimental toxocariasis of mice. Ann Trop Med Parasitol. 1989;83:621–624. doi: 10.1080/00034983.1989.11812396. [DOI] [PubMed] [Google Scholar]
- 7.De Moura KCG, Emery FS, Neves-Pinto C, Pinto MCFR, Dantas AP, Salomão K, et al. Trypanocidal activity of isolated naphthoquinones from Tabebuia and some heterocyclic derivatives: a review from an interdisciplinary study. J Braz Chem Soc. 2001;12:325–338. [Google Scholar]
- 8.De Moura KCG, Salomão K, Menna-Barreto RF, Emery FS, Pinto MC, Pinto AV, et al. Studies on the trypanocidal activity of semi-synthetic pyran[b-4,3]naphtho[1,2-d] imidazoles from β-lapachone. Eur J Med Chem. 2004;39:639–645. doi: 10.1016/j.ejmech.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003;16:265–272. doi: 10.1128/CMR.16.2.265-272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubin M, Fernandez-Villamil SH, Stoppani AO. Cytotoxicity of β-lapachone, an o-naphthoquinone with possible therapeutic use. Medicina (B Aires) 2001;61:343–350. [PubMed] [Google Scholar]
- 11.Ettlinger MG. Hydroxynaphthoquinones. III. The structure of lapachol peroxide. J Am Chem Soc. 1950;72:3472–3474. [Google Scholar]
- 12.Falkenberg MB. Quinonas. In: Simões CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR, editors. Farmacognosia: da planta ao medicamento. Porto Alegre: Ed. UFRGS; 2004. pp. 1102–1102. [Google Scholar]
- 13.Fan CK, Liao CW, Cheng YC. Factors affecting disease manifestation of toxocarosis in humans: genetics and environment. Vet Parasitol. 2013;193:342–352. doi: 10.1016/j.vetpar.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 14.Fieser LF, Fieser M. Naphthoquinone antimalarials. XII. The hooker oxidation reaction. J Am Chem Soc. 1948;70:3215–3222. doi: 10.1021/ja01190a005. [DOI] [PubMed] [Google Scholar]
- 15.Glickman LT, Schantz PM, Cypess RH. Canine and human toxocariasis: review of transmission, pathogenesis, and clinical disease. J Am Vet Med Assoc. 1979;175:1265–1269. [PubMed] [Google Scholar]
- 16.Hooker SC. Lomatiol. Part II. Its occurrence, constitution, relation to and conversion into lapachol. Also a synthesis of lapachol. J Am Chem Soc. 1936;58:1181–1190. [Google Scholar]
- 17.Hooker SC. The constitution of lapachol and its derivatives. Part IV. Oxidation with potassium permanganate. J Am Chem Soc. 1936;58:1168–1173. [Google Scholar]
- 18.Hotez PJ, Wilkins PP. Toxocariasis: America's most common neglected infection of poverty and a helminthiasis of global importance? PLOS Negl Trop Dis. 2009;3:400–400. doi: 10.1371/journal.pntd.0000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussain H, Krohn K, Ahmad VU, Miana GA, Green IR. Lapachol: an overview. Arkivoc. 2007;2:145–171. [Google Scholar]
- 20.Hussain H, Specht S, Sarite SR, Saeftel M, Hoerauf A, Schulz B, et al. A new class of phenazines with activity against a chloroquine resistant Plasmodium falciparum strain and antimicrobial activity. J Med Chem. 2011;54:4913–4917. doi: 10.1021/jm200302d. [DOI] [PubMed] [Google Scholar]
- 21.Laursen JB, Nielsen J. Phenazine natural products: biosynthesis, synthetic analogues and biological activity. Chem Rev. 2004;104:1663–1685. doi: 10.1021/cr020473j. [DOI] [PubMed] [Google Scholar]
- 22.Lescano SZ, Chieffi PP, Amato V, Neto, Ikai DK, Ribeiro MCSA. Anthelmintics in experimental toxocariasis: effects on larval recovery of Toxocara canis and on immune response. J Bras Patol Med Lab. 2005;41:21–24. [Google Scholar]
- 23.Lima NMF, Correia CS, Leon LL, Machado GMC, Madeira MF, Santana AEG, et al. Antileishmanial activity of lapachol analogues. Mem Inst Oswaldo Cruz. 2004;99:757–761. doi: 10.1590/s0074-02762004000700017. [DOI] [PubMed] [Google Scholar]
- 24.Lopes JN, Cruz FS, Docampo R, Vasconcellos ME, Sampaio MC, Pinto AV, Gilbert B. In vitro and in vivo evaluation of the toxicity of 1,4-naphthoquinone and 1,2-naphthoquinone derivatives against Trypanosoma cruzi . Ann Trop Med Parasitol. 1978;72:523–531. doi: 10.1080/00034983.1978.11719356. [DOI] [PubMed] [Google Scholar]
- 25.Magnaval JF. Comparative efficacy of diethylcarbamazine and mebendazole for the treatment of human toxocariasis. Parasitology. 1995;110:529–533. doi: 10.1017/s0031182000065240. [DOI] [PubMed] [Google Scholar]
- 26.Maizels RM. Toxocara canis: molecular basis of immune recognition and evasion. Vet Parasitol. 2013;193:365–374. doi: 10.1016/j.vetpar.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunes CM. Imunodiagnóstico da larva migrans visceral através de um método de ELISA indireto competitivo. São Paulo: Universidade de São Paulo, Faculdade de Medicina Veterinária e Zootecnia; 1996. Tese. [Google Scholar]
- 28.Othman AA. Therapeutic battle against larval toxocariasis: are we still far behind? Acta Trop. 2012;124:171–178. doi: 10.1016/j.actatropica.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Pardee AB, Li YZ, Li CJ. Cancer therapy with beta-lapachone. Current Cancer Drug Targets. 2002;2:227–242. doi: 10.2174/1568009023333854. [DOI] [PubMed] [Google Scholar]
- 30.Pawlowski Z. Toxocariasis in humans: clinical expression and treatment dilemma. J Helminthol. 2001;75:299–305. doi: 10.1017/s0022149x01000464. [DOI] [PubMed] [Google Scholar]
- 31.Quattrocchi G, Nicoletti A, Marin B, Bruno E, Druet-Cabanac M, Preux PM. Toxocariasis and epilepsy: systematic review and meta-analysis. PLOS Negl Trop Dis. 2012;6:1775–1775. doi: 10.1371/journal.pntd.0001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reis M, Trinca A, Ferreira MJ, Monsalve-Puello AR, Grácio MAA. Toxocara canis: potential activity of natural products against second-stage larvae in vitro and in vivo . Exp Parasitol. 2010;126:191–197. doi: 10.1016/j.exppara.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 33.Satou T, Horiuchi A, Akao N, Koike K, Futja K, Nikaido T. Toxocara canis: search for a potential drug amongst β-carboline alkaloids - in vivo and mouse studies. Exp Parasitol. 2005;110:134–139. doi: 10.1016/j.exppara.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Schneier AJ, Durand ML. Ocular toxocariasis: advances in diagnosis and treatment. Int Ophthalmol Clin. 2011;51:135–144. doi: 10.1097/IIO.0b013e31822d6a5a. [DOI] [PubMed] [Google Scholar]
- 35.Silva RSF. Síntese de fenazinas e derivados lactônicos e halogenados a partir de naftoquinonas naturais com atividade contra Mycobacterium tuberculosis. Rio de Janeiro: Universidade Federal do Rio de Janeiro, Núcleo de Pesquisa de Produtos Naturais; 2009. Tese. [Google Scholar]
- 36.Smith H, Holland C, Taylor M, Magnaval JF, Schantz P, Maizels R. How common is human toxocariasis? Towards standardizing our knowledge. Trends Parasitol. 2009;25:182–188. doi: 10.1016/j.pt.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Stürchler D, Schubarth P, Gualzata M, Gottstein B, Oettli A. Thiabendazole vs. albendazole in treatment of toxocariasis: a clinical trial. Ann Trop Med Parasitol. 1989;83:473–478. doi: 10.1080/00034983.1989.11812374. [DOI] [PubMed] [Google Scholar]
- 38.Torrado S, Torrado S, Cadorniga R, Torrado JJ. Formulation parameters of albendazole solution. Int J Pharm. 1996;140:45–50. [Google Scholar]
- 39.Wang GX, Luo ZJ. A novel method for the recovery of Toxocara canis in mice. J Helminthol. 1998;72:183–184. doi: 10.1017/s0022149x00016382. [DOI] [PubMed] [Google Scholar]
- 40.Wisniewska-Ligier M, Wozniakowska-Gesicka T, Sobolewska-Drylariska J, Markiewicz-Jógwiak A, Wieczorek M. Analysis of the course and treatment of toxocariasis in children: a long term observation. Parasitol Res. 2012;110:2363–2371. doi: 10.1007/s00436-011-2772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


