Abstract
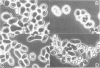
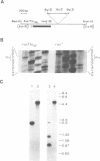
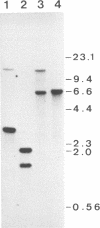
The ras1- mutation of the fission yeast Schizosaccharomyces pombe interferes with sexual differentiation by preventing conjugation and causing inefficient sporulation. From a gene library, we have isolated a gene, byr1+, which when in high copy number restores efficient sporulation to ras1- strains. byr1+ encodes a putative 340-amino acid protein product, the sequence of which strongly suggests that it functions as a protein kinase. Gene disruption experiments show that loss of byr1+ function does not interfere with mitotic growth but it completely prevents both conjugation and sporulation. byr1 is thus another important gene in the sexual differentiation pathway and we believe that at least part of ras1 function is to act directly or indirectly through byr1 to modulate protein phosphorylation.
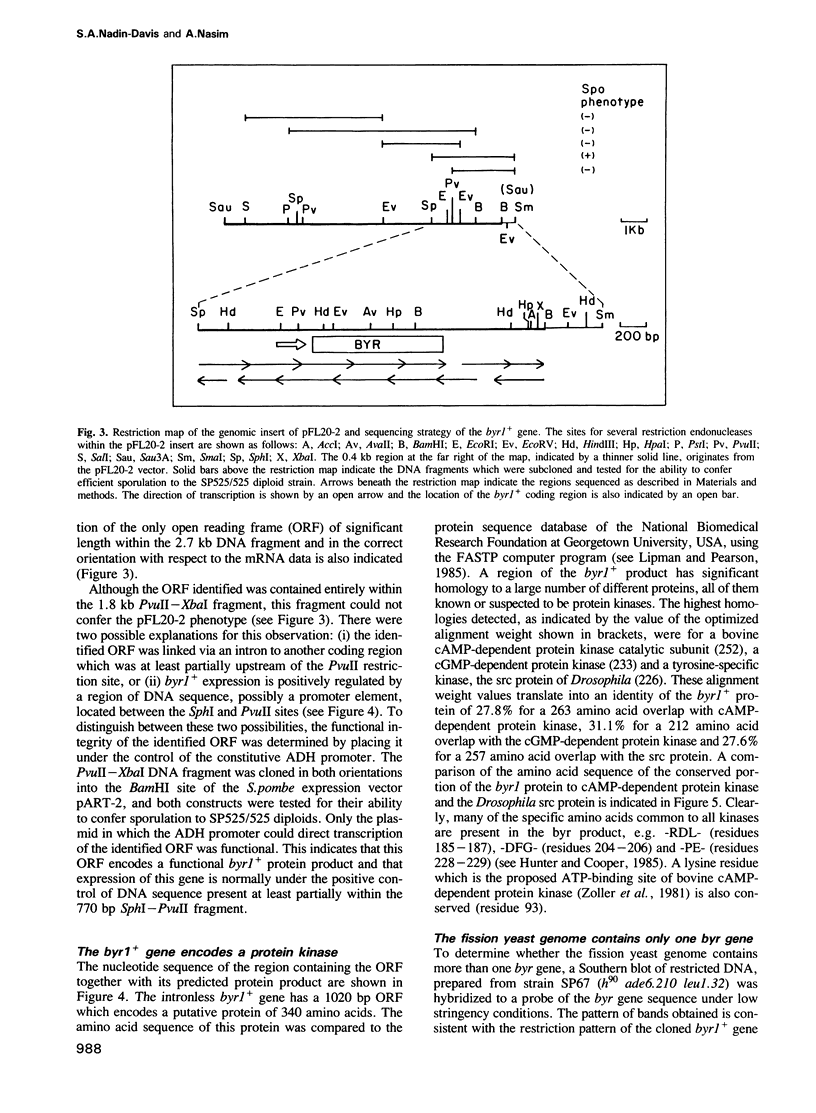
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach D., Piper M., Nurse P. Construction of a Schizosaccharomyces pombe gene bank in a yeast bacterial shuttle vector and its use to isolate genes by complementation. Mol Gen Genet. 1982;187(2):326–329. doi: 10.1007/BF00331138. [DOI] [PubMed] [Google Scholar]
- Beckner S. K., Hattori S., Shih T. Y. The ras oncogene product p21 is not a regulatory component of adenylate cyclase. Nature. 1985 Sep 5;317(6032):71–72. doi: 10.1038/317071a0. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier C., Broek D., Wigler M. ras proteins can induce meiosis in Xenopus oocytes. Cell. 1985 Dec;43(3 Pt 2):615–621. doi: 10.1016/0092-8674(85)90233-8. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sullivan K. A. Mammalian G proteins: models for ras proteins in transmembrane signalling? Cancer Surv. 1986;5(2):257–274. [PubMed] [Google Scholar]
- Broek D., Samiy N., Fasano O., Fujiyama A., Tamanoi F., Northup J., Wigler M. Differential activation of yeast adenylate cyclase by wild-type and mutant RAS proteins. Cell. 1985 Jul;41(3):763–769. doi: 10.1016/s0092-8674(85)80057-x. [DOI] [PubMed] [Google Scholar]
- Cannon J. F., Gibbs J. B., Tatchell K. Suppressors of the ras2 mutation of Saccharomyces cerevisiae. Genetics. 1986 Jun;113(2):247–264. doi: 10.1093/genetics/113.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Nieto A., Koller R., DeFeo-Jones D., Scolnick E. M. Nucleotide sequence of two rasH related-genes isolated from the yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1984 Apr 25;12(8):3611–3618. doi: 10.1093/nar/12.8.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano O., Aldrich T., Tamanoi F., Taparowsky E., Furth M., Wigler M. Analysis of the transforming potential of the human H-ras gene by random mutagenesis. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4008–4012. doi: 10.1073/pnas.81.13.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks D. J., Whitfield J. F., Durkin J. P. A viral K-RAS protein increases the stimulability of adenylate cyclase by cholera toxin in NRK cells. Biochem Biophys Res Commun. 1987 Sep 15;147(2):596–601. doi: 10.1016/0006-291x(87)90972-7. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Kaziro Y. Molecular cloning and sequence analysis of a ras gene from Schizosaccharomyces pombe. EMBO J. 1985 Mar;4(3):687–691. doi: 10.1002/j.1460-2075.1985.tb03684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Kaziro Y., Yamamoto M. Mating pheromone-like diffusible factor released by Schizosaccharomyces pombe. EMBO J. 1986 Aug;5(8):1991–1993. doi: 10.1002/j.1460-2075.1986.tb04454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Kozasa T., Kaziro Y., Takeda T., Yamamoto M. Role of a ras homolog in the life cycle of Schizosaccharomyces pombe. Cell. 1986 Jan 31;44(2):329–336. doi: 10.1016/0092-8674(86)90767-1. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hoffmann F. M., Fresco L. D., Hoffman-Falk H., Shilo B. Z. Nucleotide sequences of the Drosophila src and abl homologs: conservation and variability in the src family oncogenes. Cell. 1983 Dec;35(2 Pt 1):393–401. doi: 10.1016/0092-8674(83)90172-1. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Simon M. I., Teplow D. B., Robishaw J. D., Gilman A. G. Homologies between signal transducing G proteins and ras gene products. Science. 1984 Nov 16;226(4676):860–862. doi: 10.1126/science.6436980. [DOI] [PubMed] [Google Scholar]
- Lacal J. C., Aaronson S. A. Activation of ras p21 transforming properties associated with an increase in the release rate of bound guanine nucleotide. Mol Cell Biol. 1986 Dec;6(12):4214–4220. doi: 10.1128/mcb.6.12.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Losson R., Lacroute F. Plasmids carrying the yeast OMP decarboxylase structural and regulatory genes: transcription regulation in a foreign environment. Cell. 1983 Feb;32(2):371–377. doi: 10.1016/0092-8674(83)90456-7. [DOI] [PubMed] [Google Scholar]
- Lund P. M., Hasegawa Y., Kitamura K., Shimoda C., Fukui Y., Yamamoto M. Mapping of the ras1 gene of Schizosaccharomyces pombe. Mol Gen Genet. 1987 Oct;209(3):627–629. doi: 10.1007/BF00331175. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Nadin-Davis S. A., Nasim A., Beach D. Involvement of ras in sexual differentiation but not in growth control in fission yeast. EMBO J. 1986 Nov;5(11):2963–2971. doi: 10.1002/j.1460-2075.1986.tb04593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadin-Davis S. A., Yang R. C., Narang S. A., Nasim A. The cloning and characterization of a RAS gene from Schizosaccharomyces pombe. J Mol Evol. 1986;23(1):41–51. doi: 10.1007/BF02100997. [DOI] [PubMed] [Google Scholar]
- Powers S., Kataoka T., Fasano O., Goldfarb M., Strathern J., Broach J., Wigler M. Genes in S. cerevisiae encoding proteins with domains homologous to the mammalian ras proteins. Cell. 1984 Mar;36(3):607–612. doi: 10.1016/0092-8674(84)90340-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S., Parmelee D. C., Wade R. D., Kumar S., Ericsson L. H., Walsh K. A., Neurath H., Long G. L., Demaille J. G., Fischer E. H. Complete amino acid sequence of the catalytic subunit of bovine cardiac muscle cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Feb;78(2):848–851. doi: 10.1073/pnas.78.2.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Nelson N. C., Taylor S. S. Affinity labeling of cAMP-dependent protein kinase with p-fluorosulfonylbenzoyl adenosine. Covalent modification of lysine 71. J Biol Chem. 1981 Nov 10;256(21):10837–10842. [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]