Abstract
We reported a rare case of Budd-Chiari syndrome (BCS) associated with tamoxifen use, which was later complicated by heparin-induced thrombocytopenia and thrombosis (HITT). The patient was a 44 year-old woman with a medical history of lobular carcinoma in situ, who had been on tamoxifen for 2 years, presented with abdominal pain and distention. Imaging studies followed by a liver biopsy confirmed the diagnosis of BCS. On extensive work-up, the patient was found to have an unclassified myeloproliferative disorder with positive JAK-2 V617 mutation. After discontinuing tamoxifen, the patient was started on intravenous heparin. However, later in the course, she developed HITT. Myeloproliferative disorder, in conjunction with tamoxifen, predisposed the patient to be highly thrombophilic resulting in BCS. HITT was found to be relatively common in BCS. Anticoagulation and blood count need to be carefully monitored, and the possibility of HITT emergence in these patients should always be kept in mind.
Background
Budd-Chiari syndrome (BCS) is a disease spectrum characterised by hepatic venous outflow tract obstruction at any level from hepatic venule to the right atrium, regardless of the cause of obstruction. If left untreated, BCS is a lethal disease with a mortality close to 80%.1 Myeloproliferative disorders, including polycythaemia vera (PV) and essential thrombocytosis, are some of the most common causes of BCS with a prevalence of 50%.2–4 Hence, all patients with BCS should undergo a hypercoagulable work-up to identify a predisposing factor for venous thrombosis. Other causes include pregnancy, oestrogen therapy such as oral contraceptives and malignancy.1 It is understood that in the presence of an underlying hypercoagulable state, addition of an acquired thrombogenic stimulus like oral contraceptives could result in hepatic venous thrombosis and outflow obstruction.1
Tamoxifen, a selective oestrogen receptor modulator, is used for the treatment of oestrogen receptor-positive breast cancer and for risk reduction in high-risk patients. It is known to increase the risk of venous thromboembolism (VTE) in cancer as well as non-cancer patients.5–8 In our case, the patient developed BCS, a type of visceral venous thrombosis, after having been on tamoxifen for almost 2 years. To the best of our knowledge, the association between tamoxifen and BCS has not been previously reported in the published literature and there are invaluable learning points in the management of this case.
Case presentation
A 44-year-old Hispanic female presented to our hospital with worsening right upper quadrant abdominal pain and increased abdominal girth for 2 months prior to admission. Her medical history was significant for lobular carcinoma in situ of the left breast with microcalcifications and atypical ductal hyperplasia, diagnosed 21 months ago. She underwent left lumpectomy and had been taking tamoxifen since then as a risk reduction therapy. She also complained of bilateral leg swelling and orthopnea secondary to her distended abdomen. Review of the system was otherwise unremarkable. She denied any alcohol or illicit drug use, diabetes, history of liver, kidney or heart disease. However, she did admit to being a heavy smoker in the past and had a significant family history of cancer including ovarian, breast and possible endometrial cancer.
The physical examination was significant for abdominal distention with fluid shift and a palpable liver edge of 2 cm below the right costal margin, which was mildly tender to palpation, mild splenomegaly and 1+pedal oedema. The patient had no signs of chronic liver stigmata on the exam. No flapping tremors were noted. Other physical exam including cardiac exam was normal.
Investigations
Results of routine laboratory studies and hepatitis serology are demonstrated in table 1. Two-dimensional echo did not show any valve abnormalities or systolic or diastolic dysfunctions ruling out cardiac origin of ascites. Ascitic fluid analysis showed serum ascites-albumin gradient of 0.4 and non-neutrocytic bacteroascites with positive fluid culture for Escherichia coli. The patient received a 7-day course of ceftriaxone for bacteroascites. Imaging studies including CT of the abdomen and hepatic ultrasound with dopplers demonstrated large volume intra-abdominal ascites with a mildly enlarged liver measuring 19 cm in length with coarsened heterogeneous enhancement of the hepatic parenchyma. These studies also showed mild caudate lobe hypertrophy, poor visualisation of hepatic veins and intrahepatic inferior vena cava narrowing (figures 1 and 2) consistent with subacute BCS.
Table 1.
Results of routine laboratory studies and hepatitis serology
| Test name | Result |
|---|---|
| Haemoglobin | 0.1 mmol/l (0.07–0.1 mmol/l) |
| Haematocrit | 0.46 (0.35–0.43) |
| Platelet | 196×109/l (140–400×109/l) |
| White cell count | 6.6×109/l (4.8–10.8×109/l) |
| Alkaline phosphatase | 234 IU/l (34–125 IU/l) |
| ALT | 32 IU/l (30–65 IU/l) |
| AST | 43 IU/l (8–54 IU/l) |
| Albumin | 23 g/l (35–52 g/l) |
| Globulin | 57 g/l (60–80 g/l) |
| HBs Ag | Negative |
| Anti-HBs | Negative |
| Anti-HCV Ab IgG, IgM | Negative |
| HCV RNA | Non-detected |
| ANA | Negative |
| ASMA | Negative |
| AMA | Negative |
Figure 1.
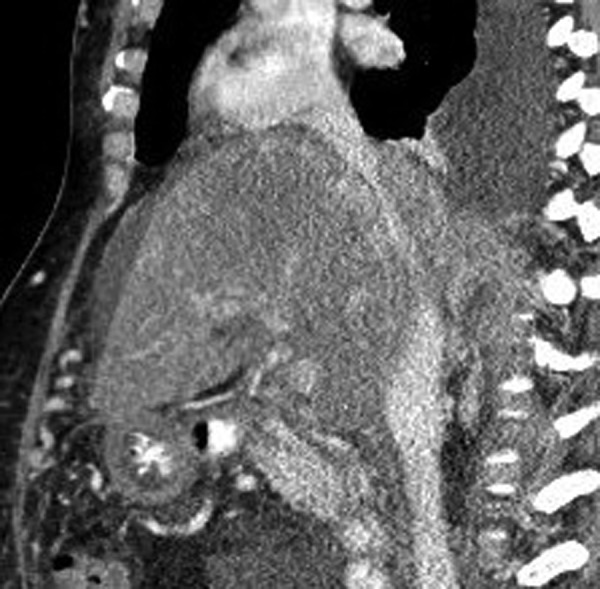
CT abdomen showing intra-abdominal IVC narrowing.
Figure 2.
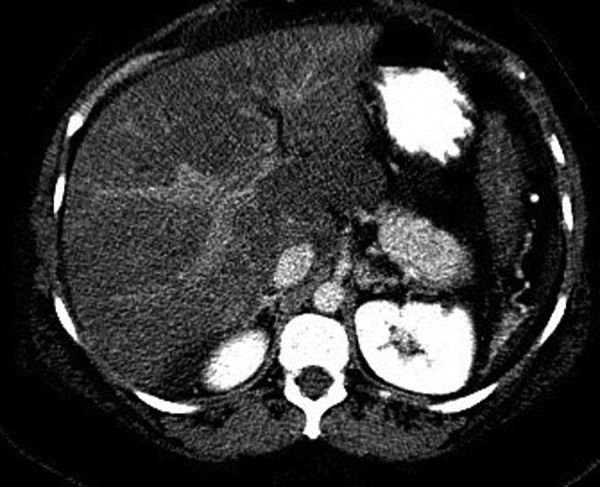
CT abdomen showing caudate lobe hypertrophy.
The patient underwent a transjugular liver biopsy for confirmation. During the procedure, the guide wire could not be advanced into the hepatic vein, suggesting hepatic vein occlusion. Contrast injection showed a ‘spider web-type appearance’ as seen in the BCS (figure 3). Liver biopsy was successfully done transcavally, which confirmed the diagnosis of BCS. It showed extensive centrovenous and sinusoidal congestion, liver plate atrophy involving zones 2 and 3 with periportal sparing. No nodular regeneration or evidence of cirrhosis was identified on the biopsy (figure 4).
Figure 3.
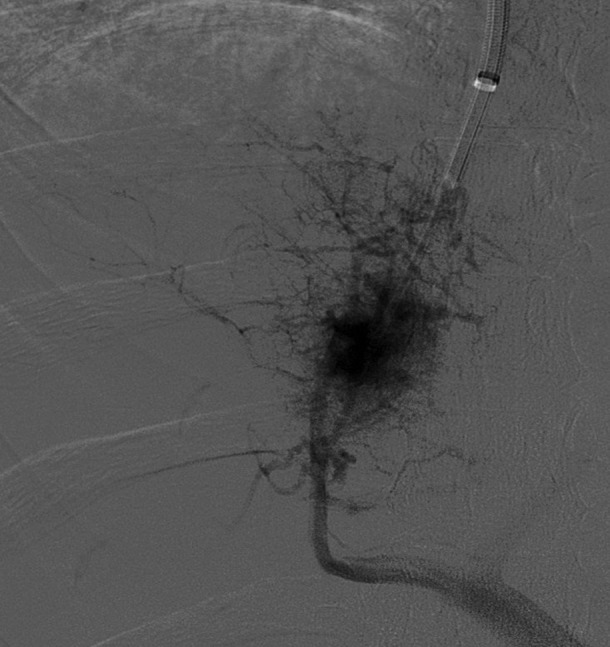
Angiogram spider web appearance of intrahepatic veins.
Figure 4.
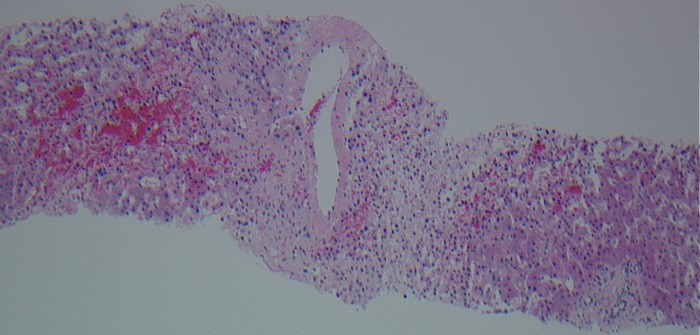
Liver biopsy.
Once the definitive diagnosis was made, the patient was worked up for underlying hypercoagulable conditions. Results are shown in table 2. All results were unremarkable except for a positive JAK-2 V617F mutation. Due to positive JAK-2 mutation, bone marrow biopsy was done to rule out myeloproliferative disorder. Bone marrow aspiration showed mild dyserythropoietic changes including nuclear budding and nuclear irregularities. Biopsy showed hypercellular marrow with normal myeloid to erythroid (M–E) ratio, foci of loosely clustered and slightly increased megakaryocytes (figure 5) and mild reticulin fibrosis(2+/3) consistent with a myeloproliferative neoplasm. Flow cytometry showed no evidence of lymphoma or leukaemia and bcr–abl gene mutation was not detected. Laboratory results revealed positive JAK-2 V617F mutation both in peripheral blood and in bone marrow with a high erythropoietin level. These findings were most consistent with an unclassifiable occult myeloproliferative disorder.
Table 2.
Results of hypercoagulability work-up
| Test name | Result |
|---|---|
| Protein C activity | 80% (74–151) |
| Protein S activity | 224 (60–145) |
| Antithrombin activity | 90% (75–135) |
| Lupus anticoagulant | Non-detected |
| Factor V leiden | Negative |
| Prothrombin gene mutation | Negative |
| BCR-ABL1 translocation | Non-detected |
| Cardiolipin Ig G and IgM | <9 GPL unit/ml (0–14) |
| Erythropoietin level | 116 mIU/ml (4.2–27.8) |
| Flow cytometry | No evidence of non-Hodgkin's lymphoma or leukaemia |
| JAK-2 V617F mutation | Positive |
Figure 5.
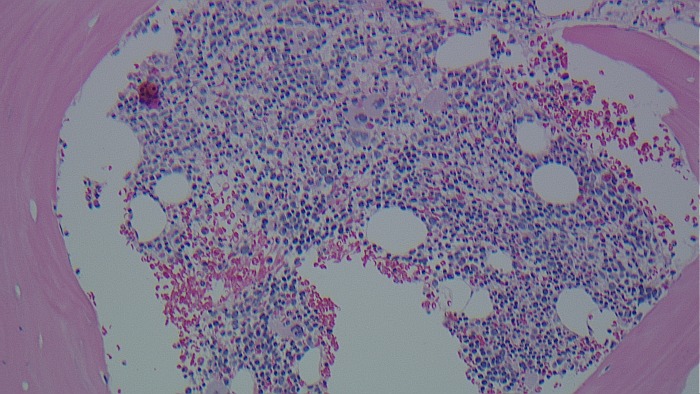
Bone marrow biopsy.
Treatment
With the diagnosis of BCS, tamoxifen was discontinued and the patient was started on intravenous heparin infusion. Since the patient did not have evidence of cirrhosis on the liver biopsy, a decision was made to attempt transjugular intrahepatic portosystemic shunt (TIPS) for decompression. The TIPS was successful.
Outcome and follow-up
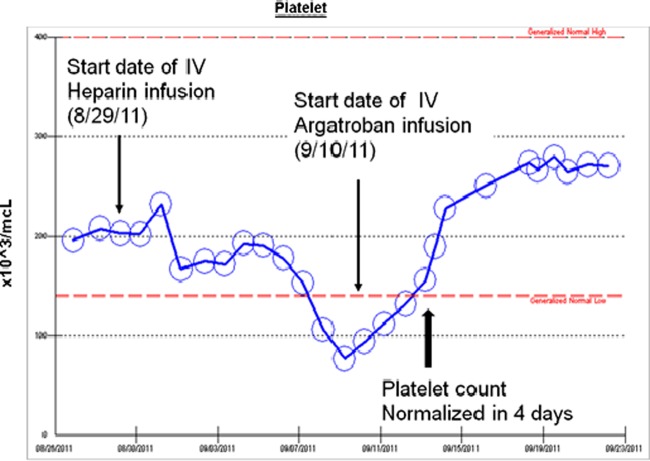
A few days after the TIPS placement, her platelet counts started to drop. Platelet trends are shown in figure 6. A repeat ultrasound not only showed an occluded TIPS, but also showed a concern for extension of the thrombus. A heparin-induced thrombocytopenia (HIT) panel (heparin/platelet factor-4 antibody assay) was positive (2.8 OD (0–0.4 OD)) in the setting of high clinical probability (4 T's score was 8 with the details of 4 T's score shown in table 3). She was diagnosed with heparin-induced thrombocytopenia and thrombosis. Heparin infusion was discontinued and switched to intravenous argatroban. The patient subsequently underwent TIPS revision. During the procedure, several areas of stenosis within the portal vein with extension of clot into the proximal TIPS stent and the superior mesenteric vein were noted. Intravenous thrombolytic agent was used for portal vein thrombosis and angioplasty was performed in several regions of stenosis.
Figure 6.
The trend of platelet count throughout the admission.
Table 3.
4 T's score for heparin-induced thrombocytopenia
| Category | 2 points | 1 point | 0 point |
|---|---|---|---|
| Thrombocytopaenia | >50% fall or nadir ≥20 ×109/l | 30–50% fall or nadir 10–19×109/l | <30% fall or nadir < 10 ×109/l |
| Timing of the decrease in platelet count | Day 5 to 10 or ≤day 1 with recent heparin (past 30 days) | >Day 10 or timing unclear or < day 1 if heparin exposure within 30–100 days | <Day 4 with no recent heparin use |
| Thrombosis or other sequelae | Proven thrombosis, skin necrosis, or acute systemic reaction after heparin bolus | Progressive, recurrent, or silent thrombosis; erythematous skin lesions | None |
| Other causes of thrombocytopenia | None evident | Possible | Definitive |
The platelet count increased to normal in 4 days on intravenous argatroban. In addition, a follow-up hepatic doppler ultrasound showed patent TIPS. Her abdominal pain decreased and she was successfully bridged to warfarin.
Discussion
BCS is a rare disorder with an incidence of 1 in 2.5 million persons per year.9 It is defined by hepatic venous outflow obstruction, which leads to increased sinusoid pressure, sinusoid congestion, hepatomegaly, portal hypertension and ascites. Ascitic fluid analysis typically reveals portal hypertension with a high total protein similar to cardiac ascites. Obstruction is usually caused by a thrombus but may also result from membranous webs or extrinsic compression by tumours. In the western countries, thrombosis is the major cause of hepatic venous obstruction. Around 80% of patients with BCS were actually found to have at least one or more hereditary or acquired hypercoagulable states.4 It is therefore necessary that all patients diagnosed with BCS undergo a work-up for hypercoagulable states.
Work-up for hypercoagulability includes protein C, S, antithrombin III levels, Factor V leiden mutation, prothrombin gene mutation and antiphospholipid antibodies. A bone marrow biopsy should be considered to identify occult myeloproliferative disorders as illustrated in a case series of BCS patients who underwent liver transplantation and by subsequent bone marrow evaluation 71% were found to have a myeloproliferative disorder.10 Some authors recommend testing for JAK-2 V617F mutations in all patients with BCS in order to reveal a possible latent form of myeloproliferative disorder.11 In addition, patients who develop BCS without history of liver disease may be evaluated for paroxysmal nocturnal haemoglobinuria, which could be responsible for up to 9% of the cases.4
However, work-up should always be preceded by thorough history including history of VTE, malignancy, family history of cancer or thrombophilia and the use of any oestrogen-containing drugs including contraceptives or, like in our patient, tamoxifen. Hormonal factors in women such as oral contraceptives or pregnancy are known to increase the risk of VTE and BCS.3 Many patients, who develop BCS in association with these hormonal factors, may also have an underlying hypercoagulable state.3 Likewise, though it has not been reported to increase the risk of BCS as of yet, tamoxifen, a selective oestrogen receptor modulator (a hormonal factor), has been shown to increase VTE.8 12 A result from International Breast Cancer Intervention Study (IBIS-I) showed that tamoxifen tripled the incidence of VTE.13 The pathological process underlying the development of VTE due to tamoxifen is unclear, but there have been several hypotheses. A study by Cushman et al6 demonstrated a significant decline in antithrombin III and protein C in the tamoxifen group; trial from Chang et al14 showed that antithrombin III levels were reduced by 8% in a tamoxifen-treated arm. It seems like tamoxifen decreases the effect of natural anticoagulants, resulting in an increased risk of VTE, especially in patients with underlying inherited or acquired thrombophilias or malignancies including myeloproliferative disorders.3 7
In our case, the patient was found to have JAK-2 V617F mutation both from peripheral blood leucocytes and from bone marrow progenitor cells. However, increased erythropoietin level with normal peripheral blood counts, and the results of bone marrow biopsy did not fully meet the 2001 WHO criteria for PV, essential thrombocytosis (ET) or primary myelofibrosis (PF).15 The patient was thought to have an unclassifiable myeloproliferative disorder, a clonal, BCR-ABL-negative syndrome. It is worth noting that normal or mildly elevated haemoglobin and platelet count do not rule out myeloproliferative disorder. Especially in the case of patients with splenomegaly, this diagnosis should be highly suspected. There are several proposed explanations for this finding. Splenic enlargement and extensive thrombus formation might result in low normal platelet count. Furthermore, it can be caused by the dilutional effect of increased plasma volume in patients with liver damage and portal hypertension.2
Medical management of BCS centres on aggressive treatment of the underlying cause as well as controlling ascites and symptoms associated with BCS. BCS can be managed medically with diuretics, sodium restriction, paracentesis for large-volume ascites and anticoagulation especially in patients with a few symptoms, normal liver function and easy-to-control ascites.16 However, some physicians consider BCS as a surgical disease and medical treatment alone is associated with high mortality (80%).1 TIPS, a minimally invasive intervention, is becoming the first choice in the treatment of BCS especially in patients who do not respond to medical treatment alone and those with worsening liver function and refractory ascites.4 TIPS has been associated with good short-term outcome,4 16 and can be used as a bridge to liver transplantation. Liver transplantation is a treatment of choice in patients with BCS and fulminant hepatic failure and those with decompensated end-stage liver disease associated with poor synthetic function.4
In patients with underlying hypercoagulable states, long-term anticoagulation is warranted. However, it is unclear as to whether patients with latent myeloproliferative disorder and BCS need cytoreductive treatment in addition to anticoagulation. Recent study showed that anticoagulation therapy alone was not associated with reduced survival in patients with latent myeloproliferative disorder and positive JAK-2 V617F mutation.17
In our case, the patient developed HIT which complicated her course of treatment by the thrombosis of TIPS stent and superior mesenteric vein. It is interesting to note that the incidence of HIT in patients with BCS is 10 times higher than in the normal population according to a previous report.18 Considering a relatively high incidence of HIT in BCS, it is important to closely monitor blood counts in these patients who are being treated with heparin or enoxaparin. HIT should be highly suspected if there is a drop in platelet count or a development of new thrombosis. Since, low-molecular-weight heparin has a lower incidence of HIT compared to unfractionated heparin, we recommend considering the use of the former for treating BCS if there are no contraindications. However, HIT rates on various heparin modalities from centres with a high volume of BCS patients need to be compared further. In addition, more data are needed to evaluate the cost-effectiveness of using direct thrombin inhibitors such as argatroban or dabigatran as first-line anticoagulants in patients with BCS.
Learning points.
Tamoxifen increases not only the risk of deep vein thrombosis or pulmonary embolism but also hepatic venous thrombosis.
Patients with a latent myeloproliferative disorder who may have normal peripheral blood cell counts are still at risk for thrombotic events, especially in the presence of other thrombogenic risk factors.
Since the prevalence of JAK-2 mutation and myeloproliferative disorder is high in patients with Budd-Chiari syndrome (BCS), it may be useful to look for an occult disease that may influence the long-term treatment.
Patients with BCS are more prone to develop heparin-induced thrombocytopenia (thrombosis); therefore, anticoagulation and blood count need to be carefully monitored and the possibility of heparin-induced thrombocytopenia emergence should always be kept in mind.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Zimmerman MA, Cameron AM, Ghobrial RM. Budd-Chiari syndrome. Clin Liver Dis 2006;10:259–73. [DOI] [PubMed] [Google Scholar]
- 2.Valla D, Casadevall N, Lacombe C, et al. Primary myeloproliferative disorder and hepatic vein thrombosis. A prospective study of erythroid colony formation in vitro in 20 patients with Budd-Chiari syndrome. Ann Intern Med 1985;103:329–34. [DOI] [PubMed] [Google Scholar]
- 3.Aydinli M, Bayraktar Y. Budd-Chiari syndrome: etiology, pathogenesis and diagnosis. World J Gastroenterol 2007;13:2693–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darwish Murad S, Plessier A, Hernandez-Guerra M, et al. Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med 2009;151:167–75. [DOI] [PubMed] [Google Scholar]
- 5.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet 2003;361:296–300. [DOI] [PubMed] [Google Scholar]
- 6.Cushman M, Costantino JP, Bovill EG, et al. Effect of tamoxifen on venous thrombosis risk factors in women without cancer: the Breast Cancer Prevention Trial. Br J Haematol 2003;120:109–16. [DOI] [PubMed] [Google Scholar]
- 7.Duggan C, Marriott K, Edwards R, et al. Inherited and acquired risk factors for venous thromboembolic disease among women taking tamoxifen to prevent breast cancer. J ClinOncol 2003;21:3588–93. [DOI] [PubMed] [Google Scholar]
- 8.Decensi A, Maisonneuve P, Rotmensz N, et al. Effect of tamoxifen on venous thromboembolic events in a Breast Cancer Prevention Trial. Circulation 2005;111:650–6. [DOI] [PubMed] [Google Scholar]
- 9.Valla DC. Hepatic venous outflow obstruction etiopathogenesis: Asia versus the West. J Gastroenterol Hepatol 2004;19:S204–11. [Google Scholar]
- 10.Melear JM, Goldstein RM, Levy MF, et al. Hematologic aspects of liver transplantation for Budd–Chiari syndrome with special reference to myeloproliferative disorders. Transplantation 2002;74:1090–5. [DOI] [PubMed] [Google Scholar]
- 11.Chung RT, Iafrate AJ, Amrein PC, et al. Case records of the Massachusetts general hospital. Case 15–2006. A 46-year-old woman with sudden onset of abdominal distention. N Engl J Med 2006;354:2166–75. [DOI] [PubMed] [Google Scholar]
- 12.Meier CR, Jick H. Tamoxifen and risk of idiopathic venous thromboembolism. Br J Clin Pharmacol 1998;45:608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet 2002;360:817–24. [DOI] [PubMed] [Google Scholar]
- 14.Chang J, Powles TJ, Ashley SE, et al. The effect of tamoxifen and hormone replacement therapy on serum cholesterol, bone mineral density and coagulation factors in healthy postmenopausal women participating in a randomised, controlled tamoxifen prevention study. Ann Oncol 1996;7:671–5. [DOI] [PubMed] [Google Scholar]
- 15.Tefferi A, Thiele J, Orazi A, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood 2007;110:1092–7. [DOI] [PubMed] [Google Scholar]
- 16.Horton JD, San Miguel FL, Membreno F, et al. Budd-Chiari syndrome: illustrated review of current management. Liver Int 2008;28:455–66. [DOI] [PubMed] [Google Scholar]
- 17.Patel RK, Lea NC, Heneghan MA, et al. Prevalence of the activating JAK2 tyrosine kinase mutation V617F in the Budd-Chiari syndrome. Gastroenterology 2006;130:2031–8. [DOI] [PubMed] [Google Scholar]
- 18.Plessier A, Sibert A, Consigny Y, et al. Aiming at minimal invasiveness as a therapeutic strategy for Budd-Chiari syndrome. Hepatology 2006;44:1308–16. [DOI] [PubMed] [Google Scholar]