Abstract
Background:
chemotherapy-induced nausea and vomiting (CINV) has been commonly reported as one of the most distressing adverse effects among treated patients with cancer. Inadequately treated, CINV can lead to increased resource utilization and severely impair patients’ daily functioning and quality of life.
Direct costs include acquisition cost of antiemetic drugs and rescue medication, administration devices, add-on treatments, such as hydration, and additional patient care, that is, nursing and physician time, unscheduled office visits, emergency room admissions, and, in some cases, extended hospitalization or readmission. There are many reports on the cost-effectiveness of antiemetic drugs, but information on the total cost per patient associated with CINV is limited. The costs associated with severe CINV episodes are considered responsible for the most significant part of the expenditures.
Scope:
The aim of this study was to investigate the management of CINV episodes in three European health-care environments and to estimate direct costs associated with severe CINV episodes.
Methods:
An online survey addressed to Italian, German, and French oncologists and oncology nurses was performed. The survey included 41 questions about the management and the resource utilization for patients experiencing any CINV episode during the 6-month period preceding the survey. Furthermore, the cost associated with severe CINV episode management was estimated by adopting the National Health Service’s perspective.
Findings:
A large proportion of patients receiving chemotherapy experienced a CINV episode (34.4% in Italy, 50.2% in France, and 40.4% in Germany); among those, 8.8% in Italy, 11.6% in France, and 19.2% in Germany experienced a severe CINV episode. Compared with Italy, Germany and France presented a greater hospitalization rate following an unplanned visit to the oncology ward or an emergency room access due to CINV. In Italy, the mean cost per patient with a severe CINV episode resulted in approximately €389, about half of the mean cost in France (€750) and a third of the mean cost in Germany (€1,017).
Conclusions:
Severe CINV episodes requiring hospitalization, day hospital, or hospitalization extension involve a significant cost for the National Health Services; additional studies should be conducted in order to evaluate potential ways to offset these expenses.
Keywords: CINV, chemotherapy-induced nausea and vomiting, antiemetic, resource utilization, direct cost, Italy, France, Germany, CINV management
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a common and severe complication experienced by patients undergoing cancer treatment [1], with an incidence rate ranging from 10% in patients treated with low emetogenic chemotherapeutic agents up to more than 90% in patients treated with highly emetogenic chemotherapeutic agents [2,3]. Although not life-threatening, CINV has a major impact on a patient’s quality of life and ranks high on the list of factors most feared by patients receiving chemotherapy [4], even compromising adherence to chemotherapy treatment [5].
Despite the introduction of more effective antiemetics, up to 20% of cancer patients still suffer from moderate to severe CINV (≥ grade 2) [6,7]. Published literature indicates that part of the burden of CINV can be attributed to practitioners’ underestimation of CINV incidence and low adherence to treatment guidelines for its prevention [8,9]. Inadequate CINV prevention can lead to increased resource utilization, as well as patient suffering [10].
CINV-related direct costs include acquisition cost of antiemetic drugs and rescue medication, administration devices, add-on treatments, such as hydration, and additional patient care (i.e., nursing and physician time, unscheduled office visits, emergency room (ER) admissions, and, in some cases, extended hospitalization or readmission) [9,11]. Specifically, costs associated with severe CINV episodes are considered responsible for the most significant part of expenditures [12]. There are many reports on the cost-effectiveness of antiemetic drugs, but information on the total cost per patient associated with CINV is limited [13].
The aim of this study was to investigate the management of CINV episodes in three main European health-care environments and to estimate direct costs per patient associated with severe CINV episodes.
Methods
An online survey addressed to Italian, German, and French oncologists and oncology nurses was performed to assess management and resource utilization for patients experiencing a CINV episode. Potential respondents were part of an existing panel of health-care providers belonging to different regions and working in different premises and institutions who volunteered for online research in the period between June and July 2014. Inclusion criteria were as follows: at least three types of cancer managed in clinical practice, at least ten patients treated with chemotherapy in the previous 6 months, and, among them, at least five patients who had experienced a CINV episode independent of its severity.
The survey preliminarily asked the health-care providers to report, based on their ability to recall, the number of patients treated with chemotherapy in the previous 6 months and, among them, the number of patients who experienced at least one episode of CINV, irrespective of its severity. Following this preliminary section of the survey, 41 questions specifically investigated the type of management and resource utilization for CINV patients.
Possible types of CINV management investigated in the survey included the following: general practitioner (GP) management, telephone call to oncology ward, unplanned oncology visit, ER access, or prolonged hospitalization. Furthermore, it was also investigated if the episode was managed autonomously by the patient and reported later. For each type of management and depending on its nature, the following resource utilization was investigated:
increase of the originally prescribed antiemetic dosage
change of antiemetic treatment
prescription of corticosteroids
prescription of psycholeptic drugs
rehydration
laboratory tests to evaluate electrolytes
day hospital
hospitalization
ambulance transportation
hospitalization extension.
The questionnaire was validated by a group of three oncologists during face-to-face interviews. A CINV episode was defined as severe if the patient was hospitalized (both ordinary hospitalization and day hospital) following an unplanned visit to the oncology ward or following an ER access, or if the patient required hospitalization extension (in the case that the patient was already hospitalized).
The costs associated with a severe CINV episode were estimated by adopting the perspective of National Health Services (NHSs). Therefore, only direct medical costs reimbursed by the Italian, German, and French NHSs were considered (Table 1). Unit costs for each country were retrieved from TUC 2013, Tarif GHS 2013, and Fallpauschalen-Katalog 2014 for Italy, France, and Germany, respectively. Results were expressed in terms of frequencies and mean values; no statistical analyses were performed. All analyses were performed using SAS® software version 9.4.
Table 1.
Health-care service costs.
| Resource utilization (cost per unit) | ||||
|---|---|---|---|---|
| Day hospital | Hospitalization | Additional hospitalization day | Source | |
| Italy | 190.20 € | 1,170.28 € | 154.91 € | TUC 2013, Code 183 |
| France | 735.47 € | 1,540.54 € | 127.09 € | Tarif GHS 2013, Code 06M091 |
| Germany | 767.88 € | 1,403.04 € | 252.80 € | Fallpauschalen-Katalog 2014, Code G67C |
Results
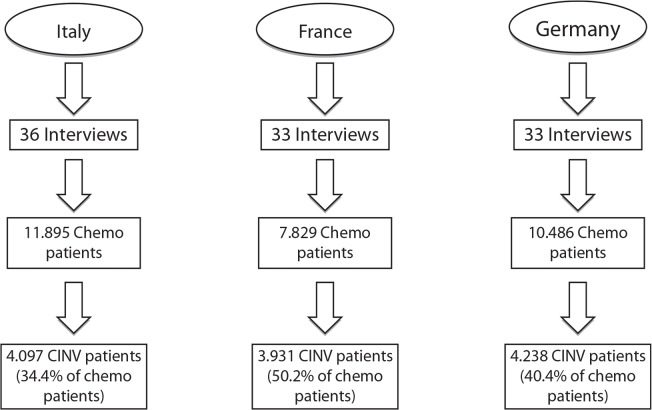
In Italy, France, and Germany, 36, 33, and 33, respectively, respondents took part in the survey. Figure 1 illustrates the number of patients who received chemotherapy (11.895 in Italy, 7.829 in France, and 10.486 in Germany) and the incidence of CINV episodes among those patients (34.4% in Italy, 50.2% in France, and 40.4% in Germany) as reported by the survey respondents in the preliminary section of the survey.
Figure 1.
Number of chemotherapy and CINV patients.
Table 2 reports the several types of CINV management for Italian, French, and German patients. In all three countries, a surprisingly high proportion of patients managed CINV autonomously (29.7% in Italy, 36.7% in France, and 22% in Germany); nevertheless, a significant number of patients referred to the oncologist, either by telephone or through an unplanned visit. Among patients managed by the GP and by a telephone call to the oncology specialist, results showed no substantial differences in resource utilization by country (data not shown), whereas these differences were more noticeable among patients who required an unplanned visit to the oncology ward, who required an ER access, and whose CINV episode occurred when they were already hospitalized (Table 3).
Table 2.
CINV episode management (patients %).
| Type of management1 | Italy | France | Germany |
|---|---|---|---|
| General practitioner | 6.7 | 12.3 | 19.7 |
| Telephone | 23.7 | 14.3 | 12.4 |
| Unplanned visit to the oncology ward | 23.7 | 14.1 | 23.6 |
| Emergency room | 7.0 | 10.7 | 11.0 |
| Already hospitalized | 11.0 | 19.7 | 21.7 |
| Autonomous | 29.7 | 36.7 | 22.0 |
Total percentage by country can exceed 100% because each CINV episode could have required more than one type of management.
Table 3.
Resource utilization by management (patients %).
| Management1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unplanned visit to the oncology ward | Emergency room | Hospitalized patients | |||||||
| Resource utilization | IT | FR | DE | IT | FR | DE | IT | FR | DE |
| Antiemetic increase | 25.2 | 34.6 | 30.1 | 31.7 | 26.1 | 33.4 | 38.5 | 36.2 | 40.8 |
| Change of antiemetic | 24.3 | 45.5 | 52.3 | 23.4 | 47.8 | 51.2 | 29.5 | 54.5 | 51.8 |
| Corticosteroid prescription | 34.0 | 32.6 | 21.2 | 40.0 | 37.6 | 26.6 | 34.8 | 42.1 | 37.2 |
| Psycholeptic prescription | 3.4 | 14.0 | 11.5 | 8.0 | 13.6 | 17.7 | 7.3 | 23.1 | 14.0 |
| Rehydration | 29.9 | 23.9 | 31.5 | 59.6 | 41.7 | 41.6 | 37.7 | 42.3 | 41.6 |
| Laboratory tests | 29.2 | 29.7 | 63.1 | 57.2 | 54.3 | 69.7 | 33.5 | 53.0 | 61.3 |
| Day hospital | 19.4 | 15.6 | 13.1 | 11.4 | 21.3 | 20.4 | – | – | – |
| Hospitalization | 5.3 | 19.0 | 29.1 | 13.8 | 40.0 | 42.1 | – | – | – |
| Transfer by ambulance | – | – | – | 11.8 | 30.1 | 36.6 | – | – | – |
| Additional hospitalization day/s | – | – | – | – | – | – | 10.9 | 19.2 | 44.2 |
Total percentage by country can exceed 100% because each CINV episode could have required more than one resource.
As expected, irrespective of the type of management, pharmacological treatment (either antiemetic change or dosage increase) together with usual interventions was the most common therapeutic option in comparison with hospital stay or its prolongation considered as associated with a severe CINV episode.
Among patients who had an unplanned visit, the percentage of those who required a day hospital was slightly higher in Italy than in France and Germany (19.4 vs 15.6% and 13.1%); however, hospitalization was more common in France and Germany than in Italy (19.0% and 29.1% vs 5.3%). Among patients coming from the ER, both day hospital and hospitalization were less frequent in Italy compared with France and Germany (day hospital: 11.4% vs 21.3% and 20.4%; hospitalization: 13.8% vs 40.0% and 42.1%).
Furthermore, for patients who were already hospitalized, in Italy, only 10.9% of them required at least one additional hospitalization day, compared with 19.2% in France and 44.2% in Germany. Therefore, irrespective of the provenience of patients (ER or unplanned visit), results seem to indicate a lower tendency to hospitalize patients due to a CINV episode in Italy compared with the other two European countries.
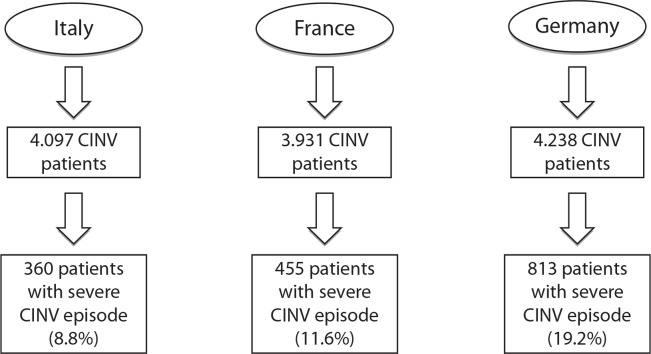
Figure 2 illustrates the flowchart that defines the subgroup of patients who experienced a severe CINV episode. The highest proportion of patients with a severe CINV episode was found in Germany (19.2%), followed by France and Italy (11.6% and 8.8% of CINV patients, respectively). Resource utilization in the management of severe CINV episodes confirmed a greater tendency to hospitalize patients in Germany and in France than in Italy (Table 4).
Figure 2.
Flowchart: focus on patients with severe CINV episode.
Table 4.
Resource utilization for patients with severe CINV episode (patients %).
| Resource utilization | Italy | France | Germany |
|---|---|---|---|
| Hospitalization following an unplanned visit to the oncology ward | 8.9 | 16.9 | 22.3 |
| Day hospital following an unplanned visit to the oncology ward | 48.8 | 19.8 | 16.5 |
| Hospitalization following an ER access | 6.7 | 25.1 | 14.7 |
| Day hospital following an ER access | 9.5 | 10.4 | 20.3 |
| Hospitalization extension for patients already hospitalized | 26.1 | 27.8 | 26.2 |
In Italy day hospital was the most common intervention for severe CINV patients coming from an unplanned visit, when compared with France and Germany (48.8% vs 19.8% and 16.5%), whereas, considering day hospital following an ER access, the percentages are 20.3% in Germany, 10.4% in France, and 9.5% in Italy. In Italy the highest percentage of patients who required a day hospital following an unplanned visit was found (48.8%), followed by France and Germany (19.8% and 16.5%, respectively). On the other hand, the observed percentages of patients who required a hospitalization extension do not show relevant differences among the three EU countries.
Table 5 reports estimated costs associated with a severe CINV episode by country, in terms of both average cost per patient and total expenditure for the NHSs. The total cost related to the entire patient population considered amounts to approximately €140,000 in Italy, €341,000 in France, and €827,000 in Germany. Consequently, in Italy the mean cost per patient with a severe CINV episode results in €389, about half of the average cost in France (€750), and a third of the average cost in Germany (€1,017). Again, total costs classified by resource utilization suggest a lower cost due to hospitalization in Italy compared with the other two European countries. Furthermore, these cost differences among the three countries are imputable to both different health-care service unit costs and different percentages of patients who required hospitalization, day hospital, and hospitalization extension.
Table 5.
Severe CINV episode cost.
| Resource utilization | Italy | France | Germany |
|---|---|---|---|
|
| |||
| Total cost | €140,047.8 | €341,305.1 | €826,583.30 |
| of which: | |||
| hospitalization following an unplanned visit to the oncology ward | €29,257.0 | €79,023.0 | €281,226.8 |
| day hospital following an unplanned visit to the oncology ward | €33,855.3 | €55,162.5 | €162,026.9 |
| hospitalization following an ER access | €49,151.6 | €107,820.0 | €174,203.8 |
| day hospital following an ER access | €11,982.1 | €55,898.0 | €46,074.0 |
| hospitalization extension for patients already hospitalized | €15,801.8 | €43,401.6 | €163,053.8 |
|
| |||
| Number of patients with severe CINV episode | 360 | 455 | 813 |
|
| |||
| Average cost per patient | €389.0 | €750.1 | €1.016.70 |
Discussion
This study aimed at investigating the management of CINV episodes in three European countries and at quantifying NHSs’ expenditure for severe CINV management. Our first finding confirmed that despite the currently available antiemetic options, a high percentage of patients still suffer from CINV; in our study, 34.4% of patients in Italy, 50.2% in France, and 40.4% in Germany experienced a CINV episode independently of its severity during a 6-month period.
Looking at the different types of management for CINV, our results showed that in France and Germany there is a higher number of hospitalizations and day hospital as compared with Italy. Ihbe-Heffinger et al. [9] found that the most frequently used resources in Germany, in three hospitals, and in three office-based facilities during a 5-day observation period were the need for rescue medication, additional office physician, outpatient hospital visits, and hospital admissions. These resources, especially attributable to severe patients, entail higher NHS costs as stated in the study by Haiderali et al., where it was found that patients who reported severe nausea had higher average costs due to health-care utilization (about €725 per patient) than patients who reported moderate (about €29 per patient) or mild nausea (about €6 per patient) [12]. These results are in line with our findings and support the choice to focus on costs associated with severe CINV episodes.
In the three European countries assessed in this study, severe CINV episodes represented a relevant percentage of the overall CINV episodes (Germany: 19.2%; France: 11.6%, and Italy: 8.8%). Although the quantification of the costs and impact of CINV in clinical practice is extremely relevant for resource allocation, studies that deal with the cost of the disease are limited [9,14], while most of the studies simply consider costs of antiemetics, and often only in relation to acute CINV [15]. In general, poorly controlled CINV was found to be associated with higher medical costs due to events such as unscheduled office visits, need for hydration, and ER admissions [11,16]. In our study, the average cost per patient with a severe CINV episode was €389, €750, and €1,017 in Italy, France, and Germany, respectively.
One limitation in our study was the information collection through an electronic survey based on health providers’ recall structured to capture the disease management without focusing on patient-level data. As a consequence, we were not able to differentiate between acute and delayed CINV or among cancer types and chemotherapy. However, we accepted this limitation in order to maintain the heterogeneity of cancer patients with a variety of malignancies and a broad range of chemotherapies. Another possible limitation could be the assumption to define severe CINV episodes on the basis of CINV management rather than on the clinical severity of the episode. The design of the study, different from an observational study, doesn’t foresee the collection of patient data. Moreover, it is important to consider that results coming from the present study are based on a sample of health providers who voluntarily took part in the survey and, being so, may not reflect the general population experience.
In conclusion, this study highlights a significant impact of CINV on NHS budget, mainly due to CINV episodes requiring hospitalization, day hospital, or hospitalization extension. These costs could be offset by optimizing the management of CINV episodes, by providing education to patients in order to limit hospital access and by improving utilization of existing antiemetic agents and new, more efficacious treatments. Such approaches can help to achieve not only an improvement in patient well-being but also a significant reduction of the budgetary impact of CINV on NHS.
Abbreviations:
- CINV
chemotherapy-induced nausea and vomiting
- ER
emergency room
- NHS
National Health Service
- GP
general practitioner
- TUC
Tariffa Unica Convenzionale
- GHS
Globally Harmonized System
References
- 1.Underhill ML, Chicko L, Berry DL. A nurse-led evidence-based practice project to monitor and improve the management of chemotherapy-induced nausea and vomiting. Clin J Oncol Nurs. 2015;19(1):38–40. doi: 10.1188/15.CJON.38-40. [DOI] [PubMed] [Google Scholar]
- 2.Gralla RJ, Osoba D, Kris MG, Kirkbride P, Hesketh PJ, Chinnery PW, Clark-Snow R, Gill DP, Groshen S, Grunberg S, Koeller JM, Morrow GR, Perez EA, Silber JH, Pfister DG. Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical Oncology. J Clin Oncol. 1999;17(9):2971–94. doi: 10.1200/JCO.1999.17.9.2971. [DOI] [PubMed] [Google Scholar]
- 3.Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (MASCC) Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol. 2006;17(1):20–8. doi: 10.1093/annonc/mdj078. [DOI] [PubMed] [Google Scholar]
- 4.Hesketh PJ, Kris MG, Grunberg SM, Beck T, Hainsworth JD, Harker G, Aapro MS, Gandara D, Lindley CM. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15(1):103–9. doi: 10.1200/JCO.1997.15.1.103. [DOI] [PubMed] [Google Scholar]
- 5.Feyer P, Jordan K. Update and new trends in antiemetic therapy: the continuing need for novel therapies. Ann Oncol. 2011;22(1):30–8. doi: 10.1093/annonc/mdq600. [DOI] [PubMed] [Google Scholar]
- 6.Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H. Chemotherapy-induced nausea and vomiting: Incidence and impact on patient quality of life at community oncology settings. Support Care Cancer. 2007;15(5):497–503. doi: 10.1007/s00520-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 7.Bouganim N, Dranitsaris G, Hopkins S, Vandermeer L, Godbout L, Dent S, Wheatley-Price P, Milano C, Clemons M. Prospective validation of risk prediction indexes for acute and delayed chemotherapy-induced nausea and vomiting. Curr Oncol. 2012;19(6):e414–21. doi: 10.3747/co.19.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, Daniele B, De Pouvourville G, Rubenstein EB, Daugaard G. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100(10):2261–8. doi: 10.1002/cncr.20230. [DOI] [PubMed] [Google Scholar]
- 9.Ihbe-Heffinger A, Ehlken B, Bernard R, Berger K, Peschel C, Eichler HG, Deuson R, Thödtmann J, Lordick F. The impact of delayed chemotherapy-induced nausea and vomiting on patients, health resource utilization and costs in German cancer centers. Ann Oncol. 2004;15(3):526–36. doi: 10.1093/annonc/mdh110. [DOI] [PubMed] [Google Scholar]
- 10.Viale PH, Grande C, Moore S. Efficacy and cost: avoiding undertreatment of chemotherapy-induced nausea and vomiting. Clin J Oncol Nurs. 2012;16(4):E133–41. doi: 10.1188/12.CJON.E133-E141. [DOI] [PubMed] [Google Scholar]
- 11.Stewart DJ, Dahrouge S, Coyle D, Evans WK. Costs of treating and preventing nausea and vomiting in patients receiving chemotherapy. J Clin Oncol. 1999;17(1):344–51. doi: 10.1200/JCO.1999.17.1.344. [DOI] [PubMed] [Google Scholar]
- 12.Haiderali A, Menditto L, Good M, Teitelbaum A, Wegner J. Impact on daily functioning and indirect/direct costs associated with chemotherapy-induced nausea and vomiting (CINV) in a U.S. population. Support Care Cancer. 2011;19(6):843–51. doi: 10.1007/s00520-010-0915-9. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien BJ, Rusthoven J, Rocchi A, Latreille J, Fine S, Vandenberg T, Laberge F. Impact of chemotherapy-associated nausea and vomiting on patients’ functional status and on costs: survey of five Canadian centres. CMAJ. 1993;149(3):296–302. [PMC free article] [PubMed] [Google Scholar]
- 14.Ballatori E, Roila F, Ruggeri B, Betti M, Sarti S, Soru G, Cruciani G, Di Maio M, Andrea B, Dueson RR. The impact of chemotherapy-induced nausea and vomiting on health-related quality of life. Support Care Cancer. 2007;15(2):179–85. doi: 10.1007/s00520-006-0109-7. [DOI] [PubMed] [Google Scholar]
- 15.Pradelli L, Eandi M. Palonosetron nella prevenzione della CINV in Italia: aspetti farmacologici clinici e farmacoeconomici. Farmeconomia e Percorsi Terapeutici. 2007;8:71. [PubMed] [Google Scholar]
- 16.Vanscoy GJ, Fortner B, Smith R, Weber R, Rihn TL. Preventing chemotherapy-induced nausea and vomiting: the economic implications of choosing antiemetics. Commun Oncol. 2005;2:127–32. doi: 10.1016/S1548-5315(11)70865-8. [DOI] [Google Scholar]